南方医科大学学报 ›› 2026, Vol. 46 ›› Issue (1): 183-190.doi: 10.12122/j.issn.1673-4254.2026.01.20
赵铖( ), 李稳, 郑宝寿, 王光明, 肖芝松, 李云鹏(
), 李稳, 郑宝寿, 王光明, 肖芝松, 李云鹏( )
)
收稿日期:2025-06-18
出版日期:2026-01-20
发布日期:2026-01-16
通讯作者:
李云鹏
E-mail:3243330487@qq.com;lyp0872@163.com
作者简介:赵 铖,在读硕士研究生,E-mail: 3243330487@qq.com
基金资助:
Cheng ZHAO( ), Wen LI, Baoshou ZHENG, Guangming WANG, Zhisong XIAO, Yunpeng LI(
), Wen LI, Baoshou ZHENG, Guangming WANG, Zhisong XIAO, Yunpeng LI( )
)
Received:2025-06-18
Online:2026-01-20
Published:2026-01-16
Contact:
Yunpeng LI
E-mail:3243330487@qq.com;lyp0872@163.com
摘要:
目的 探讨lncRNA SNHG12对前列腺癌(PCa)多西他赛(DTX)耐药的影响及潜在机制。 方法 使用梯度DTX处理PC-3细胞获得DTX耐药的PC-3细胞(PC-3R),通过在雄性BALB/c裸鼠左背部注射PC-3R细胞构建PCa荷瘤模型。动物实验分为对照组(NC),DTX组,sh-SNHG12组,DTX+sh-SNHG12组, 5只/组。通过RT-qPCR、Western blotting、免疫荧光、免疫组化检测关键基因和蛋白的表达,通过CCK-8、克隆形成以及Transwell迁移实验评估细胞的增殖情况,通过RIP-qPCR检测RNA与蛋白之间的结合。 结果 SNHG12在PC-3R细胞中表达上调(P<0.001),敲低SNHG12可抑制PC-3R细胞的增殖和细胞迁移能力以及裸鼠荷瘤组织生长(P<0.001),10 nmol/L DTX处理对PC-3R细胞增殖及迁移没有显著影响,而在DTX处理的基础上敲低SNHG12可抑制PC-3R细胞增殖、迁移以及裸鼠荷瘤组织的生长(P<0.001)。在PC-3R细胞中ELAVL1的表达上调(P<0.001),PC-3R细胞及荷瘤组织中PI3K/AKT信号通路活化水平也上调,并且额外PI3K激活剂740 Y-P处理可削弱敲低SNHG12的作用。PC-3R细胞中的SNHG12可与ELAVL1结合。机制研究发现,SNHG12通过与ELAVL1结合来激活PI3K/AKT信号通路,进而导致PCa DTX耐药。 结论 敲低SNHG12可以抑制PCa DTX耐药,为PCa DTX增敏疗法的开发提供了潜在干预靶点。
赵铖, 李稳, 郑宝寿, 王光明, 肖芝松, 李云鹏. lncRNA SNHG12与ELAVL1相互作用激活PI3K/AKT信号通路促进前列腺癌细胞多西他赛的耐药机制[J]. 南方医科大学学报, 2026, 46(1): 183-190.
Cheng ZHAO, Wen LI, Baoshou ZHENG, Guangming WANG, Zhisong XIAO, Yunpeng LI. Overexpression of lncRNA SNHG12 promotes docetaxel resistance of prostate cancer cells by activating PI3K/AKT signaling via interacting with ELAVL1[J]. Journal of Southern Medical University, 2026, 46(1): 183-190.
| Genes | Primer (5'-3′) |
|---|---|
| SNHG12 | F:ATGAAATGCAGGGGACCTGG |
| R:TGTAACATGAATCTTAAAGCACAGC | |
| ELAVL1 | F:AACTACGTGACCGCGAAGG R:CGCCCAAACCGAGAGAACA |
| β-actin | F:CATGTACGTTGCTATCCAGGC |
| R:CTCCTTAATGTCACGCACGAT |
表1 引物序列信息列表
Tab.1 Primer sequences for RT-qPCR
| Genes | Primer (5'-3′) |
|---|---|
| SNHG12 | F:ATGAAATGCAGGGGACCTGG |
| R:TGTAACATGAATCTTAAAGCACAGC | |
| ELAVL1 | F:AACTACGTGACCGCGAAGG R:CGCCCAAACCGAGAGAACA |
| β-actin | F:CATGTACGTTGCTATCCAGGC |
| R:CTCCTTAATGTCACGCACGAT |
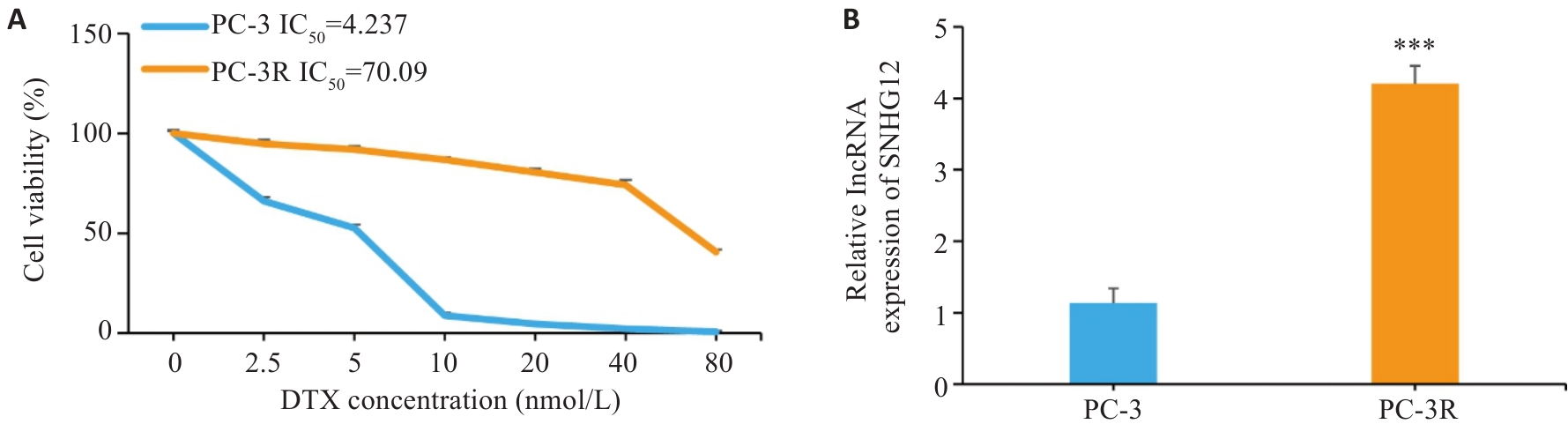
图1 多西他赛耐药细胞的构建以及SNHG12的表达鉴定
Fig.1 Construction of docetaxel-resistant PC-3 cells and identification of SNHG12 expression. A: CCK-8 assay for detecting cell viability. B: RT-qPCR for detecting expression of SNHG12 in PC-3 and PC-3R cells. n=3, ***P<0.001 vs PC-3 group.
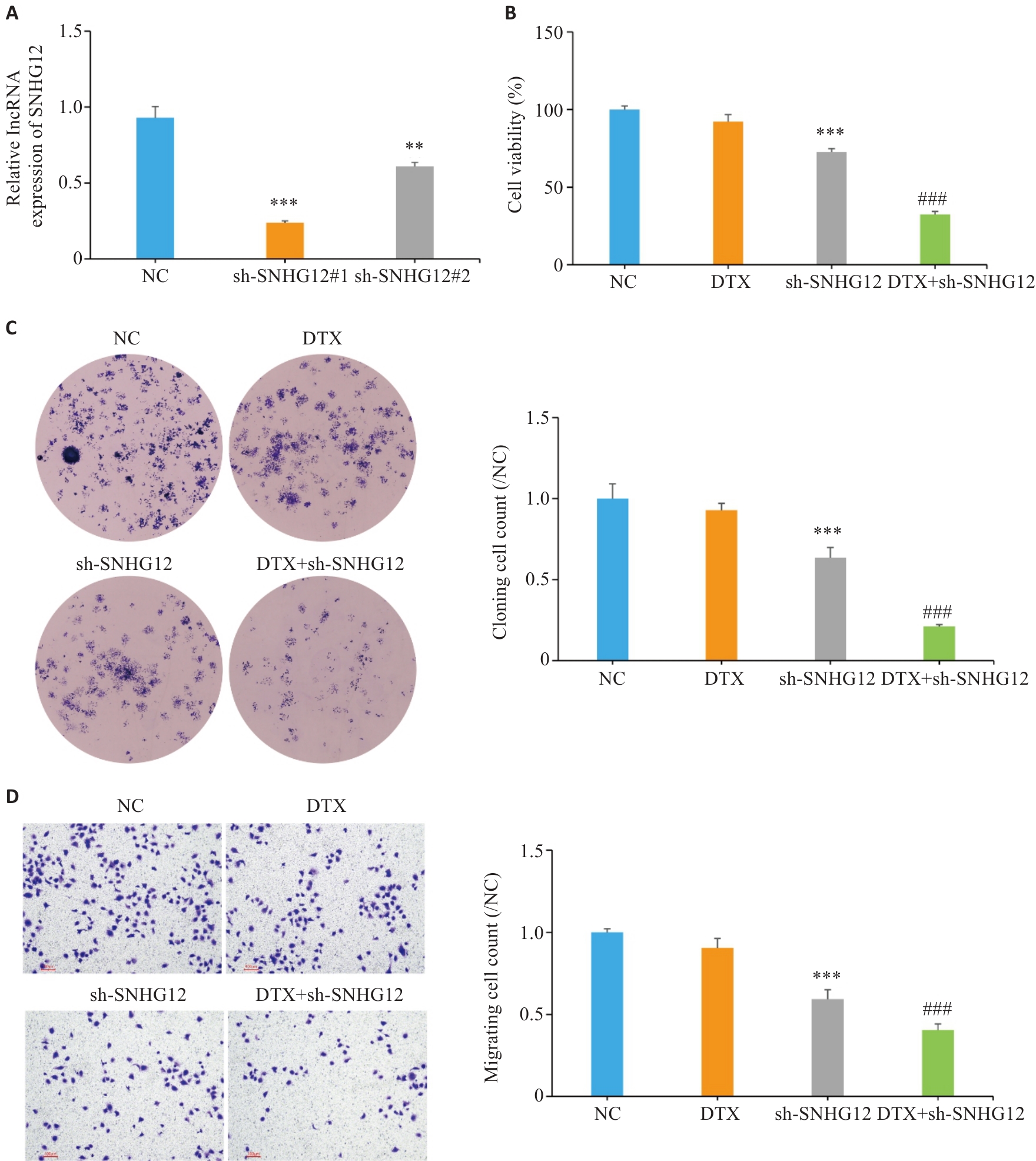
图2 敲低SNHG12抑制前列腺癌细胞的多西他赛耐药
Fig.2 Knockdown of SNHG12 inhibits docetaxel resistance in prostate cancer cells. A: Transfection efficiency of sh-SNHG12 detected by RT-qPCR. B: CCK-8 assay for assessing cell viability. C: Clone formation assay for assessing cell clone formation ability. D: Transwell assay for assessing cell migration ability (Scale bar=100 μm). n=3, **P<0.001, ***P<0.001 vs NC group; ###P<0.001 vs DTX group.
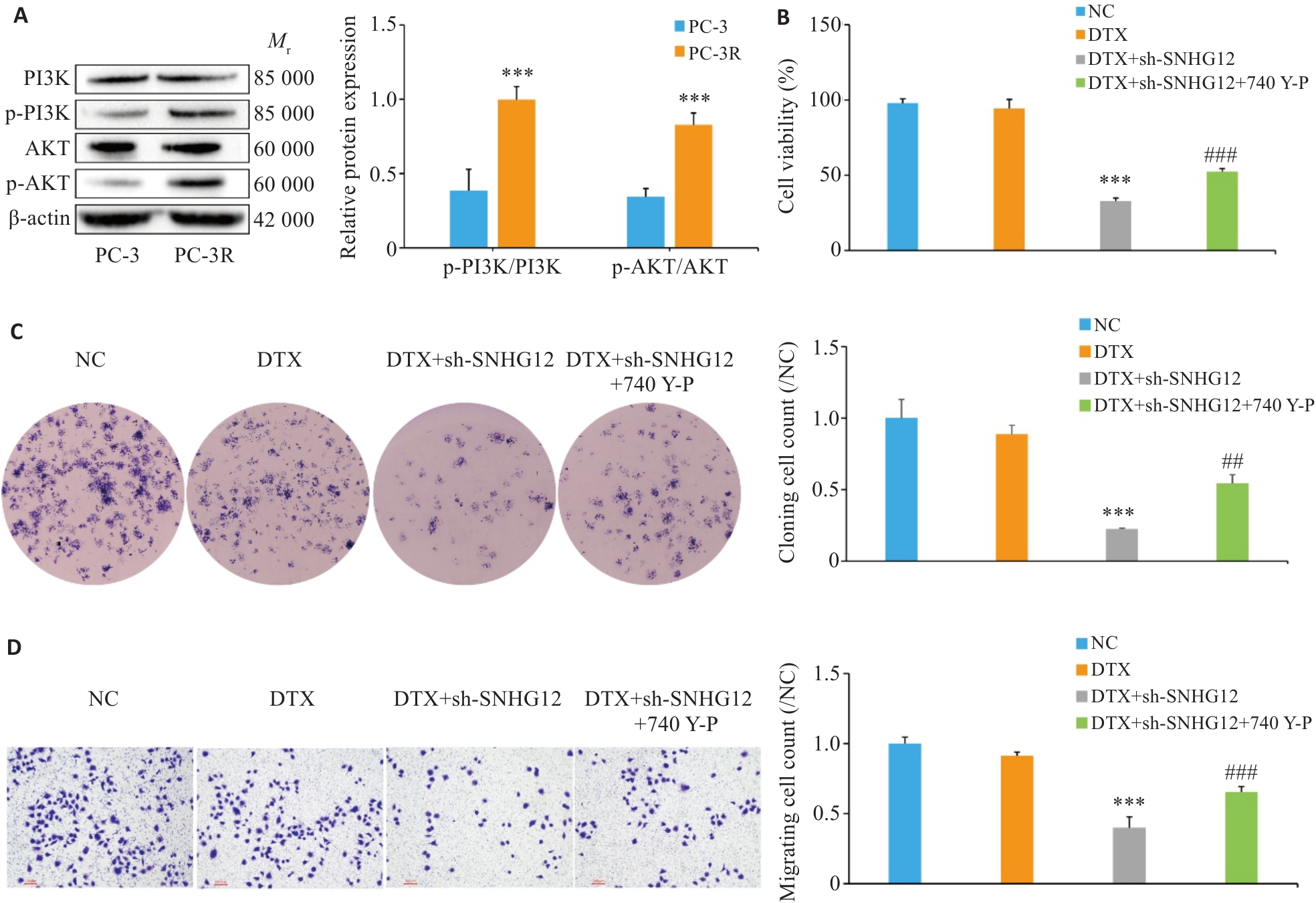
图3 敲低SNHG12通过PI3K/AKT信号通路抑制前列腺癌细胞的多西他赛耐药
Fig.3 Knockdown of SNHG12 inhibits docetaxel resistance in prostate cancer cells through the PI3K/AKT signaling pathway. A: Western blotting for detecting expressions of p-PI3K/PI3K and p-AKT/AKT in PC-3 and PC-3R cells. B: CCK-8 assay for assessing viability of PC-3R cells. C: Clone formation assay for assessing clone formation ability of PC-3R cells. D: Transwell assay for assessing migration ability of PC-3R cells (Scale bar=100 μm). n=3, ***P<0.001 vs PC-3 group/DTX group; ##P<0.01, ###P<0.001 vs DTX+sh-SNHG12 group.
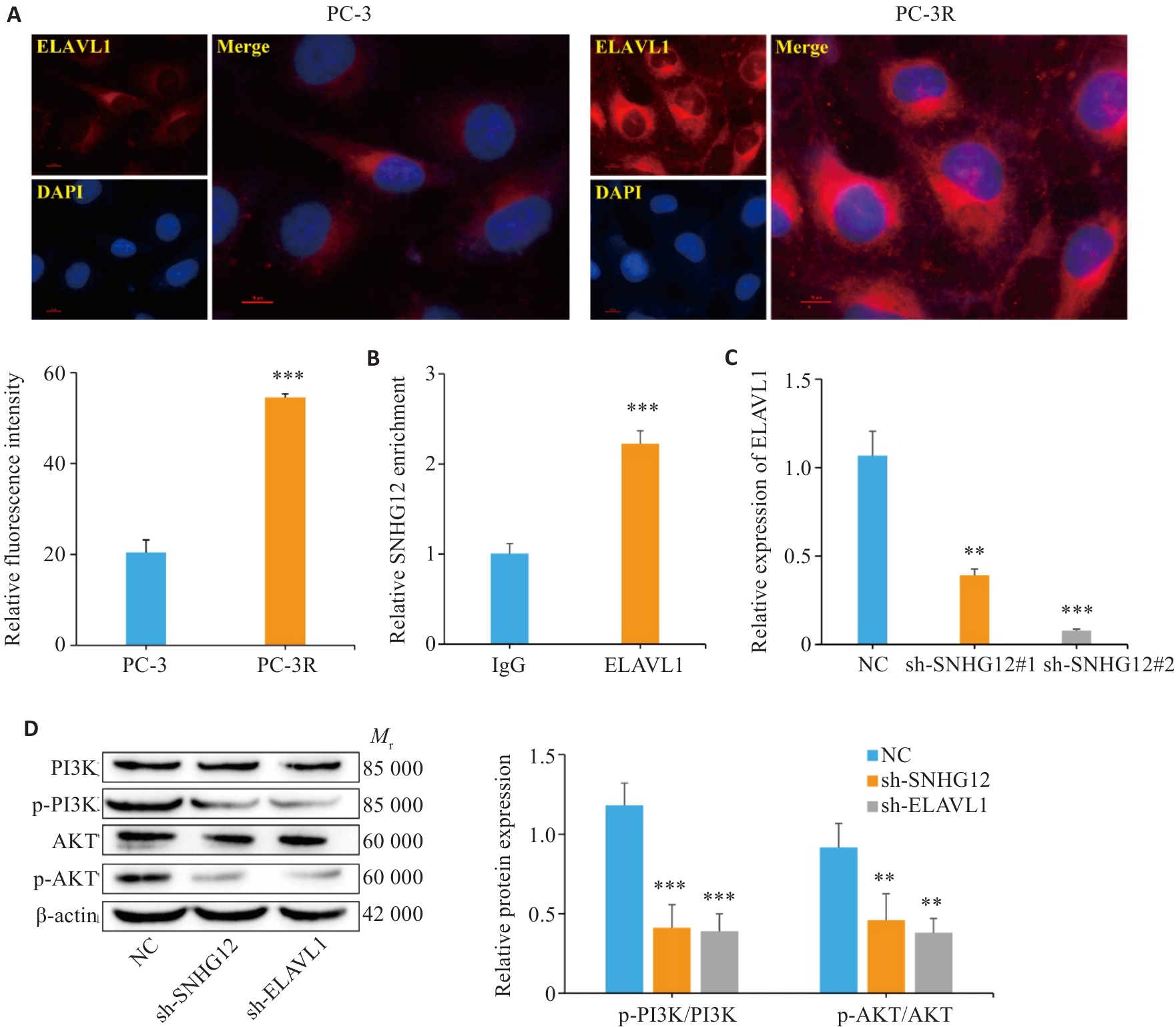
图4 SNHG12与ELAVL1相互作用激活PI3K/AKT信号通路
Fig.4 SNHG12 interacts with ELAVL1 to activate the PI3K/AKT signaling pathway. A: Immunofluorescence staining for detecting ELAVL1 expression in PC-3 and PC-3R cells (Scale bar=10 μm). B: RIP-qPCR for detecting enrichment level of SNHG12 on ELAVL1 in PC-3 and PC-3R cells. C: RT-qPCR for detecting transfection efficiency of sh-ELAVL1. D: Western blotting for detecting expressions of p-PI3K/PI3K and p-AKT/AKT in PC-3R cells. n=3, **P<0.01, ***P<0.001 vs PC-3 group/IgG group/NC group.
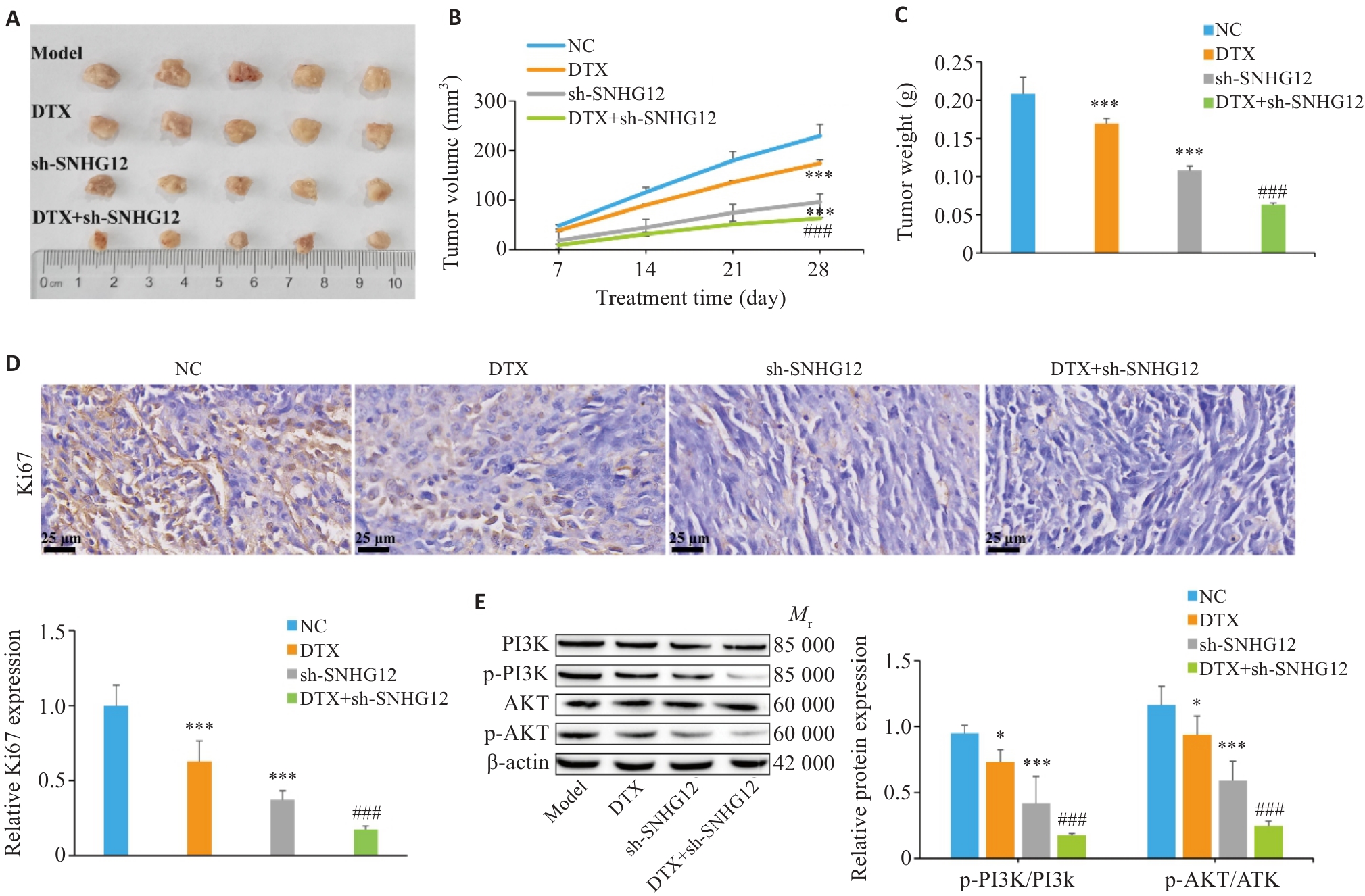
图5 敲低SNHG12抑制前列腺癌多西他赛耐药
Fig.5 Knockdown of SNHG12 inhibits docetaxel resistance in prostate cancer. A: Gross observation of the dissected tumors from the nude mouse models. B: Tumor volume changes in each group. C: Tumor weight changes in each group. D: Immunohistochemical detection of Ki67 expression in the tumor tissues in each group (Scale bar=25 μm). E: Western blotting for detecting expressions of p-PI3K/PI3K and p-AKT/AKT in the tumor tissues. n=5, *P<0.05, ***P<0.001 vs NC group; ###P<0.001 vs DTX group.
| [1] | Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-63. doi:10.3322/caac.21834 |
| [2] | Chen HT, Pang BR, Zhou C, et al. Prostate cancer-derived small extracellular vesicle proteins: the hope in diagnosis, prognosis, and therapeutics[J]. J Nanobiotechnology, 2023, 21(1): 480. doi:10.1186/s12951-023-02219-0 |
| [3] | Graham LS, Lin JK, Lage DE, et al. Management of prostate cancer in older adults[J]. Am Soc Clin Oncol Educ Book, 2023, 43: e390396. doi:10.1200/edbk_390396 |
| [4] | Sun XC, Zhang Y, Xin SY, et al. NOTCH3 promotes docetaxel resistance of prostate cancer cells through regulating TUBB3 and MAPK signaling pathway[J]. Cancer Sci, 2024, 115(2): 412-26. doi:10.1111/cas.16040 |
| [5] | Lu JH, Zou QR, Li Y, et al. FTH1P8 induces and transmits docetaxel resistance by inhibiting ferroptosis in prostate cancer[J]. Biomed Pharmacother, 2024, 180: 117472. doi:10.1016/j.biopha.2024.117472 |
| [6] | Hashemi M, Zandieh MA, Talebi Y, et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies[J]. Biomed Pharmacother, 2023, 160: 114392. doi:10.1016/j.biopha.2023.114392 |
| [7] | Zhang YG. LncRNA-encoded peptides in cancer[J]. J Hematol Oncol, 2024, 17(1): 66. doi:10.1186/s13045-024-01591-0 |
| [8] | Tamang S, Acharya V, Roy D, et al. SNHG12: an LncRNA as a potential therapeutic target and biomarker for human cancer[J]. Front Oncol, 2019, 9: 901. doi:10.3389/fonc.2019.00901 |
| [9] | Cheng G, Song ZS, Liu YN, et al. Long noncoding RNA SNHG12 indicates the prognosis of prostate cancer and accelerates tumorigenesis via sponging miR-133b[J]. J Cell Physiol, 2020, 235(2): 1235-46. doi:10.1002/jcp.29039 |
| [10] | Song JN, Wu XH, Ma R, et al. Long noncoding RNA SNHG12 promotes cell proliferation and activates Wnt/β-catenin signaling in prostate cancer through sponging microRNA-195[J]. J Cell Biochem, 2019, 120(8): 13066-75. doi:10.1002/jcb.28578 |
| [11] | Li KY, Wang Q, Tang XY, et al. Advances in prostate cancer biomarkers and probes[J]. Cyborg Bionic Syst, 2024, 5: 0129. doi:10.34133/cbsystems.0129 |
| [12] | Wang P, Chen D, Ma HB, et al. LncRNA SNHG12 contributes to multidrug resistance through activating the MAPK/Slug pathway by sponging miR-181a in non-small cell lung cancer[J]. Oncotarget, 2017, 8(48): 84086-101. doi:10.18632/oncotarget.20475 |
| [13] | Lebedeva S, Jens M, Theil K, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR[J]. Mol Cell, 2011, 43(3): 340-52. doi:10.1016/j.molcel.2011.06.008 |
| [14] | Ma S, Xu YH, Qin XY, et al. RUNX1, FUS, and ELAVL1-induced circPTPN22 promote gastric cancer cell proliferation, migration, and invasion through miR-6788-5p/PAK1 axis-mediated autophagy[J]. Cell Mol Biol Lett, 2024, 29(1): 95. doi:10.1186/s11658-024-00610-9 |
| [15] | Kanzaki H, Chiba T, Kaneko T, et al. The RNA-binding protein ELAVL1 regulates hepatitis B virus replication and growth of hepatocellular carcinoma cells[J]. Int J Mol Sci, 2022, 23(14): 7878. doi:10.3390/ijms23147878 |
| [16] | Mao GX, Mu ZM, Wu DA. Exosomal lncRNA FOXD3-AS1 upregulates ELAVL1 expression and activates PI3K/Akt pathway to enhance lung cancer cell proliferation, invasion, and 5-fluorouracil resistance[J]. Acta Biochim Biophys Sin (Shanghai), 2021, 53(11): 1484-94. doi:10.1093/abbs/gmab129 |
| [17] | Cai ZL, Xu H, Bai G, et al. ELAVL1 promotes prostate cancer progression by interacting with other m6A regulators[J]. Front Oncol, 2022, 12: 939784. doi:10.3389/fonc.2022.939784 |
| [18] | Wei LJ, Zhang Q, Zhong CC, et al. Functional inhibition of the RNA-binding protein HuR sensitizes triple-negative breast cancer to chemotherapy[J]. Mol Oncol, 2023, 17(10): 1962-80. doi:10.1002/1878-0261.13478 |
| [19] | Huang YS, Xia L, Tan XW, et al. Molecular mechanism of lncRNA SNHG12 in immune escape of non-small cell lung cancer through the HuR/PD-L1/USP8 axis[J]. Cell Mol Biol Lett, 2022, 27(1): 43. doi:10.1186/s11658-022-00343-7 |
| [20] | Li MX, Che N, Liu XZ, et al. Dauricine regulates prostate cancer progression by inhibiting PI3K/AKT-dependent M2 polarization of macrophages[J]. Biochem Pharmacol, 2023, 217: 115838. doi:10.1016/j.bcp.2023.115838 |
| [21] | Eberlein C, Williamson SC, Hopcroft L, et al. Capivasertib combines with docetaxel to enhance anti-tumour activity through inhibition of AKT-mediated survival mechanisms in prostate cancer[J]. Br J Cancer, 2024, 130(8): 1377-87. doi:10.1038/s41416-024-02614-w |
| [22] | Lu XX, Yang FY, Chen DX, et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways[J]. Int J Biol Sci, 2020, 16(7): 1121-34. doi:10.7150/ijbs.41686 |
| [23] | Brady SN, Maggi LB Jr, Winkeler CL, et al. Nucleophosmin protein expression level, but not threonine 198 phosphorylation, is essential in growth and proliferation[J]. Oncogene, 2009, 28(36): 3209-20. doi:10.1038/onc.2009.178 |
| [24] | Shi Q, Zhu YS, Ma J, et al. Prostate Cancer-associated SPOP mutations enhance cancer cell survival and docetaxel resistance by upregulating Caprin1-dependent stress granule assembly[J]. Mol Cancer, 2019, 18(1): 170. doi:10.1186/s12943-019-1096-x |
| [25] | Sun D, Fan XH. LncRNA SNHG12 accelerates the progression of ovarian cancer via absorbing miRNA-129 to upregulate SOX4[J]. Eur Rev Med Pharmacol Sci, 2019, 23(6): 2345-52. |
| [26] | Wang JZ, Xu CL, Wu H, et al. LncRNA SNHG12 promotes cell growth and inhibits cell apoptosis in colorectal cancer cells[J]. Braz J Med Biol Res, 2017, 50(3): e6079. doi:10.1590/1414-431x20176079 |
| [27] | Peng Y, Wang YY, Zhou C, et al. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway?[J]. Front Oncol, 2022, 12: 819128. doi:10.3389/fonc.2022.819128 |
| [28] | Li HY, Shen X, Ma MJ, et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway[J]. J Exp Clin Cancer Res, 2021, 40(1): 340. doi:10.1186/s13046-021-02146-8 |
| [29] | Okuno K, Xu CM, Pascual-Sabater S, et al. Berberine overcomes gemcitabine-associated chemoresistance through regulation of Rap1/PI3K-Akt signaling in pancreatic ductal adenocarcinoma[J]. Pharmaceuticals (Basel), 2022, 15(10): 1199. doi:10.3390/ph15101199 |
| [30] | Yin HL, Qin HX, Yang L, et al. circCYP24A1 promotes Docetaxel resistance in prostate Cancer by Upregulating ALDH1A3[J]. Biomark Res, 2022, 10(1): 48. doi:10.1186/s40364-022-00393-1 |
| [31] | Li YZ, Guo SQ, Liu WC, et al. Silencing of SNHG12 enhanced the effectiveness of MSCs in alleviating ischemia/reperfusion injuries via the PI3K/AKT/mTOR signaling pathway[J]. Front Neurosci, 2019, 13: 645. doi:10.3389/fnins.2019.00645 |
| [32] | Cai ZL, Zhai XX, Xu JD, et al. ELAVL1 regulates PD-L1 mRNA stability to disrupt the infiltration of CD4-positive T cells in prostate cancer[J]. Neoplasia, 2024, 57: 101049. doi:10.1016/j.neo.2024.101049 |
| [33] | Wei LJ, Kim SH, Armaly AM, et al. RNA-binding protein HuR inhibition induces multiple programmed cell death in breast and prostate cancer[J]. Cell Commun Signal, 2024, 22(1): 580. doi:10.1186/s12964-024-01916-z |
| [1] | 李艳宇, 李晨, 戴传君, 郭润之, 韩浩宇, 卢林明, 周芳芳, 支慧. 皖南蝮蛇抑瘤组分-Ι通过调控RAI14抑制顺铂耐药胃癌细胞的增殖与侵袭[J]. 南方医科大学学报, 2026, 46(1): 113-121. |
| [2] | 饶璐, 丁家和, 魏江平, 阳勇, 张小梅, 王计瑞. 槐花通过抑制PI3K/AKT通路减轻炎症反应治疗银屑病[J]. 南方医科大学学报, 2025, 45(9): 1989-1996. |
| [3] | 陈子贤, 周家伟, 谭磊, 黄志鹏, 薛康颐, 陈明坤. 基于免疫抑制性Neu_2中性粒细胞亚群模型精准预测前列腺癌生存预后及免疫治疗应答[J]. 南方医科大学学报, 2025, 45(8): 1643-1653. |
| [4] | 张兆君, 吴琼, 谢苗苗, 叶洳吟, 耿晨晨, 石纪雯, 杨清玲, 王文锐, 石玉荣. 层状双氢氧化物负载si-NEAT1通过miR-133b/PD-L1轴调控乳腺癌紫杉醇耐药及巨噬细胞极化[J]. 南方医科大学学报, 2025, 45(8): 1718-1731. |
| [5] | 马振南, 刘福全, 赵雪峰, 张晓微. DTX2促进奥沙利铂耐药的结直肠癌细胞增殖、侵袭和上皮间质转化[J]. 南方医科大学学报, 2025, 45(4): 829-836. |
| [6] | 张夏玮, 杨晶晶, 温亚男, 刘青阳, 窦立萍, 高春记. METTL3介导的m6A修饰通过调节自噬促进急性髓性白血病细胞中FOXO3表达及蒽环类药物耐药性[J]. 南方医科大学学报, 2025, 45(3): 470-478. |
| [7] | 徐皓男, 张放, 黄钰莹, 姚其盛, 管悦琴, 陈浩. 百蕊草通过调节肠道菌群和调控EGFR/PI3K/Akt信号通路改善小鼠抗生素相关性腹泻[J]. 南方医科大学学报, 2025, 45(2): 285-295. |
| [8] | 裴月娇, 刘慧敏, 昕宇, 刘波. miR-124通过调控PI3K/AKT信号通路改善睡眠剥夺大鼠认知功能[J]. 南方医科大学学报, 2025, 45(2): 340-346. |
| [9] | 张芡, 刘博文, 雷丽, 王晔, 张馨月, 毛樟坤, 唐鹏, 张金梅, 杨佳宜, 彭彦茜, 刘泽. 丝氨酸蛋白酶抑制剂E1过表达通过诱导M2型巨噬细胞极化促进三阴性乳腺癌细胞增殖与紫杉醇耐药[J]. 南方医科大学学报, 2025, 45(12): 2551-2560. |
| [10] | 郭旋, 刘洋, 熊燕, 刘镖水, 宋婷, 李云飞. 图像引导下前列腺癌放疗固定装置的摆位误差与临床因素相关性分析[J]. 南方医科大学学报, 2025, 45(12): 2718-2725. |
| [11] | 刘柳青, 王坤, 王雪晴, 杜冰心. 枸杞多糖通过下调miR-23a减轻顺铂诱导的颗粒细胞损伤[J]. 南方医科大学学报, 2025, 45(11): 2340-2349. |
| [12] | 张可妮, 乔通, 尹林, 黄菊, 耿志军, 左芦根, 胡建国, 李静. 球松素靶向肠上皮细胞PI3K/AKT/CCL2轴抑制巨噬细胞肠道浸润缓解葡聚糖硫酸钠诱导的小鼠结肠炎[J]. 南方医科大学学报, 2025, 45(10): 2199-2209. |
| [13] | 张玉如, 万磊, 方昊翔, 李方泽, 王丽文, 李柯霏, 闫佩文, 姜辉. miR-155-5p介导PIK3R1负调控PI3K/AKT信号通路促进原发性干燥综合征人唾液腺上皮细胞增殖[J]. 南方医科大学学报, 2025, 45(1): 65-71. |
| [14] | 杜越, 张秀森, 周克旭, 金星, 原翔, 高社干. RgpB通过抑制自噬小体与溶酶体融合避免Cx43降解参与食管癌化疗耐药[J]. 南方医科大学学报, 2024, 44(9): 1670-1676. |
| [15] | 张先恒, 刘健, 韩琦, 陈一鸣, 丁香, 陈晓露. 黄芩清热除痹胶囊通过PTEN/PI3K/AKT信号通路改善痛风性关节炎大鼠的炎症反应及尿酸、脂质代谢失衡[J]. 南方医科大学学报, 2024, 44(8): 1450-1458. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||