南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (9): 1889-1902.doi: 10.12122/j.issn.1673-4254.2025.09.09
• • 上一篇
杨子为1( ), 吕畅2, 董柱1, 计书磊1, 毕生辉1, 张雪花1, 王晓武1(
), 吕畅2, 董柱1, 计书磊1, 毕生辉1, 张雪花1, 王晓武1( )
)
收稿日期:2025-04-17
出版日期:2025-09-20
发布日期:2025-09-28
通讯作者:
王晓武
E-mail:a_1779874531@smu.edu.cn;xzwkwxw@smu.edu.cn
作者简介:杨子为,在读硕士研究生,E-mail: a_1779874531@smu.edu.cn
基金资助:
Ziwei YANG1( ), Chang LÜ2, Zhu DONG1, Shulei JI1, Shenghui BI1, Xuehua ZHANG1, Xiaowu WANG1(
), Chang LÜ2, Zhu DONG1, Shulei JI1, Shenghui BI1, Xuehua ZHANG1, Xiaowu WANG1( )
)
Received:2025-04-17
Online:2025-09-20
Published:2025-09-28
Contact:
Xiaowu WANG
E-mail:a_1779874531@smu.edu.cn;xzwkwxw@smu.edu.cn
Supported by:摘要:
目的 探讨传统中药金樱子(RLM)治疗肺动脉高压(PAH)的协同机制,通过网络药理学预测其活性成分与作用靶点,并通过体内外实验验证其抗增殖效应 降低细胞内钙离子浓度作用。 方法 网络药理学分析:筛选金樱子活性成分及PAH疾病靶点,构建“成分-靶点-疾病”互作网络,进行基因富集分析与分子对接验证。体外实验:设置对照组、缺氧组+溶剂对照、缺氧+金樱子(100 mg/mL)、缺氧+金樱子(200 mg/mL)、缺氧+金樱子(300 mg/mL),通过Western blotting和免疫荧光检测细胞增殖。动物实验:将大鼠随机分为5组:阴性对照组、野百合碱(MCT)+溶剂对照、野百合碱+金樱子(100 mg/mL),MCT+金樱子(200 mg/mL)、MCT+金樱子(300 mg/mL)。HE染色、免疫荧光染色观察肺血管形态学变化。 结果 网络分析筛选出7种核心活性成分(如β-谷甾醇、山奈酚)及39个关键靶点,分子对接显示Src为高亲和力靶点。KEGG富集分析显示,差异基因显著富集于钙信号通路和PI3K-AKT通路。体外实验表明,与对照组相比,Hypo组PCNA上调(P<0.001)。金樱子显著抑制PASMCs增殖(PCNA表达下调)。Western blotting实验证实金樱子可以抑制Src和AKT1的磷酸化。动物实验证实,金樱子治疗组肺动脉平均压(P<0.001)和右心室肥厚指数降低,右心室射血功能改善,肺血管壁增厚和纤维化减轻。 结论 金樱子通过调控Src-AKT1轴,发挥抗平滑肌增殖的作用,为肺动脉高压的治疗提供了新型天然药物候选策略。
杨子为, 吕畅, 董柱, 计书磊, 毕生辉, 张雪花, 王晓武. 金樱子通过调控Src-AKT1轴抑制肺动脉高压平滑肌增殖[J]. 南方医科大学学报, 2025, 45(9): 1889-1902.
Ziwei YANG, Chang LÜ, Zhu DONG, Shulei JI, Shenghui BI, Xuehua ZHANG, Xiaowu WANG. Rosa laevigata Michx. inhibits pulmonary arterial smooth muscle cell proliferation in hypertension by modulating the Src-AKT1 axis[J]. Journal of Southern Medical University, 2025, 45(9): 1889-1902.
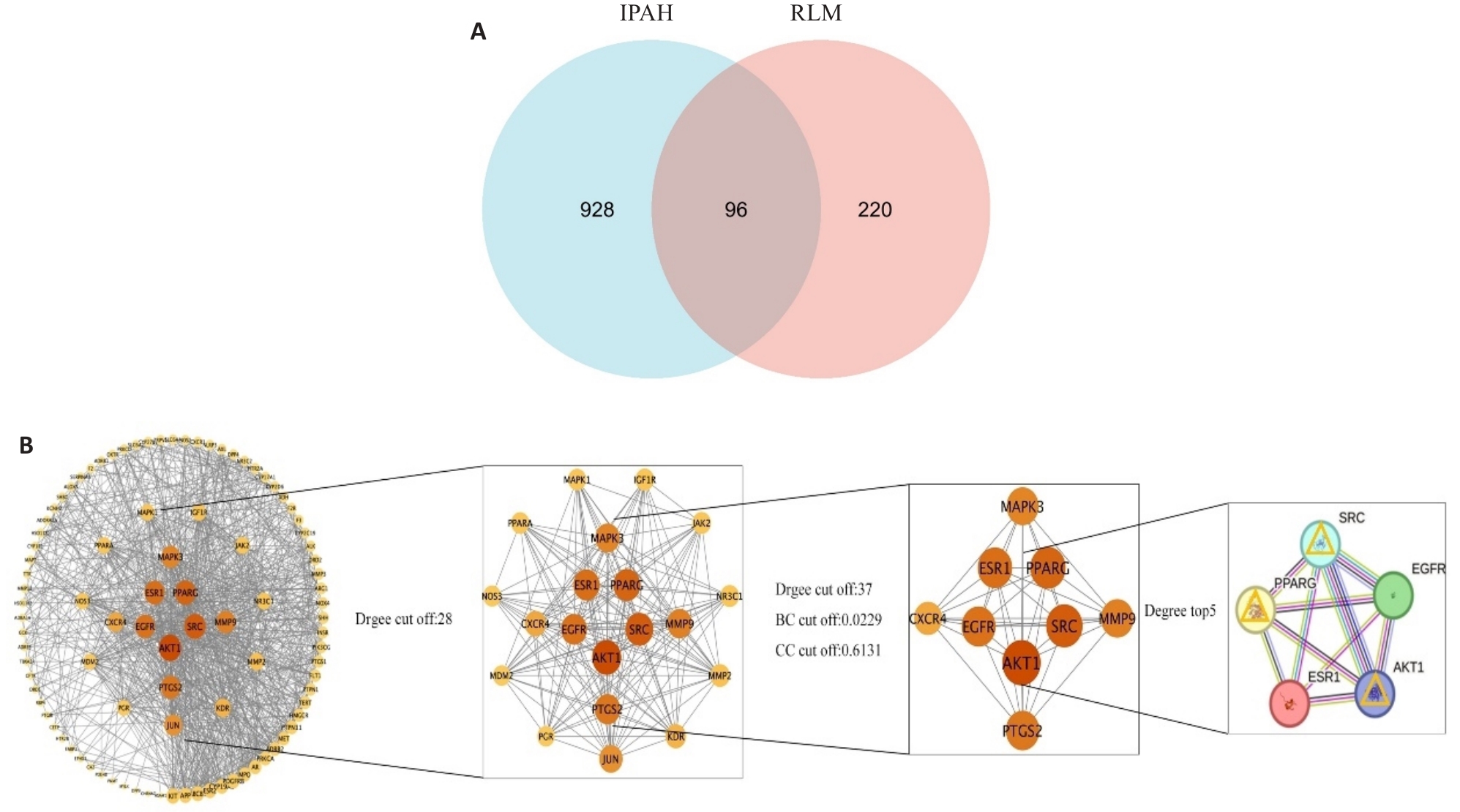
图2 金樱子靶点筛选与特发性肺动脉高压相关性研究
Fig.2 Target screening of RLM for treatment of pulmonary hypertension. A: Venn diagram of the intersecting targets of RLM and idiopathic pulmonary hypertension. B: Construction and screening process of the component-target network. The targets such as SRC and AKT1 show prominent network topological parameters (degree, betweenness centrality), indicating their core roles in regulating smooth muscle cell proliferation.
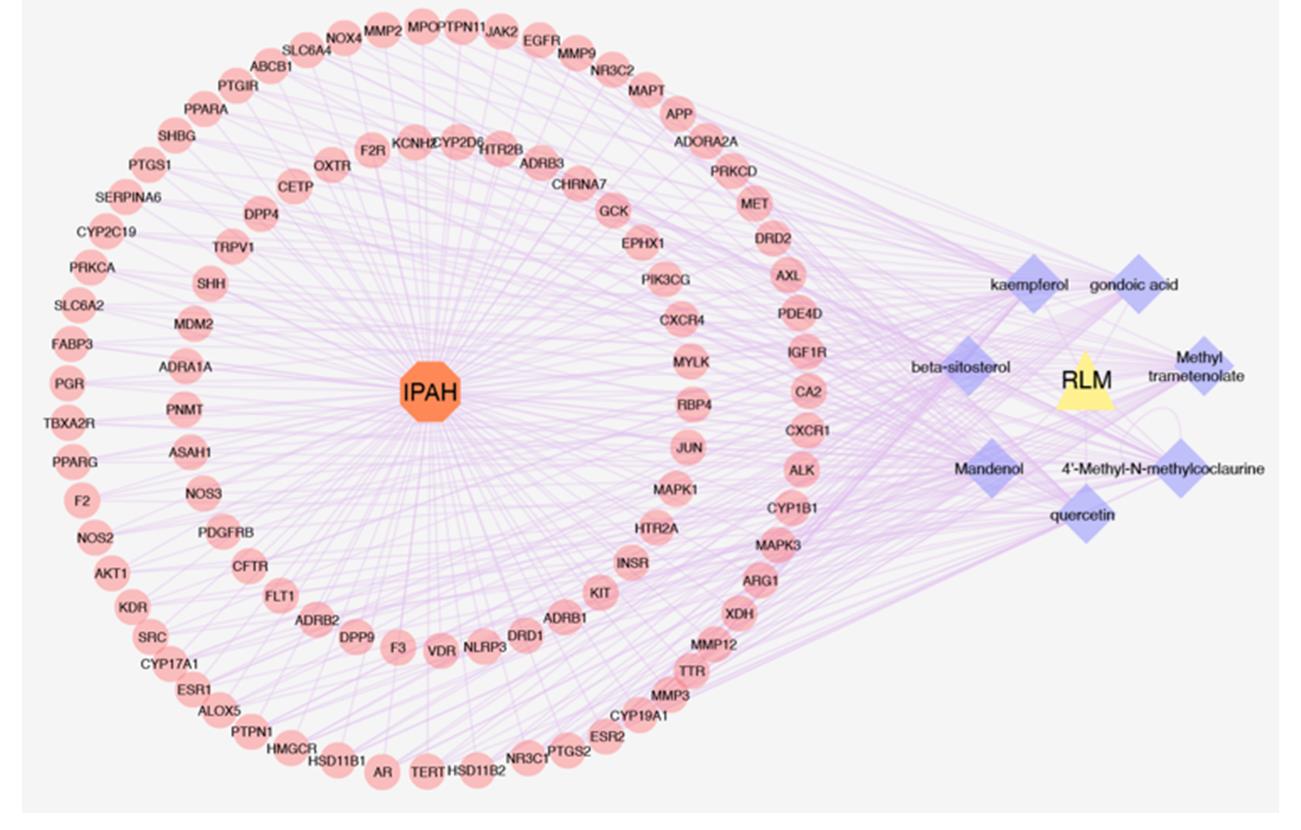
图3 基于集合靶标构建蛋白质相互作用网络, 并针对核心靶标建立独立网络
Fig.3 Development of the PPI network using the collective targets and a separate PPI network focusing on the core targets.
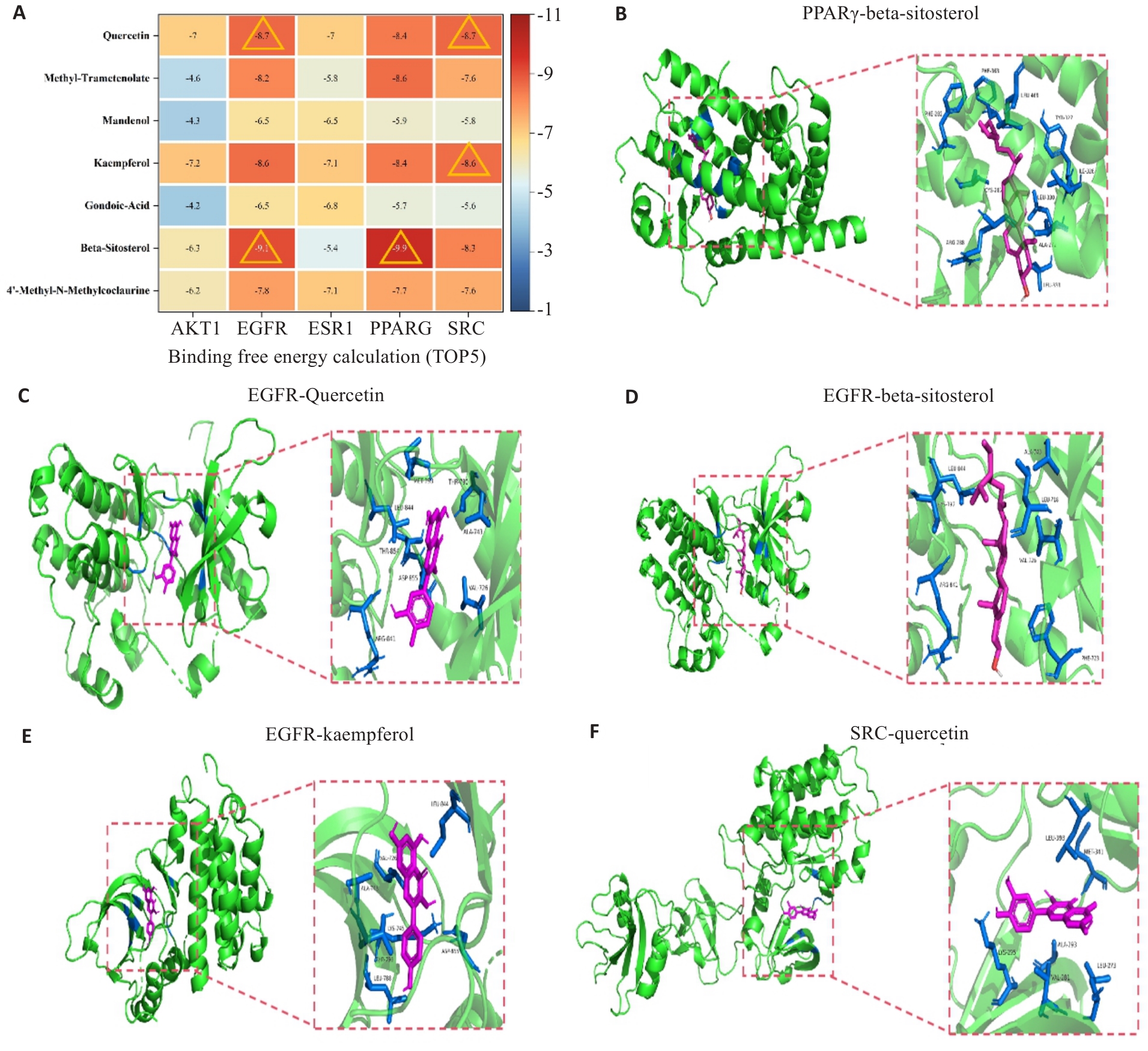
图5 分子对接分析展示了金樱子核心化合物与PAH中RLM治疗靶点之间的潜在结合模式和相互作用的可靠性
Fig.5 Molecular docking analysis of potential binding modes and reliability of interactions between the core compounds and the targets in RLM treatment of PAH. A: Calculation of the binding energy between 5 targets and 7 active components. The triangles indicate the top 5 binding energies. B: Schematic diagram of the three-dimensional structure of PPARγ-Beta-Sitosterol molecular docking result. C: EGFR-Kaempferol molecular docking result. D: EGFR-Beta-Sitosterol molecular docking result. E: EGFR-Kaempferol molecular docking result. F: SRC-Quercetin molecular docking result.
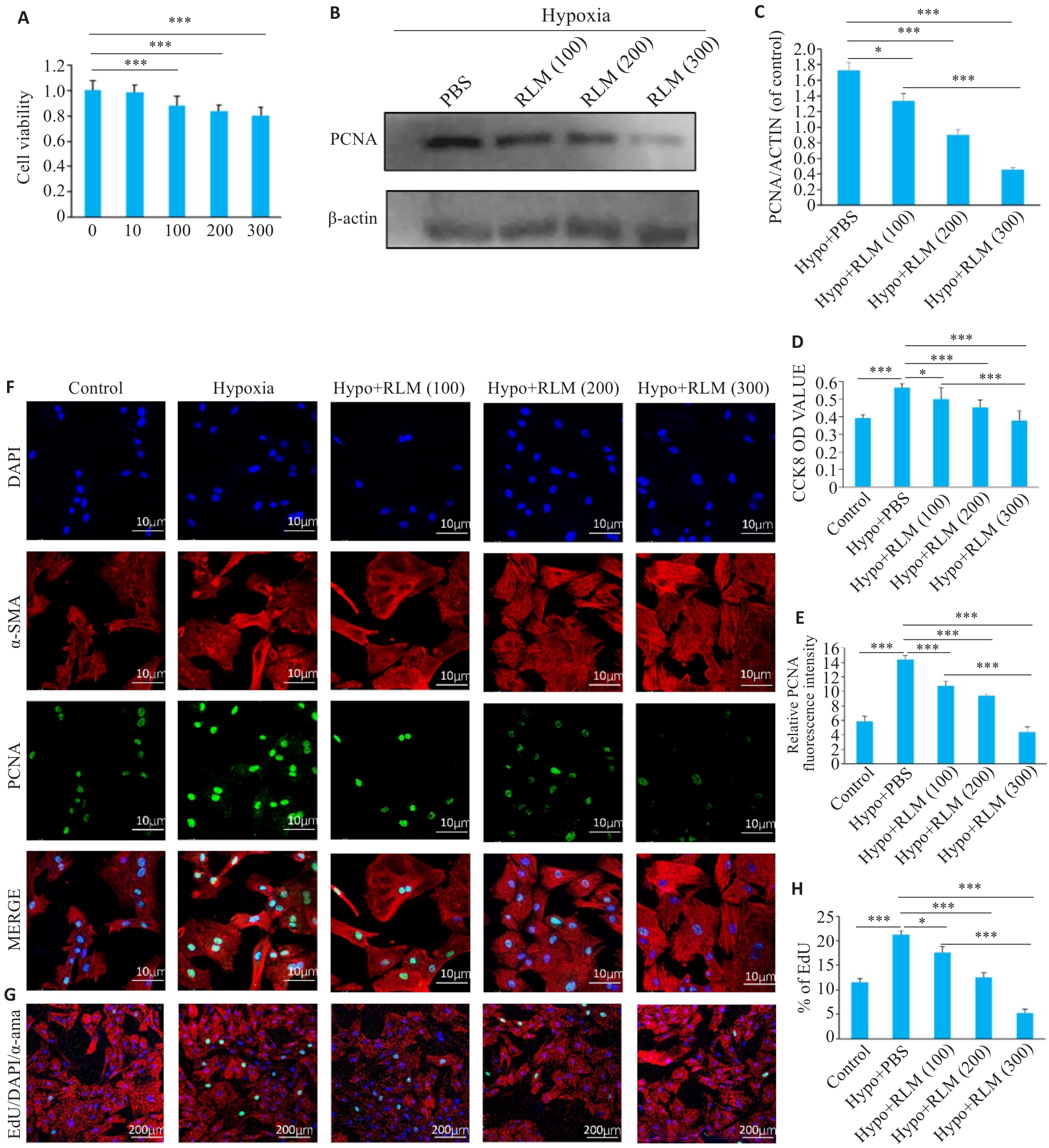
图 6 金樱子抑制缺氧导致的肺血管平滑肌细胞的增殖
Fig.6 Inhibitory effect of RLM on proliferation of rat pulmonary arterial smooth muscle cells. A: Bar chart showing the effect of different concentrations of RLM on cell viability measured by CCK8 assay. B, C: Western blotting of PCNA in RLM-treated cells. D: Results of CCK-8 assay of RLM-treated rat pulmonary arterial smooth muscle cells under hypoxia condition. E: Quantitative analysis of PCNA expression by immunofluorescence after RLM treatment. F: Immunofluorescence assay of PCNA in RLM-treated cells (scale bar=10 μm). G:Representive image of EdU assay. H: Quantitative analysis bar graph of EdU. All data were analyzed by one-way ANOVA plus Dunnett multiple comparison test. *P<0.05, ***P<0.001.
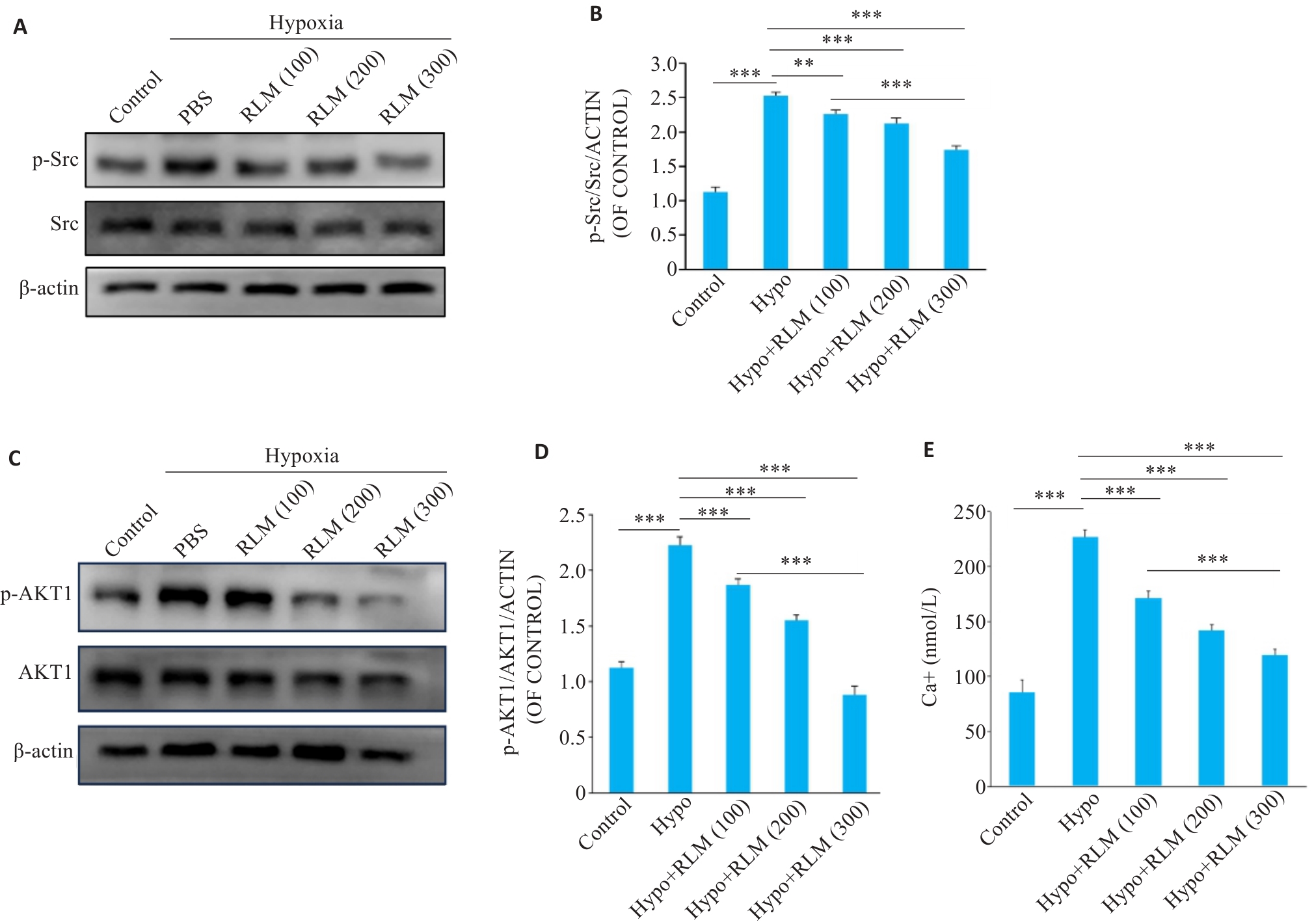
图 7 RLM在体外抑制低氧诱导的Src 、AKT1磷酸化和降低胞内钙离子浓度
Fig.7 RLM inhibits hypoxia-induced phosphorylation of AKT1 and Src. A: Western blotting of Src and p-Src in RLM-treated cells. B: Quantitative analysis of p-Src/Src expression. C: Western blotting of AKT1 and p-AKT1 in RLM-treated cells. D: Quantitative analysis of p-AKT1/AKT1 expression. E: Calcium ion concentration measurements show a decrease in intracellular calcium levels following RLM treatment. All data were analyzed by one-way ANOVA plus Dunnett multiple comparison test. **P<0.01, ***P<0.001.
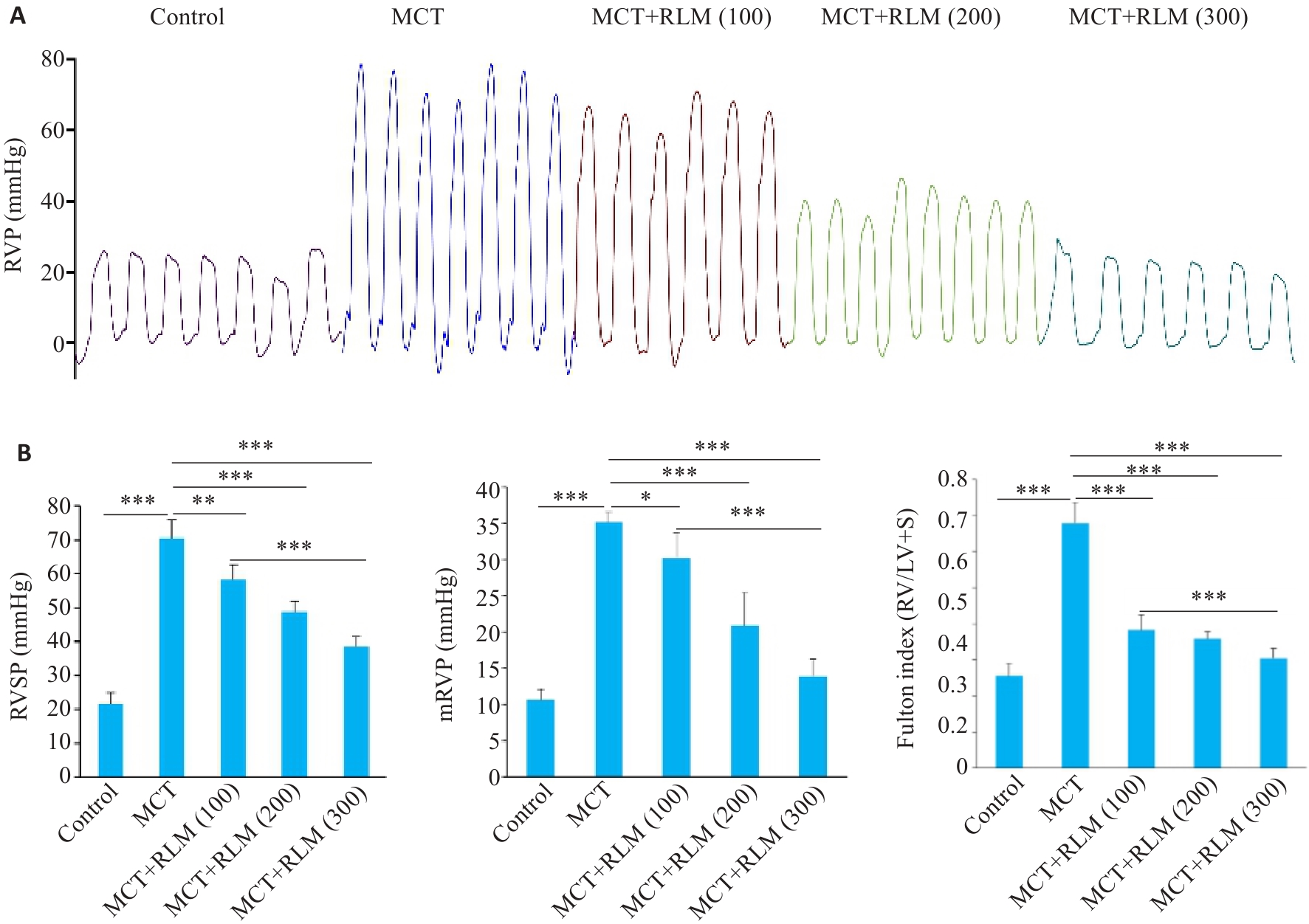
图 8 RLM在MCT诱导的肺动脉高压大鼠模型中改善了血流动力学变化
Fig.8 RLM relieves pulmonary hypertension in MCT-induced PAH rat model. A: Schematic diagram and trace of animal experiments. B: Data analysis of RVSP, mRVP and Fulton Index [RV/(LV+S)] in the 5 groups of rats. All data were analyzed by one-way ANOVA plus Dunnett multiple comparison test. *P<0.05, **P<0.01, ***P<0.001.
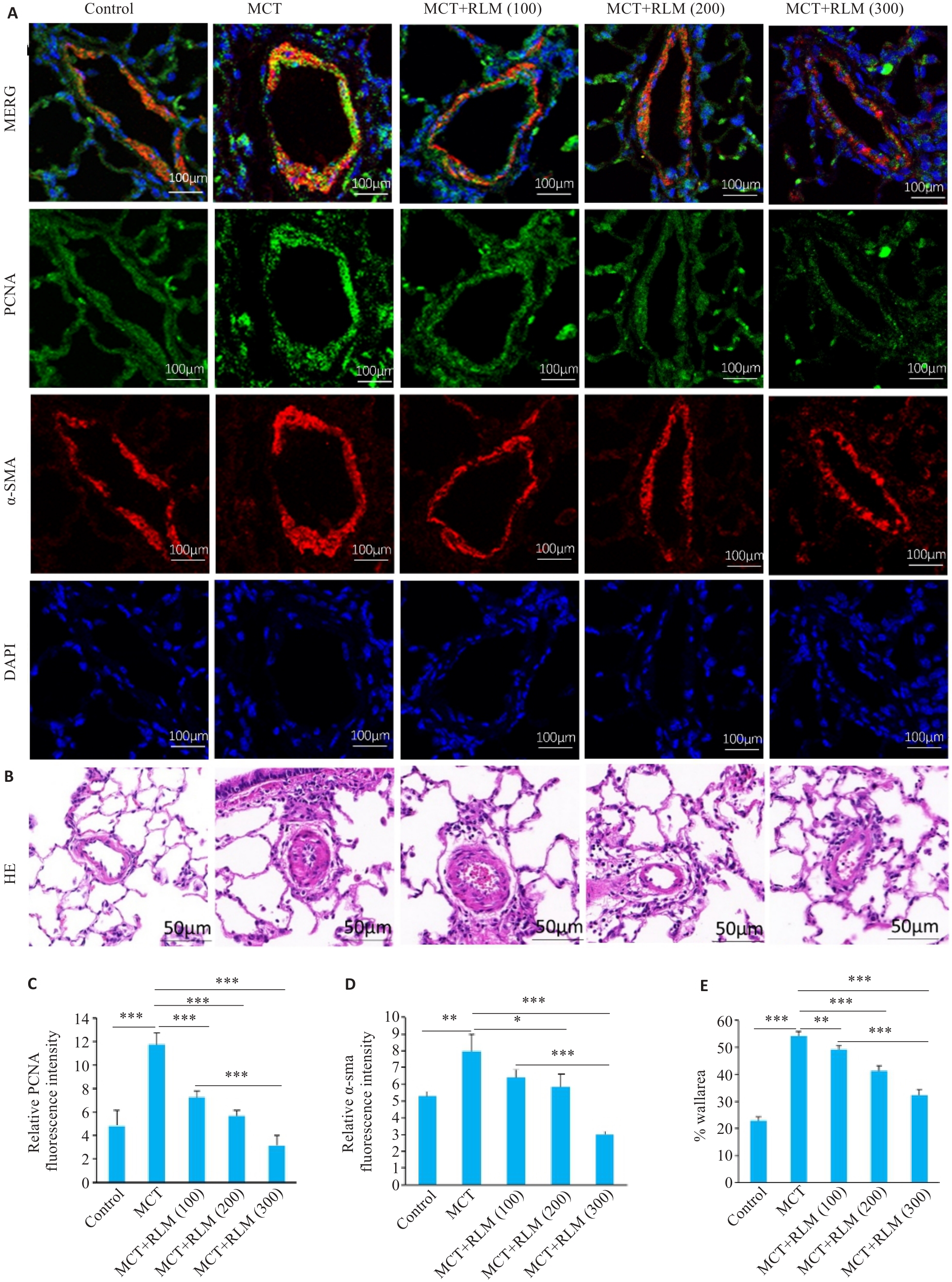
图 10 RLM抑制了MCT诱导的肺动脉高压大鼠模型中肺血管的增殖
Fig.10 RLM inhibits hypoxia-induced proliferation in MCT-induced PAH rat model. A: Representative images of PCNA and α-SMA immunofluorescence post‐RLM treatment. B: Representative images of HE staining. C. Quantitative analysis of PCNA immunofluorescence post‐RLM treatment.D: Quantitative analysis of α-SMA immunofluorescence post‐RLM treatment. E. Quantitative analysis of HE staining. All data were analyzed by one-way ANOVA plus Dunnett multiple comparison test. *P<0.05, **P<0.01, ***P<0.001.
| [1] | Hassoun PM. Pulmonary arterial hypertension[J]. N Engl J Med, 2021, 385(25): 2361-76. doi:10.1056/nejmra2000348 |
| [2] | Gallardo-Vara E, Ntokou A, Dave JM, et al. Vascular pathobiology of pulmonary hypertension[J]. J Heart Lung Transplant, 2023, 42(5): 544-52. doi:10.1016/j.healun.2022.12.012 |
| [3] | Xu WL, Janocha AJ, Erzurum SC. Metabolism in pulmonary hypertension[J]. Annu Rev Physiol, 2021, 83: 551-76. doi:10.1146/annurev-physiol-031620-123956 |
| [4] | Ghofrani HA, Gomberg-Maitland M, Zhao L, et al. Mechanisms and treatment of pulmonary arterial hypertension[J]. Nat Rev Cardiol, 2025, 22(2): 105-20. doi:10.1038/s41569-024-01064-4 |
| [5] | Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: a review[J]. JAMA, 2022, 327(14): 1379-91. doi:10.1001/jama.2022.4402 |
| [6] | Zhang JR, Ouyang X, Hou C, et al. Natural ingredients from Chinese materia Medica for pulmonary hypertension[J]. Chin J Nat Med, 2021, 19(11): 801-14. doi:10.1016/s1875-5364(21)60092-4 |
| [7] | Yu ZJ, Xiao J, Chen X, et al. Bioactivities and mechanisms of natural medicines in the management of pulmonary arterial hypertension[J]. Chin Med, 2022, 17(1): 13. doi:10.1186/s13020-022-00568-w |
| [8] | Quan XX, Huang YY, Chen L, et al. Traditional uses, phytochemical, pharmacology, quality control and modern applications of two important Chinese medicines from Rosa laevigata Michx. a review[J]. Front Pharmacol, 2022, 13: 1012265. doi:10.3389/fphar.2022.1012265 |
| [9] | Ding XR, Zheng RF, Kaderyea K, et al. Chinese herbal for mula Regan Saibisitan alleviates airway inflammation of chronic bronchitis via inhibiting the JAK2/STAT3 pathway[J]. J Ethnopharmacol, 2025, 341: 119336. doi:10.1016/j.jep.2025.119336 |
| [10] | Zhang BB, Zeng MN, Wang R, et al. Plantaginis Herba attenuates adriamycin-induced nephropathy: Molecular mechanism insights by integrated transcriptomic and experimental validation[J]. J Ethnopharmacol, 2025, 341: 119331. doi:10.1016/j.jep.2025.119331 |
| [11] | Bagchi A, Raha A, Das C, et al. Hepatoprotective efficacy of Argemone mexicana L. root in paracetamol-induced hepatotoxicity in a rat model[J]. J Ethnopharmacol, 2025, 341: 119329. doi:10.1016/j.jep.2025.119329 |
| [12] | Liang CX, Chen SJ, Liu CQ, et al. Active components unveiling and pharmacodynamic research on Valeriana jatamansi Jones for ameliorating ulcerative colitis based on pharmacokinetics and network pharmacology[J]. J Ethnopharmacol, 2025, 341: 119299. doi:10.1016/j.jep.2024.119299 |
| [13] | Ko HM, Choi SH, Kim Y, et al. Effect of Rosa laevigata on PM10-induced inflammatory response of human lung epithelial cells[J]. Evid Based Complement Alternat Med, 2020, 2020: 2893609. doi:10.1155/2020/2893609 |
| [14] | Liu XG, Liu J, Liu CF, et al. Selenium-containing polysaccharides isolated from Rosa laevigata Michx fruits exhibit excellent anti-oxidant and neuroprotective activity in vitro [J]. Int J Biol Macromol, 2022, 209(Pt A): 1222-33. doi:10.1016/j.ijbiomac.2022.04.146 |
| [15] | Zhang S, Lu BN, Han X, et al. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice[J]. Food Chem Toxicol, 2013, 55: 60-9. doi:10.1016/j.fct.2012.12.041 |
| [16] | Chen S, Wang L, Rong S, et al. Extraction, purification, chemical characterization, and in vitro hypoglycemic activity of polysaccharides derived from Rosa laevigata Michx[J]. Int J Biol Macromol, 2024, 279(Pt 1): 135116. doi:10.1016/j.ijbiomac.2024.135116 |
| [17] | Zhuang YX, Coppock JD, Haugrud AB, et al. Dichloroacetate and quercetin prevent cell proliferation, induce cell death and slow tumor growth in a mouse model of HPV-positive head and neck cancer[J]. Cancers (Basel), 2024, 16(8): 1525. doi:10.3390/cancers16081525 |
| [18] | Du L, Li CC, Qian X, et al. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway[J]. Pharmacol Res, 2019, 146: 104320. doi:10.1016/j.phrs.2019.104320 |
| [19] | Ali F, Wang D, Cheng Y, et al. Quercetin attenuates angiotensin II-induced proliferation of vascular smooth muscle cells and p53 pathway activation in vitro and in vivo[J]. Biofactors, 2023, 49(4): 956-70. doi:10.1002/biof.1959 |
| [20] | Hou XM, Zhang MS, Qin XJ. Vasodilation of quercetin on rat renal artery and the relationship with L-type voltage-gated Ca2+ channels and protein kinase C[J]. Sheng Li Xue Bao Acta Physiol Sin, 2017, 69(6): 775-80. |
| [21] | Blaškovič D, Zižková P, Držík F, et al. Modulation of rabbit muscle sarcoplasmic reticulum Ca(2+)-ATPase activity by novel quercetin derivatives[J]. Interdiscip Toxicol, 2013, 6(1): 3-8. doi:10.2478/intox-2013-0001 |
| [22] | Baran I, Ganea C. RyR3 in situ regulation by Ca(2+) and quercetin and the RyR3-mediated Ca(2+) release flux in intact Jurkat cells[J]. Arch Biochem Biophys, 2013, 540(1/2): 145-59. doi:10.1016/j.abb.2013.10.024 |
| [23] | Yang DP, Dong WP, Yang YC, et al. Tetramethylpyrazine improves monocrotaline-induced pulmonary hypertension through the ROS/iNOS/PKG-1 axis[J]. J Healthc Eng, 2022, 2022: 1890892. doi:10.1155/2022/1890892 |
| [24] | Ma XM, Cao YQ, Yang DP, et al. Inhibition of RUNX1 slows the progression of pulmonary hypertension by targeting CBX5[J]. Biomol Biomed, 2025, 25(2): 472-81. doi:10.17305/bb.2024.10720 |
| [25] | Kazmirczak F, Vogel NT, Prisco SZ, et al. Ferroptosis-mediated inflammation promotes pulmonary hypertension[J]. Circ Res, 2024, 135(11): 1067-83. doi:10.1161/circresaha.123.324138 |
| [26] | Yung LM, Nikolic I, Paskin-Flerlage SD, et al. A selective transforming growth factor‑β ligand trap attenuates pulmonary hypertension[J]. Am J Respir Crit Care Med, 2016, 194(9): 1140-51. doi:10.1164/rccm.201510-1955oc |
| [27] | Kim K, Kim S, Moh SH, et al. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression[J]. Mol Cell Biochem, 2015, 407(1/2): 143-9. doi:10.1007/s11010-015-2464-5 |
| [28] | Wang D, Ali F, Liu HX, et al. Quercetin inhibits angiotensin II-induced vascular smooth muscle cell proliferation and activation of JAK2/STAT3 pathway: a target based networking pharmacology approach[J]. Front Pharmacol, 2022, 13: 1002363. doi:10.3389/fphar.2022.1002363 |
| [29] | Wang J, Wu QB, Ding L, et al. Therapeutic effects and molecular mechanisms of bioactive compounds against respiratory diseases: traditional Chinese medicine theory and high-frequency use[J]. Front Pharmacol, 2021, 12: 734450. doi:10.3389/fphar.2021.734450 |
| [30] | Ding YY, Che DL, Li CM, et al. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations[J]. Int Immunopharmacol, 2019, 66: 185-97. doi:10.1016/j.intimp.2018.11.025 |
| [31] | Zhou C, Townsley MI, Alexeyev M, et al. Endothelial hyperpermeability in severe pulmonary arterial hypertension: role of store-operated calcium entry[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 311(3): L560-9. doi:10.1152/ajplung.00057.2016 |
| [32] | Wang J, Xu CY, Zheng QY, et al. Orai1, 2, 3 and STIM1 promote store-operated calcium entry in pulmonary arterial smooth muscle cells[J]. Cell Death Discov, 2017, 3: 17074. doi:10.1038/cddiscovery.2017.74 |
| [33] | Weatherald J, Hemnes AR, Maron BA, et al. Phenotypes in pulmonary hypertension[J]. Eur Respir J, 2024, 64(3): 2301633. doi:10.1183/13993003.01633-2023 |
| [34] | Egom EE. Pulmonary arterial hypertension due to NPR-C mutation: a novel paradigm for normal and pathologic remodeling[J]? Int J Mol Sci, 2019, 20(12): 3063. doi:10.3390/ijms20123063 |
| [35] | Wang J, Niu YQ, Luo LJ, et al. Decoding CeRNA regulatory network in the pulmonary artery of hypoxia-induced pulmonary hypertension (HPH) rat model[J]. Cell Biosci, 2022, 12(1): 27. doi:10.1186/s13578-022-00762-1 |
| [36] | Klouda T, Tsikis ST, Hirsch TI, et al. Smooth muscle Cxcl12 contributions to vascular remodeling in flow and hypoxia-induced pulmonary hypertension[J]. J Biol Chem, 2025, 301(6): 110207. doi:10.1016/j.jbc.2025.110207 |
| [37] | Huang HJ, Lin DH, Hu L, et al. RNA binding protein quaking promotes hypoxia-induced smooth muscle reprogramming in pulmonary hypertension[J]. Am J Respir Cell Mol Biol, 2023, 69(2): 159-71. doi:10.1165/rcmb.2022-0349oc |
| [38] | Tang LX, Cai Q, Wang X, et al. Canagliflozin ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting pulmonary arterial smooth muscle cell proliferation[J]. Clin Exp Hypertens, 2023, 45(1): 2278205. doi:10.1080/10641963.2023.2278205 |
| [39] | Wang LL, Moonen JR, Cao AQ, et al. Dysregulated smooth muscle cell BMPR2-ARRB2 axis causes pulmonary hypertension[J]. Circ Res, 2023, 132(5): 545-64. doi:10.1161/circresaha.121.320541 |
| [40] | Ji L, Su SS, Xin MY, et al. Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2α-Arg-NO axis and PI3K-AKT-ENOS-NO signaling pathway[J]. Phytomedicine, 2022, 104: 154329. doi:10.1016/j.phymed.2022.154329 |
| [41] | Wang J, Xuan B, Song BM, et al. MTMR7 attenuates the proliferation and migration of pulmonary arterial smooth muscle cells in pulmonary hypertension by suppressing ERK/STAT3 signaling[J]. Mol Cell Biochem, 2025, 480(6): 3799-812. doi:10.1007/s11010-025-05217-y |
| [42] | Huang L, Li HY, Huang SQ, et al. Notopterol attenuates monocrotaline-induced pulmonary arterial hypertension in rat[J]. Front Cardiovasc Med, 2022, 9: 859422. doi:10.3389/fcvm.2022.859422 |
| [43] | Manning BD, Toker A. AKT/PKB signaling: navigating the network[J]. Cell, 2017, 169(3): 381-405. doi:10.1016/j.cell.2017.04.001 |
| [44] | Luo J, Zeng BL, Tao CF, et al. ClpP regulates breast cancer cell proliferation, invasion and apoptosis by modulating the Src/PI3K/Akt signaling pathway[J]. PeerJ, 2020, 8: e8754. doi:10.7717/peerj.8754 |
| [45] | Cai BR, Yang L, Jung YD, et al. PTEN: an emerging potential target for therapeutic intervention in respiratory diseases[J]. Oxid Med Cell Longev, 2022, 2022: 4512503. doi:10.1155/2022/4512503 |
| [46] | Sala V, Margaria JP, Murabito A, et al. Therapeutic targeting of PDEs and PI3K in heart failure with preserved ejection fraction (HFpEF)[J]. Curr Heart Fail Rep, 2017, 14(3): 187-96. doi:10.1007/s11897-017-0331-2 |
| [47] | Mirhadi E, Roufogalis BD, Banach M, et al. Resveratrol: Mechanistic and therapeutic perspectives in pulmonary arterial hypertension[J]. Pharmacol Res, 2021, 163: 105287. doi:10.1016/j.phrs.2020.105287 |
| [48] | Hsieh MW, Wang WT, Yeh JL, et al. The potential application and promising role of targeted therapy in pulmonary arterial hypertension[J]. Biomedicines, 2022, 10(6): 1415. doi:10.3390/biomedicines10061415 |
| [49] | Kim OH, Choi YW, Park JH, et al. Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis[J]. Life Sci, 2022, 308: 120936. doi:10.1016/j.lfs.2022.120936 |
| [50] | Lan YN, Lu J, Zhang SH, et al. Piezo1-mediated mechano-transduction contributes to disturbed flow-induced atherosclerotic endothelial inflammation[J]. J Am Heart Assoc, 2024, 13(21): e035558. doi:10.1161/JAHA.123.035558 |
| [51] | Zhang S, Wang J, Qi XM, et al. Plasminogen activator Inhibitor-2 inhibits pulmonary arterial smooth muscle cell proliferation in pulmonary arterial hypertension via PI3K/Akt and ERK signaling[J]. Exp Cell Res, 2021, 398(1): 112392. doi:10.1016/j.yexcr.2020.112392 |
| [1] | 呼琴, 金华. 清肾颗粒通过调控miR-23b及Nrf2通路改善慢性肾脏病湿热证患者的肾功能:基于网络药理学和临床试验[J]. 南方医科大学学报, 2025, 45(9): 1867-1879. |
| [2] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [3] | 王立明, 陈宏睿, 杜燕, 赵鹏, 王玉洁, 田燕歌, 刘新光, 李建生. 益气滋肾方通过抑制PI3K/Akt/NF-κB通路改善小鼠慢性阻塞性肺疾病的炎症反应[J]. 南方医科大学学报, 2025, 45(7): 1409-1422. |
| [4] | 朱胤福, 李怡燃, 王奕, 黄颖而, 龚昆翔, 郝文波, 孙玲玲. 桂枝茯苓丸活性成分常春藤皂苷元通过抑制JAK2/STAT3通路抑制宫颈癌细胞的生长[J]. 南方医科大学学报, 2025, 45(7): 1423-1433. |
| [5] | 何丽君, 陈晓菲, 闫陈昕, 师林. 扶正化积汤治疗非小细胞肺癌的分子机制:基于网络药理学及体外实验验证[J]. 南方医科大学学报, 2025, 45(6): 1143-1152. |
| [6] | 李国永, 黎仁玲, 刘艺婷, 柯宏霞, 李菁, 王新华. 牛蒡子治疗小鼠病毒性肺炎后肺纤维化的机制:基于代谢组学、网络药理学和实验验证方法[J]. 南方医科大学学报, 2025, 45(6): 1185-1199. |
| [7] | 管丽萍, 颜燕, 卢心怡, 李智峰, 高晖, 曹东, 侯晨曦, 曾靖宇, 李欣怡, 赵洋, 王俊杰, 方会龙. 复方积雪草减轻小鼠日本血吸虫引起的肝纤维化:通过调控TLR4/MyD88通路抑制炎症-纤维化级联反应[J]. 南方医科大学学报, 2025, 45(6): 1307-1316. |
| [8] | 唐培培, 谈勇, 殷燕云, 聂晓伟, 黄菁宇, 左文婷, 李玉玲. 调周滋阴方治疗早发性卵巢功能不全的疗效、安全性及作用机制[J]. 南方医科大学学报, 2025, 45(5): 929-941. |
| [9] | 梁晓涛, 熊一凡, 刘雪琪, 梁小珊, 朱晓煜, 谢炜. 活血疏风颗粒通过抑制TLR4/NF-κB通路改善慢性偏头痛小鼠的中枢敏化[J]. 南方医科大学学报, 2025, 45(5): 986-994. |
| [10] | 冉念东, 刘杰, 徐剑, 张永萍, 郭江涛. 黑骨藤正丁醇萃取成分治疗大鼠阿尔茨海默病的药效学及作用机制[J]. 南方医科大学学报, 2025, 45(4): 785-798. |
| [11] | 徐皓男, 张放, 黄钰莹, 姚其盛, 管悦琴, 陈浩. 百蕊草通过调节肠道菌群和调控EGFR/PI3K/Akt信号通路改善小鼠抗生素相关性腹泻[J]. 南方医科大学学报, 2025, 45(2): 285-295. |
| [12] | 高俊杰, 叶开, 吴竞. 槲皮素通过调控TP53基因抑制肾透明细胞癌的增殖和迁移[J]. 南方医科大学学报, 2025, 45(2): 313-321. |
| [13] | 刘莹, 李柏睿, 李永财, 常禄博, 王娇, 杨琳, 颜永刚, 屈凯, 刘继平, 张岗, 沈霞. 加味逍遥丸通过神经递质调节、抗炎抗氧化及肠道菌群调控改善大鼠的抑郁样行为[J]. 南方医科大学学报, 2025, 45(2): 347-358. |
| [14] | 褚乔, 王小娜, 续佳颖, 彭荟林, 赵裕琳, 张静, 陆国玉, 王恺. 白头翁皂苷D通过多靶点和多途径抑制三阴性乳腺癌侵袭转移[J]. 南方医科大学学报, 2025, 45(1): 150-161. |
| [15] | 龙秀鹏, 陶顺, 阳绅, 李素云, 饶利兵, 李莉, 张哲. 槲皮素通过抑制MAPK信号通路改善心力衰竭[J]. 南方医科大学学报, 2025, 45(1): 187-196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||