Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (5): 929-941.doi: 10.12122/j.issn.1673-4254.2025.05.05
Previous Articles Next Articles
Peipei TANG( ), Yong TAN(
), Yong TAN( ), Yanyun YIN, Xiaowei NIE, Jingyu HUANG, Wenting ZUO, Yuling LI
), Yanyun YIN, Xiaowei NIE, Jingyu HUANG, Wenting ZUO, Yuling LI
Received:2024-03-26
Online:2025-05-20
Published:2025-05-23
Contact:
Yong TAN
E-mail:756919091@qq.com;xijun1025@163.com
Peipei TANG, Yong TAN, Yanyun YIN, Xiaowei NIE, Jingyu HUANG, Wenting ZUO, Yuling LI. Tiaozhou Ziyin recipe for treatment of premature ovarian insufficiency: efficacy, safety and mechanism[J]. Journal of Southern Medical University, 2025, 45(5): 929-941.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.05.05
| TCM | MOL ID | Molecule name | OB (%) | DL | |
|---|---|---|---|---|---|
| Shudi baishao shanzhuyu | A1 | MOL000359 | Sitosterol | 36.91 | 0.75 |
| Shudi shanzhuyu sharen | B1 | MOL000449 | Stigmasterol | 43.83 | 0.76 |
| Baishao shanzhuyu tusizi sharen | C1 | MOL000358 | Beta-sitosterol | 36.91 | 0.75 |
| Baishao tusizi | D1 | MOL000422 | Kaempferol | 41.88 | 0.24 |
| Shanzhuyu danshen sharen | E1 | MOL001771 | Poriferast-5-en-3beta-ol | 36.91 | 0.75 |
| Baishao | BS1 | MOL001919 | (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5, 6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 43.56 | 0.53 |
| BS2 | MOL001924 | Paeoniflorin | 53.87 | 0.79 | |
| Shanzhuyu | SZY1 | MOL001494 | Mandenol | 42 | 0.19 |
| SZY2 | MOL001495 | Ethyl linolenate | 46.1 | 0.2 | |
| Tusizi | TSZ1 | MOL001558 | Sesamin | 56.55 | 0.83 |
| TSZ8 | MOL000098 | Quercetin | 46.43 | 0.28 | |
| Danshen | DS1 | MOL001601 | 1,2,5,6-Tetrahydrotanshinone | 38.75 | 0.36 |
| DS8 | MOL000006 | Luteolin | 36.16 | 0.25 | |
| Sharen | SR1 | MOL001755 | 24-Ethylcholest-4-en-3-one | 36.08 | 0.76 |
| SR2 | MOL001973 | Sitosteryl acetate | 40.39 | 0.85 |
Tab.1 List of effective active ingredients in tiaozhou ziyin recipe
| TCM | MOL ID | Molecule name | OB (%) | DL | |
|---|---|---|---|---|---|
| Shudi baishao shanzhuyu | A1 | MOL000359 | Sitosterol | 36.91 | 0.75 |
| Shudi shanzhuyu sharen | B1 | MOL000449 | Stigmasterol | 43.83 | 0.76 |
| Baishao shanzhuyu tusizi sharen | C1 | MOL000358 | Beta-sitosterol | 36.91 | 0.75 |
| Baishao tusizi | D1 | MOL000422 | Kaempferol | 41.88 | 0.24 |
| Shanzhuyu danshen sharen | E1 | MOL001771 | Poriferast-5-en-3beta-ol | 36.91 | 0.75 |
| Baishao | BS1 | MOL001919 | (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5, 6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 43.56 | 0.53 |
| BS2 | MOL001924 | Paeoniflorin | 53.87 | 0.79 | |
| Shanzhuyu | SZY1 | MOL001494 | Mandenol | 42 | 0.19 |
| SZY2 | MOL001495 | Ethyl linolenate | 46.1 | 0.2 | |
| Tusizi | TSZ1 | MOL001558 | Sesamin | 56.55 | 0.83 |
| TSZ8 | MOL000098 | Quercetin | 46.43 | 0.28 | |
| Danshen | DS1 | MOL001601 | 1,2,5,6-Tetrahydrotanshinone | 38.75 | 0.36 |
| DS8 | MOL000006 | Luteolin | 36.16 | 0.25 | |
| Sharen | SR1 | MOL001755 | 24-Ethylcholest-4-en-3-one | 36.08 | 0.76 |
| SR2 | MOL001973 | Sitosteryl acetate | 40.39 | 0.85 |
| Name | Degree |
|---|---|
| SRC | 104 |
| TP53 | 96 |
| STAT3 | 88 |
| PIK3CA | 86 |
| MAPK3 | 86 |
| HRAS | 84 |
| PIK3R1 | 84 |
| MAPK1 | 82 |
| HSP90AA1 | 80 |
| PTPN11 | 64 |
Tab.2 Core targets of Tiaozhou Ziyin recipe in the treatment of POI
| Name | Degree |
|---|---|
| SRC | 104 |
| TP53 | 96 |
| STAT3 | 88 |
| PIK3CA | 86 |
| MAPK3 | 86 |
| HRAS | 84 |
| PIK3R1 | 84 |
| MAPK1 | 82 |
| HSP90AA1 | 80 |
| PTPN11 | 64 |
| Sub group | GO term | -LogP |
|---|---|---|
| BP | Positive regulation of locomotion | 57.01529 |
| BP | Positive regulation of cell motility | 56.17373 |
| BP | Positive regulation of cell migration | 55.77701 |
| BP | Protein phosphorylation | 55.0968 |
| BP | Positive regulation of phosphorylation | 54.99759 |
| BP | Response to hormone | 54.48073 |
| BP | Transmembrane receptor protein tyrosine kinase signaling pathway | 47.79846 |
| BP | Positive regulation of protein phosphorylation | 47.32929 |
| BP | Response to peptide | 47.32309 |
| BP | Enzyme-linked receptor protein signaling pathway | 46.18651 |
| CC | Membrane raft | 22.13493 |
| CC | Membrane microdomain | 22.10097 |
| CC | Receptor complex | 17.86078 |
| CC | Caveola | 15.1191 |
| CC | Focal adhesion | 13.80176 |
| CC | Cell-substrate junction | 13.60269 |
| CC | Plasma membrane raft | 13.26789 |
| CC | Side of membrane | 12.02636 |
| CC | External side of plasma membrane | 10.71497 |
| CC | Extracellular matrix | 10.53049 |
| MF | Protein kinase activity | 53.06992 |
| MF | Phosphotransferase activity, alcohol group as acceptor | 48.91392 |
| MF | Kinase activity | 48.35798 |
| MF | Protein tyrosine kinase activity | 35.63223 |
| MF | Kinase binding | 28.34491 |
| MF | Transmembrane receptor protein tyrosine kinase activity | 28.34218 |
| MF | Transmembrane receptor protein kinase activity | 27.83784 |
| MF | Protein serine/threonine kinase activity | 25.19876 |
| MF | Protein serine kinase activity | 23.51593 |
| MF | Protein kinase binding | 22.3736 |
Tab.3 GO functional enrichment analysis
| Sub group | GO term | -LogP |
|---|---|---|
| BP | Positive regulation of locomotion | 57.01529 |
| BP | Positive regulation of cell motility | 56.17373 |
| BP | Positive regulation of cell migration | 55.77701 |
| BP | Protein phosphorylation | 55.0968 |
| BP | Positive regulation of phosphorylation | 54.99759 |
| BP | Response to hormone | 54.48073 |
| BP | Transmembrane receptor protein tyrosine kinase signaling pathway | 47.79846 |
| BP | Positive regulation of protein phosphorylation | 47.32929 |
| BP | Response to peptide | 47.32309 |
| BP | Enzyme-linked receptor protein signaling pathway | 46.18651 |
| CC | Membrane raft | 22.13493 |
| CC | Membrane microdomain | 22.10097 |
| CC | Receptor complex | 17.86078 |
| CC | Caveola | 15.1191 |
| CC | Focal adhesion | 13.80176 |
| CC | Cell-substrate junction | 13.60269 |
| CC | Plasma membrane raft | 13.26789 |
| CC | Side of membrane | 12.02636 |
| CC | External side of plasma membrane | 10.71497 |
| CC | Extracellular matrix | 10.53049 |
| MF | Protein kinase activity | 53.06992 |
| MF | Phosphotransferase activity, alcohol group as acceptor | 48.91392 |
| MF | Kinase activity | 48.35798 |
| MF | Protein tyrosine kinase activity | 35.63223 |
| MF | Kinase binding | 28.34491 |
| MF | Transmembrane receptor protein tyrosine kinase activity | 28.34218 |
| MF | Transmembrane receptor protein kinase activity | 27.83784 |
| MF | Protein serine/threonine kinase activity | 25.19876 |
| MF | Protein serine kinase activity | 23.51593 |
| MF | Protein kinase binding | 22.3736 |
| ID | Pathway | GeneRatio | P | Count |
|---|---|---|---|---|
| hsa05200 | Pathways in cancer | 0.453988 | 6.19E-86 | 74 |
| hsa04151 | PI3K-Akt signaling pathway | 0.300613 | 2.97E-55 | 49 |
| hsa05205 | Proteoglycans in cancer | 0.245399 | 3.2E-51 | 40 |
| hsa05215 | Prostate cancer | 0.196319 | 3.38E-49 | 32 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 0.171779 | 3.99E-44 | 28 |
| hsa05417 | Lipid and atherosclerosis | 0.214724 | 8.47E-42 | 35 |
| hsa01522 | Endocrine resistance | 0.171779 | 5.01E-41 | 28 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 0.171779 | 9.62E-41 | 28 |
| hsa05206 | MicroRNAs in cancer | 0.233129 | 1.66E-40 | 38 |
| hsa04015 | Rap1 signaling pathway | 0.202454 | 7.19E-39 | 33 |
| hsa04014 | Ras signaling pathway | 0.208589 | 1.07E-38 | 34 |
| hsa04010 | MAPK signaling pathway | 0.220859 | 2.44E-38 | 36 |
| hsa05163 | Human cytomegalovirus infection | 0.196319 | 3.1E-36 | 32 |
| hsa05230 | Central carbon metabolism in cancer | 0.141104 | 1.92E-35 | 23 |
| hsa05218 | Melanoma | 0.141104 | 4.14E-35 | 23 |
| hsa04218 | Cellular senescence | 0.171779 | 9.59E-35 | 28 |
| hsa05161 | Hepatitis B | 0.171779 | 2.98E-34 | 28 |
| hsa05207 | Chemical carcinogenesis - receptor activation | 0.184049 | 6.44E-34 | 30 |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection | 0.177914 | 1.6E-33 | 29 |
| hsa05223 | Non-small cell lung cancer | 0.134969 | 4.06E-33 | 22 |
Tab.4 KEGG pathway enrichment analysis
| ID | Pathway | GeneRatio | P | Count |
|---|---|---|---|---|
| hsa05200 | Pathways in cancer | 0.453988 | 6.19E-86 | 74 |
| hsa04151 | PI3K-Akt signaling pathway | 0.300613 | 2.97E-55 | 49 |
| hsa05205 | Proteoglycans in cancer | 0.245399 | 3.2E-51 | 40 |
| hsa05215 | Prostate cancer | 0.196319 | 3.38E-49 | 32 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 0.171779 | 3.99E-44 | 28 |
| hsa05417 | Lipid and atherosclerosis | 0.214724 | 8.47E-42 | 35 |
| hsa01522 | Endocrine resistance | 0.171779 | 5.01E-41 | 28 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 0.171779 | 9.62E-41 | 28 |
| hsa05206 | MicroRNAs in cancer | 0.233129 | 1.66E-40 | 38 |
| hsa04015 | Rap1 signaling pathway | 0.202454 | 7.19E-39 | 33 |
| hsa04014 | Ras signaling pathway | 0.208589 | 1.07E-38 | 34 |
| hsa04010 | MAPK signaling pathway | 0.220859 | 2.44E-38 | 36 |
| hsa05163 | Human cytomegalovirus infection | 0.196319 | 3.1E-36 | 32 |
| hsa05230 | Central carbon metabolism in cancer | 0.141104 | 1.92E-35 | 23 |
| hsa05218 | Melanoma | 0.141104 | 4.14E-35 | 23 |
| hsa04218 | Cellular senescence | 0.171779 | 9.59E-35 | 28 |
| hsa05161 | Hepatitis B | 0.171779 | 2.98E-34 | 28 |
| hsa05207 | Chemical carcinogenesis - receptor activation | 0.184049 | 6.44E-34 | 30 |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection | 0.177914 | 1.6E-33 | 29 |
| hsa05223 | Non-small cell lung cancer | 0.134969 | 4.06E-33 | 22 |
| Core active component | Core target | Binding energy (kcal·mol-1) |
|---|---|---|
| Kaempferol | SRC | -6.0 |
| TP53 | -8.8 | |
| STAT3 | -8.0 | |
| PIK3CA | -6.7 | |
| MAPK3 | -7.5 | |
| Beta-sitosterol | SRC | -4.9 |
| TP53 | -6.9 | |
| STAT3 | -6.3 | |
| PIK3CA | -5.4 | |
| MAPK3 | -6.5 | |
| Luteolin | SRC | -7.0 |
| TP53 | -8.5 | |
| STAT3 | -7.9 | |
| PIK3CA | -6.5 | |
| MAPK3 | -9.0 | |
| Quercetin | SRC | -7.2 |
| TP53 | -8.9 | |
| STAT3 | -8.2 | |
| PIK3CA | -6.1 | |
| MAPK3 | -8.2 |
Tab.5 Molecular docking results of core active components and core targets of Tiaozhou Ziyin recipe
| Core active component | Core target | Binding energy (kcal·mol-1) |
|---|---|---|
| Kaempferol | SRC | -6.0 |
| TP53 | -8.8 | |
| STAT3 | -8.0 | |
| PIK3CA | -6.7 | |
| MAPK3 | -7.5 | |
| Beta-sitosterol | SRC | -4.9 |
| TP53 | -6.9 | |
| STAT3 | -6.3 | |
| PIK3CA | -5.4 | |
| MAPK3 | -6.5 | |
| Luteolin | SRC | -7.0 |
| TP53 | -8.5 | |
| STAT3 | -7.9 | |
| PIK3CA | -6.5 | |
| MAPK3 | -9.0 | |
| Quercetin | SRC | -7.2 |
| TP53 | -8.9 | |
| STAT3 | -8.2 | |
| PIK3CA | -6.1 | |
| MAPK3 | -8.2 |
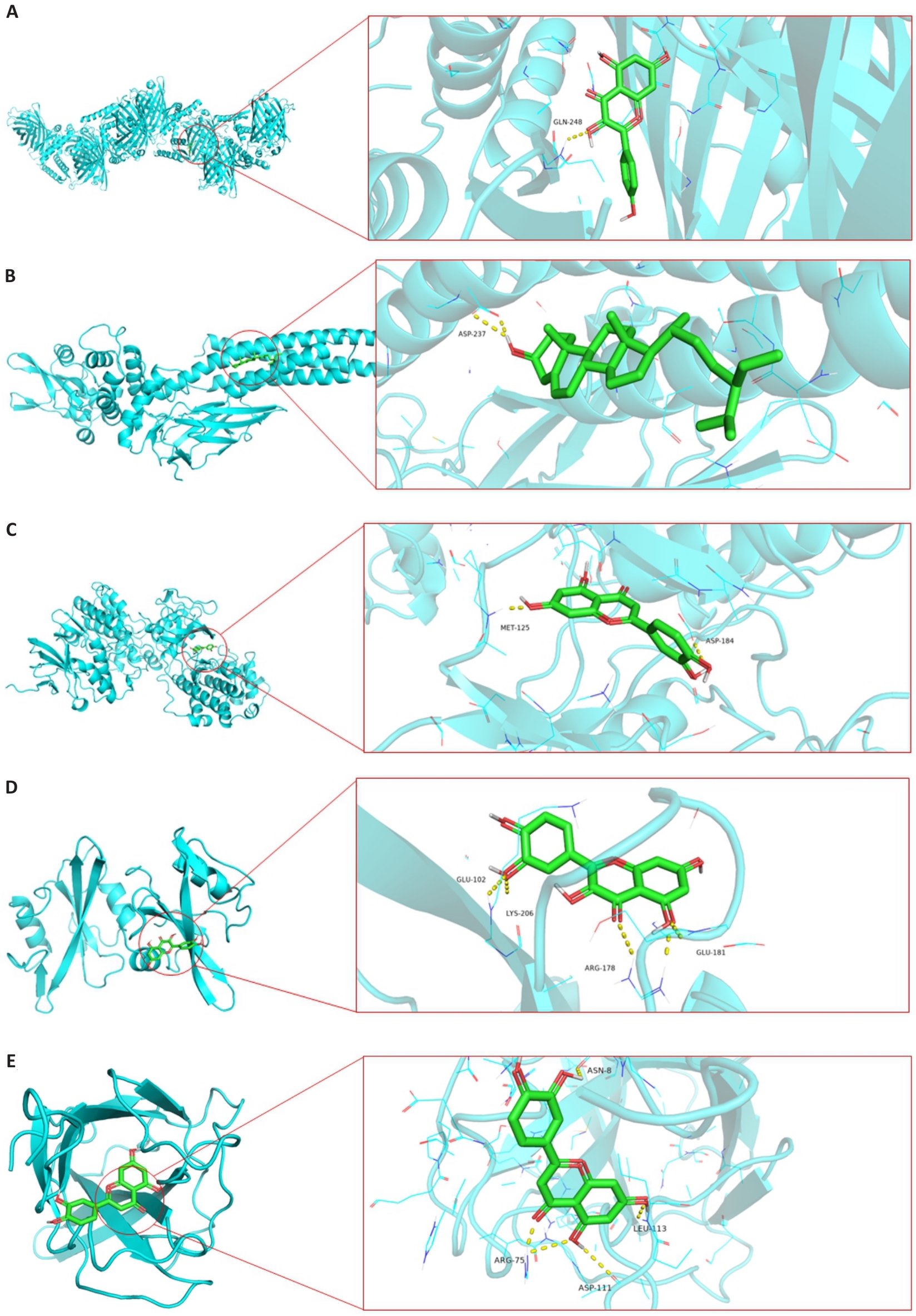
Fig.8 Schematic diagram of molecular docking of interaction between some core active components and core targets. A: Docking site between kaempferol and TP53. B: Docking site between beta-sitosterol and STAT3. C: Docking site between luteolin and MAPK3.D: Docking site between quercetin and SRC.E: Docking site between luteolin and PIK3CA.
| Group | Age (year) | Disease course (month) |
|---|---|---|
| Control group | 30.23±4.04 | 18.90±9.65 |
| Experimental group | 30.93±3.69 | 19.83±9.93 |
| t/ Z | -0.701 | -0.369 |
| P | 0.486 | 0.713 |
Tab.6 Comparison of age and disease course between the two groups (Mean±SD, n=30)
| Group | Age (year) | Disease course (month) |
|---|---|---|
| Control group | 30.23±4.04 | 18.90±9.65 |
| Experimental group | 30.93±3.69 | 19.83±9.93 |
| t/ Z | -0.701 | -0.369 |
| P | 0.486 | 0.713 |
| Hormone | Control group | Experimental group | t/Z | P | |
|---|---|---|---|---|---|
| AMH (ng/mL) | Before treatment | 0.58±0.31 | 0.55±0.31 | 0.471 | 0.64 |
| After treatment | 0.65±0.32 | 0.72±0.37# | -0.789 | 0.433 | |
| Four weeks after treatment | 0.60±0.33 | 0.69±0.35 | -0.995 | 0.324 | |
| E2 (ng/L) | Before treatment | 29.47±9.53 | 27.57±8.89 | -0.854 | 0.393 |
| After treatment | 40.63±13.35# | 47.70±13.76# | -2.019 | 0.048 | |
| Four weeks after treatment | 31.00±8.78 | 41.47±13.13* | -3.629 | <0.001 | |
| FSH (mIU/mL) | Before treatment | 33.19±7.89 | 34.51±7.60 | -0.872 | 0.383 |
| After treatment | 20.91±8.21# | 17.10±6.24# | 2.026 | 0.047 | |
| Four weeks after treatment | 30.37±7.73 | 20.70±8.46* | -4.266 | <0.001 | |
| LH (mIU/mL) | Before treatment | 21.11±5.71 | 23.14±7.18 | -1.124 | 0.261 |
| After treatment | 16.08±5.37# | 12.46±4.11# | -2.883 | 0.004 | |
| Four weeks after treatment | 19.16±6.29 | 15.27±6.28* | -2.343 | 0.019 | |
Tab.7 Comparison of sex hormone levels before, after and four weeks after treatment between the two groups (n=30)
| Hormone | Control group | Experimental group | t/Z | P | |
|---|---|---|---|---|---|
| AMH (ng/mL) | Before treatment | 0.58±0.31 | 0.55±0.31 | 0.471 | 0.64 |
| After treatment | 0.65±0.32 | 0.72±0.37# | -0.789 | 0.433 | |
| Four weeks after treatment | 0.60±0.33 | 0.69±0.35 | -0.995 | 0.324 | |
| E2 (ng/L) | Before treatment | 29.47±9.53 | 27.57±8.89 | -0.854 | 0.393 |
| After treatment | 40.63±13.35# | 47.70±13.76# | -2.019 | 0.048 | |
| Four weeks after treatment | 31.00±8.78 | 41.47±13.13* | -3.629 | <0.001 | |
| FSH (mIU/mL) | Before treatment | 33.19±7.89 | 34.51±7.60 | -0.872 | 0.383 |
| After treatment | 20.91±8.21# | 17.10±6.24# | 2.026 | 0.047 | |
| Four weeks after treatment | 30.37±7.73 | 20.70±8.46* | -4.266 | <0.001 | |
| LH (mIU/mL) | Before treatment | 21.11±5.71 | 23.14±7.18 | -1.124 | 0.261 |
| After treatment | 16.08±5.37# | 12.46±4.11# | -2.883 | 0.004 | |
| Four weeks after treatment | 19.16±6.29 | 15.27±6.28* | -2.343 | 0.019 | |
| Group | Antral follicle count | Kupperman score (points) | ||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Four weeks after treatment | Before treatment | After treatment | Four weeks after treatment | |
| Control group | 3.67±1.54 | 4.57±1.76# | 3.83±1.44 | 21.40±6.47 | 15.40±6.70# | 18.93±6.23 |
| Experimental group | 3.73±1.57 | 5.33±1.63# | 5.03±1.52* | 20.20±7.12 | 11.07±4.53# | 12.87±4.97* |
| t/Z | -0.166 | -1.755 | -2.859 | 0.683 | 2.936 | 4.170 |
| P | 0.869 | 0.085 | 0.004 | 0.497 | 0.005 | <0.001 |
Tab.8 Comparison of antral follicle count and Kupperman score before, after and 4 weeks after treatment between the two groups (n=30)
| Group | Antral follicle count | Kupperman score (points) | ||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Four weeks after treatment | Before treatment | After treatment | Four weeks after treatment | |
| Control group | 3.67±1.54 | 4.57±1.76# | 3.83±1.44 | 21.40±6.47 | 15.40±6.70# | 18.93±6.23 |
| Experimental group | 3.73±1.57 | 5.33±1.63# | 5.03±1.52* | 20.20±7.12 | 11.07±4.53# | 12.87±4.97* |
| t/Z | -0.166 | -1.755 | -2.859 | 0.683 | 2.936 | 4.170 |
| P | 0.869 | 0.085 | 0.004 | 0.497 | 0.005 | <0.001 |
| Group | Rate of adverse reaction incidence | Rate of pregnancy |
|---|---|---|
| Control group | 3 (10%) | 1 (3.3%) |
| Experimental group | 1 (3.3%) | 2 (6.7%) |
| χ2 | 1.071 | 0.351 |
| P | 0.301 | 0.554 |
Tab.9 Comparison of rate of adverse reaction incidence and pregnancy between the two groups [n (%), n=30]
| Group | Rate of adverse reaction incidence | Rate of pregnancy |
|---|---|---|
| Control group | 3 (10%) | 1 (3.3%) |
| Experimental group | 1 (3.3%) | 2 (6.7%) |
| χ2 | 1.071 | 0.351 |
| P | 0.301 | 0.554 |
| 1 | 陈子江, 田秦杰, 乔 杰, 等. 早发性卵巢功能不全的临床诊疗中国专家共识[J]. 中华妇产科杂志, 2017, 52(9): 577-81. DOI: 10.3760/cma.j.issn.0529-567x.2017.09.001 |
| 2 | Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, et al. Autoim-mune diseases in patients with premature ovarian insufficiency-our current state of knowledge[J]. Int J Mol Sci, 2021, 22(5): 2594. |
| 3 | 孙玉英, 陈淑萍, 谈 勇. 滋阴补阳序贯法联合西药对DOR大鼠卵巢Smad2、Smad3、Smad7 mRNA表达的影响[J]. 华中科技大学学报: 医学版, 2018, 47(3): 300-4. DOI: 10.3870/j.issn.1672-0741.2018.03.009 |
| 4 | 王飞虹, 谈 勇, 殷燕云, 等. 滋阴补阳方序贯联合拮抗剂方案对卵巢低反应体外受精结局的影响[J]. 中华中医药杂志, 2019, 34(7): 3327-9. |
| 5 | 罗倩倩, 谈 勇, 胡荣魁, 等. 滋阴方治疗排卵障碍性不孕症的机制及价值基于网络药理学方法[J]. 南方医科大学学报, 2021, 41(3): 319-28. DOI: 10.12122/j.issn.1673-4254.2021.03.02 |
| 6 | 唐培培, 赵 娟, 谈 勇, 等. 滋阴补阳方序贯法联合促排卵治疗肾虚型多囊卵巢综合征不孕症临床疗效[J]. 中华中医药杂志, 2022, 37(12): 7531-4. |
| 7 | Du XY, Huang J, Xu LQ, et al. The proto-oncogene c-src is involved in primordial follicle activation through the PI3K, PKC and MAPK signaling pathways[J]. Reprod Biol Endocrinol, 2012, 10: 58. |
| 8 | Chan Y, Zhu BS, Zhang JM, et al. Associations between TP53 and MDM2 polymorphisms and the follicle-stimulating hormone/luteinizing hormone ratio in infertile women[J]. Genet Test Mol Biomarkers, 2018, 22(7): 405-12. |
| 9 | Vagnini LD, Renzi A, Petersen CG, et al. Correlation of TP53 (rs1625895), TP73 (rs3765730), MMP9 (rs17576), and MTHFR (rs868014) polymorphisms with low ovarian reserve[J]. Eur J Obstet Gynecol Reprod Biol, 2022, 269: 132-7. |
| 10 | Yuan XL, Zhou XF, He YT, et al. C/EBPβ promotes STAT3 expression and affects cell apoptosis and proliferation in porcine ovarian granulosa cells[J]. Genes, 2018, 9(6): 295. |
| 11 | Zhang GF, Cui ZF, Li JJ, et al. miR-122-5p regulates proliferation and apoptosis of chicken granulosa cells of hierarchal follicles by targeting MAPK3[J]. Gene, 2022, 824: 146397. |
| 12 | Wang SH, Hao J, Zhang C, et al. KLF17 promotes human naive pluripotency through repressing MAPK3 and ZIC2[J]. Sci China Life Sci, 2022, 65(10): 1985-97. |
| 13 | Periferakis A, Periferakis K, Badarau IA, et al. Kaempferol: antimicrobial properties, sources, clinical, and traditional applications[J]. Int J Mol Sci, 2022, 23(23): 15054. |
| 14 | Nejabati HR, Roshangar L. Kaempferol: a potential agent in the pr-evention of colorectal cancer[J]. Physiol Rep, 2022, 10(20): e15488. |
| 15 | Sengupta B, Biswas P, Roy D, et al. Anticancer properties of kaempferol on cellular signaling pathways[J]. Curr Top Med Chem, 2022, 22(30): 2474-82. |
| 16 | 王明迪, 梁 天, 赵千惠, 等. 淫羊藿素和山柰酚对鸡等级前卵泡颗粒细胞发育的影响[J]. 中国家禽, 2023, 45(4): 57-63. |
| 17 | Zeng ZC, Jiang J, Wang XJ, et al. Kaempferol ameliorates in-vitro and in-vivo postovulatory oocyte ageing in mice[J]. Reprod Biomed Online, 2022, 45(6): 1065-83. |
| 18 | Khan Z, Nath N, Rauf A, et al. Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications[J]. Chem Biol Interact, 2022, 365: 110117. |
| 19 | Ma LY, Ma Y, Liu YX. β-Sitosterol protects against food allergic response in BALB/c mice by regulating the intestinal barrier function and reconstructing the gut microbiota structure[J]. Food Funct, 2023, 14(10): 4456-69. |
| 20 | Chen YK, Yin S, Liu R, et al. β-Sitosterol activates autophagy to inhibit the development of hepatocellular carcinoma by regulating the complement C5a receptor 1/alpha fetoprotein axis[J]. Eur J Pharmacol, 2023, 957: 175983. |
| 21 | Yu YY, Cao Y, Huang WL, et al. β-sitosterol ameliorates endometrium receptivity in PCOS-like mice: the mediation of gut microbiota[J]. Front Nutr, 2021, 8: 667130. |
| 22 | 赵 帅, 陈冬梅, 虎 娜, 等. β-谷甾醇通过PI3K/AKT通路影响颗粒细胞增殖及凋亡[J]. 宁夏医科大学学报, 2021, 43(4): 339-44. DOI: 10.16050/j.cnki.issn1674-6309.2021.04.004 |
| 23 | Franza L, Carusi V, Nucera E, et al. Luteolin, inflammation and cancer: Special emphasis on gut microbiota[J]. Biofactors, 2021, 47(2): 181-9. |
| 24 | Huang Y, Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress[J]. Am J Physiol Endocrinol Metab, 2021, 320(6): E1085-92. |
| 25 | Hosseini A, Razavi BM, Banach M, et al. Quercetin and metabolic syndrome: a review[J]. Phytother Res, 2021, 35(10): 5352-64. |
| 26 | Deepika, Maurya PK. Health benefits of quercetin in age-related diseases[J]. Molecules, 2022, 27(8): 2498. |
| 27 | Chen Y, Zhao Y, Miao CY, et al. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells[J]. J Ovarian Res, 2022, 15(1): 138. |
| [1] | Niandong RAN, Jie LIU, Jian XU, Yongping ZHANG, Jiangtao GUO. n-butanol fraction of ethanol extract of Periploca forrestii Schltr.: its active components, targets and pathways for treating Alcheimer's disease in rats [J]. Journal of Southern Medical University, 2025, 45(4): 785-798. |
| [2] | Haonan¹ XU, Fang³ ZHANG, Yuying² HUANG, Qisheng⁴ YAO, Yueqin⁴ GUAN, Hao CHEN. Thesium chinense Turcz. alleviates antibiotic-associated diarrhea in mice by modulating gut microbiota structure and regulating the EGFR/PI3K/Akt signaling pathway [J]. Journal of Southern Medical University, 2025, 45(2): 285-295. |
| [3] | Junjie GAO, Kai YE, Jing WU. Quercetin inhibits proliferation and migration of clear cell renal cell carcinoma cells by regulating TP53 gene [J]. Journal of Southern Medical University, 2025, 45(2): 313-321. |
| [4] | Ying LIU, Borui LI, Yongcai LI, Lubo CHANG, Jiao WANG, Lin YANG, Yonggang YAN, Kai QV, Jiping LIU, Gang ZHANG, Xia SHEN. Jiawei Xiaoyao Pills improves depression-like behavior in rats by regulating neurotransmitters, inhibiting inflammation and oxidation and modulating intestinal flora [J]. Journal of Southern Medical University, 2025, 45(2): 347-358. |
| [5] | Qiao CHU, Xiaona WANG, Jiaying XU, Huilin PENG, Yulin ZHAO, Jing ZHANG, Guoyu LU, Kai WANG. Pulsatilla saponin D inhibits invasion and metastasis of triple-negative breast cancer cells through multiple targets and pathways [J]. Journal of Southern Medical University, 2025, 45(1): 150-161. |
| [6] | Xiupeng LONG, Shun TAO, Shen YANG, Suyun LI, Libing RAO, Li LI, Zhe ZHANG. Quercetin improves heart failure by inhibiting cardiomyocyte apoptosis via suppressing the MAPK signaling pathway [J]. Journal of Southern Medical University, 2025, 45(1): 187-196. |
| [7] | Meng XU, Lina CHEN, Jinyu WU, Lili LIU, Mei SHI, Hao ZHOU, Guoliang ZHANG. Mechanism of Hedyotis diffusa-Scutellaria barbata D. Don for treatment of primary liver cancer: analysis with network pharmacology, molecular docking and in vitro validation [J]. Journal of Southern Medical University, 2025, 45(1): 80-89. |
| [8] | Qing LIU, Jing LIU, Yihang ZHENG, Jin LEI, Jianhua HUANG, Siyu LIU, Fang LIU, Qunlong PENG, Yuanfang ZHANG, Junjie WANG, Yujuan LI. Quercetin mediates the therapeutic effect of Centella asiatica on psoriasis by regulating STAT3 phosphorylation to inhibit the IL-23/IL-17A axis [J]. Journal of Southern Medical University, 2025, 45(1): 90-99. |
| [9] | Xingmei CHEN, Qinwen LIU, Yi LI, Xiaoyu ZHONG, Qiling FAN, Ke MA, Liuting LUO, Daogang GUAN, Zhibo ZHU. Analysis of core functional components in Yinchenhao Decoction and their pathways for treating liver fibrosis [J]. Journal of Southern Medical University, 2024, 44(8): 1508-1517. |
| [10] | Shanyuan ZHANG, Qiaoyan CAI, Jianghan QI, Kaixin YIN, Chenchen HE, Zhuye GAO, Ling ZHANG, Jianfeng CHU. Pharmacodynamics of Qingxin Jieyu Granules for treatment of atherosclerosis and its regulatory mechanism for lipid metabolism [J]. Journal of Southern Medical University, 2024, 44(8): 1518-1528. |
| [11] | Yuming ZHANG, Shicheng XIA, Linlin ZHANG, Mengxi CHEN, Xiaojing LIU, Qin GAO, Hongwei YE. Protective effect of Lonicerae japonicae flos extract against doxorubicin-induced liver injury in mice [J]. Journal of Southern Medical University, 2024, 44(8): 1571-1581. |
| [12] | Jinjin WANG, Wenfei CUI, Xuewei DOU, Binglei YIN, Yuqi NIU, Ling NIU, Guoli YAN. Euonymus alatus delays progression of diabetic kidney disease in mice by regulating EGFR tyrosine kinase inhibitor resistance signaling pathway [J]. Journal of Southern Medical University, 2024, 44(7): 1243-1255. |
| [13] | Linyue WANG, Wenyue QI, Jihua GAO, Maosheng TIAN, Jiancheng XU. Tongyangxiao Lotion promotes postoperative wound healing in a rat model of anal fistula by downregulating inflammatory factors and suppressing inflammation [J]. Journal of Southern Medical University, 2024, 44(7): 1256-1265. |
| [14] | Wenxiang ZHANG, Huixian GU, Pengde CHEN, Siyu WU, Hongyan MA, Lan YAO. Compound Yuye Decoction protects diabetic rats against cardiomyopathy by inhibiting myocardial apoptosis and inflammation via regulating the PI3K/Akt signaling pathway [J]. Journal of Southern Medical University, 2024, 44(7): 1306-1314. |
| [15] | Yan HUANG, Lulu QIN, Shaoxing GUAN, Yanping GUANG, Yuru WEI, Ailing CAO, Dongmei LI, Guining WEI, Qibiao SU. Therapeutic mechanism of aqueous extract of Semiliquidambar cathayensis Chang root for pancreatic cancer: the active components, therapeutic targets and pathways [J]. Journal of Southern Medical University, 2024, 44(7): 1336-1344. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||