南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (4): 837-843.doi: 10.12122/j.issn.1673-4254.2025.04.19
收稿日期:2024-10-05
出版日期:2025-04-20
发布日期:2025-04-28
通讯作者:
刘艳
E-mail:649745448@qq.com;liuyan496@126.com
作者简介:岳雅清,硕士,主治医师,E-mail: 649745448@qq.com
基金资助:
Yaqing YUE( ), Zhaoxia MU, Xibo WANG, Yan LIU(
), Zhaoxia MU, Xibo WANG, Yan LIU( )
)
Received:2024-10-05
Online:2025-04-20
Published:2025-04-28
Contact:
Yan LIU
E-mail:649745448@qq.com;liuyan496@126.com
摘要:
目的 探索敲低肌动蛋白相关蛋白2/3复合体亚基4(ARPC4)对Aurora-A过表达诱导的宫颈癌细胞增殖、迁移、侵袭及上皮间质转化的影响以及Aurora-A表达调控ARPC4的分子机制。 方法 将pCDH-NC、pCDH-Aurora-A、pCDH-Aurora-A+shRNA-ARPC4、pCDH-Aurora-A质粒转染至Hela细胞中,并在第4组细胞中加入NF-κBp65抑制剂,RT-PCR检测转染效率。根据Aurora-A、ARPC4的表达情况及NF-κBp65通路的抑制状态将其分为4组:Vector组、Aurora-A过表达质粒组、Aurora-A过表达+ARPC4敲降组、Aurora-A过表达+NF-κBp65抑制剂组。EDU免疫荧光检测Hela细胞增殖情况;结晶紫染色检测Hela细胞细胞集落形成情况;划痕实验和Transwell实验分别检测Hela细胞迁移情况;Transwell基质胶检测Hela细胞侵袭情况;Western blotting检测Hela细胞上皮间充质化(EMT)情况、NF-κBp65及ARPC4的表达。 结果 Aurora-A敲低的细胞中ARPC4表达下降,Aurora-A过表达的细胞中ARPC4表达上升(P<0.05)。过表达Aurora-A的宫颈癌细胞增殖、迁移及侵袭能力增强,而敲低ARPC4拮抗其作用(P<0.05)。Aurora-A过表达组NF-κBp65磷酸化水平增加,ARPC4表达水平增加(P<0.05)。Aurora-A可直接与NF-κBp65相互作用。与Aurora-A过表达组相比,Aurora-A过表达+NF-κBp65抑制剂组ARPC4表达下降(P<0.05)。 结论 Aurora-A通过激活NF-κBp65信号通路上调ARPC4表达,促进宫颈癌细胞的迁移、侵袭和上皮间充质化的过程。
岳雅清, 牟召霞, 王希波, 刘艳. Aurora-A过表达通过激活NF-κBp65/ARPC4信号轴促进宫颈癌细胞的侵袭和转移[J]. 南方医科大学学报, 2025, 45(4): 837-843.
Yaqing YUE, Zhaoxia MU, Xibo WANG, Yan LIU. Aurora-A overexpression promotes cervical cancer cell invasion and metastasis by activating the NF-κBp65/ARPC4 signaling axis[J]. Journal of Southern Medical University, 2025, 45(4): 837-843.
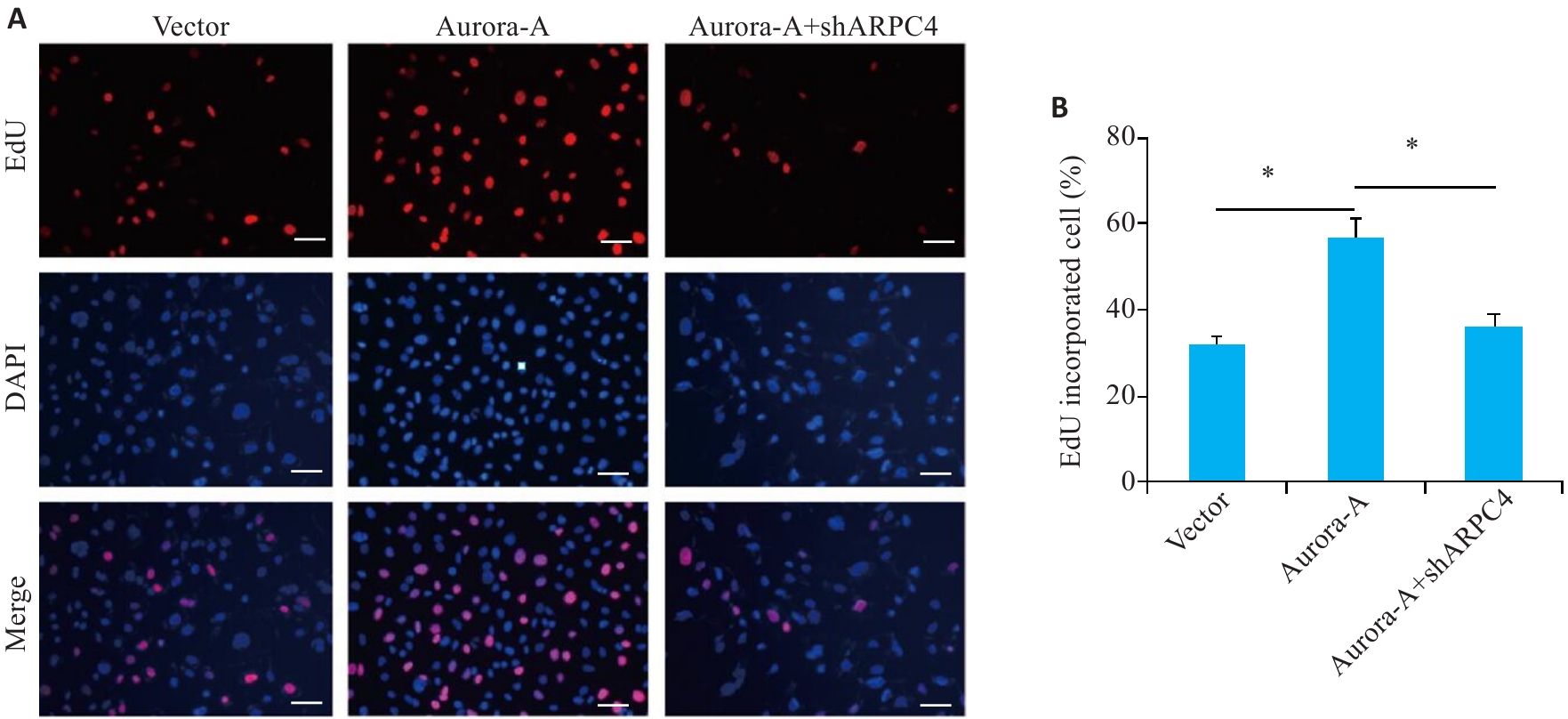
图1 敲低ARPC4拮抗Aurora-A过表达诱导的细胞增殖
Fig.1 ARPC4 knockdown antagonizes Aurora-A overexpression-induced enhancement of HeLa cell proliferation. A: EDU immunofluorescence staining for detecting cell proliferation in each group (scale bar=200 μm). B: Statistical chart of cell proliferation. *P<0.05.
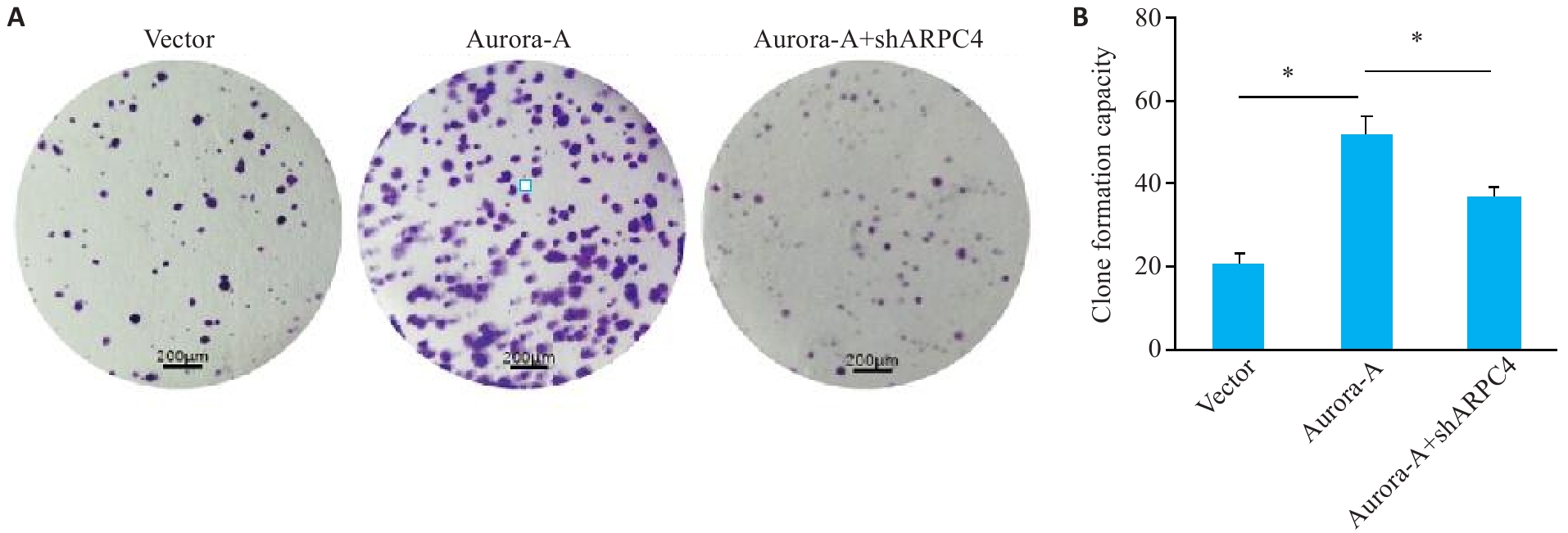
图2 敲低ARPC4拮抗Aurora-A过表达诱导的细胞集落形成
Fig.2 ARPC4 knockdown antagonizes the effect of Aurora-A overexpression for promoting cell colony formation in HeLa cells. A: Colony formation in each group (crystal violet staining, scale bar=200 μm). B: Statistical chart of colony formation. *P<0.05 .
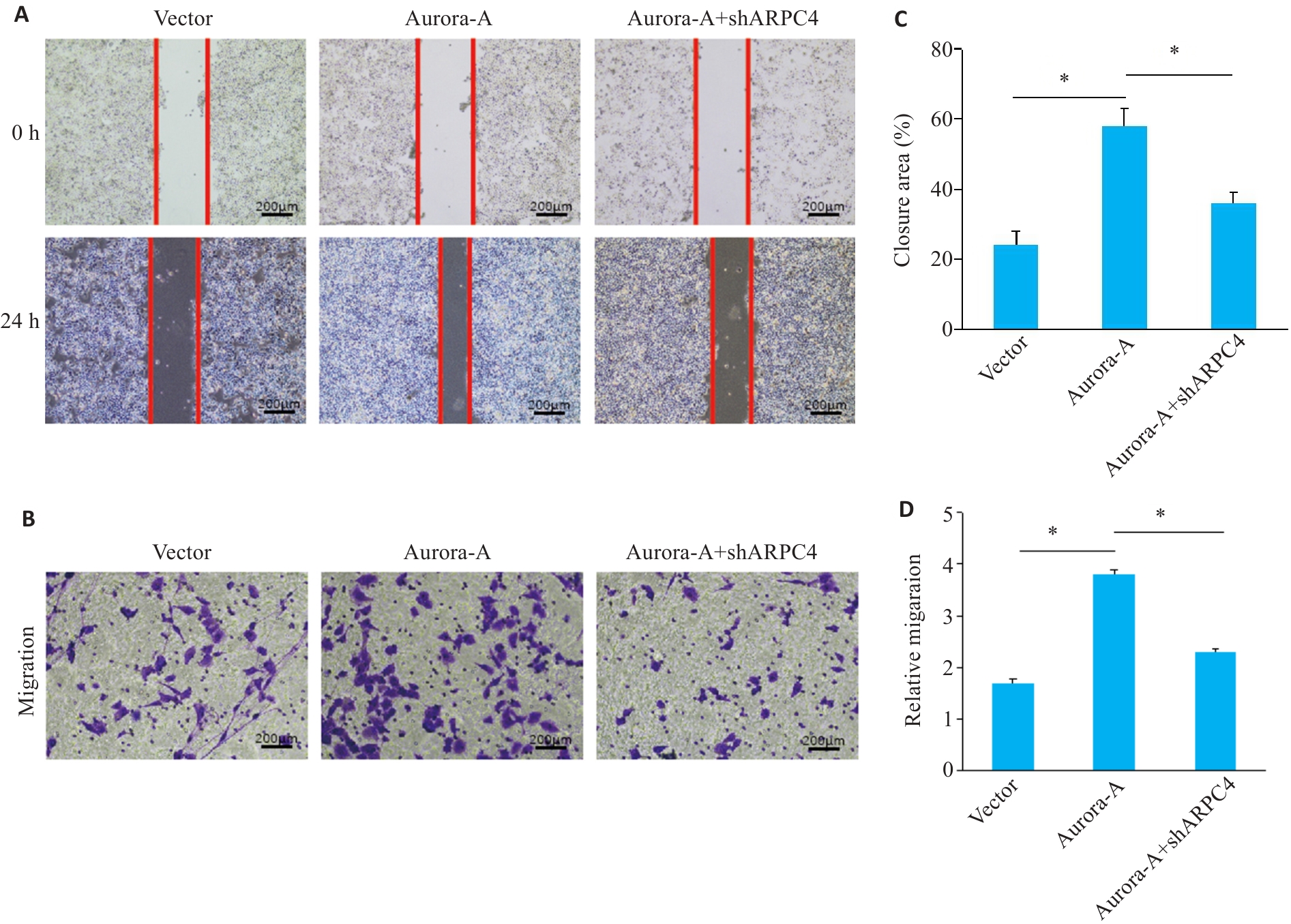
图3 敲低ARPC4拮抗Aurora-A过表达诱导的细胞迁移
Fig.3 ARPC4 knockdown antagonizes Aurora-A overexpression-induced enhancement of cell migration. A: Scratch assay for assessing migration ability of HeLa cells in each group (scale bar=200 μm). B: Transwell assay for assessing cell migration in each group (Crystal violet staining, scale bar=200 μm). C: Statistical chart of scratch assay in each group; D: Statistical chart of Transwell assay for each group. *P<0.05.
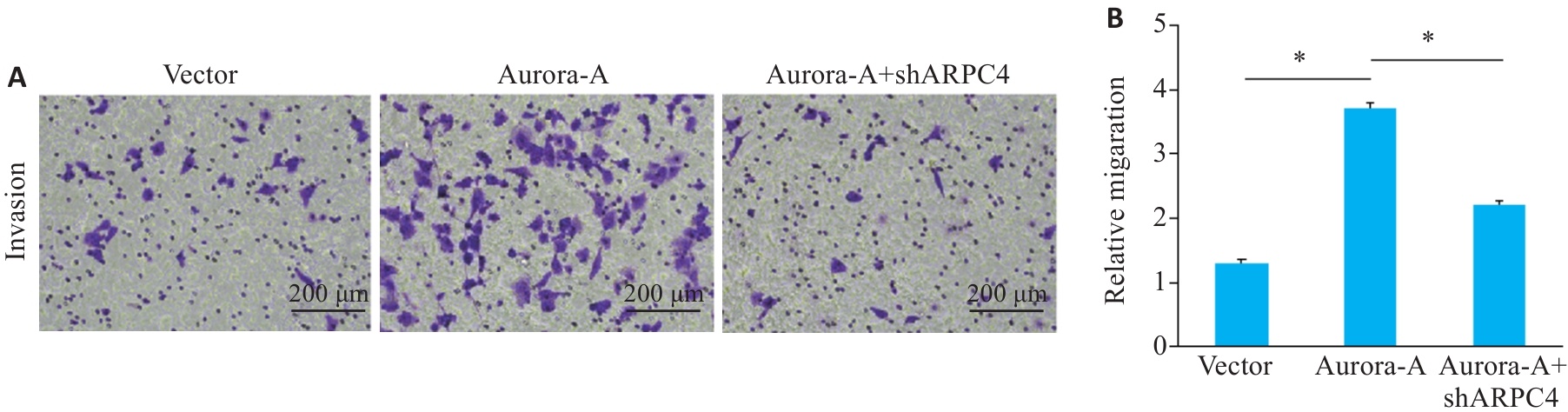
图4 敲低ARPC4拮抗Aurora-A过表达诱导的细胞侵袭
Fig.4 ARPC4 knockdown antagonizes the effect of Aurora-A overexpression for promoting cell invasion. A: Crystal violet staining for observing cell invasion in each group (scale bar=200 μm); B: Statistical chart of the invasive ability of each group. *P<0.05.
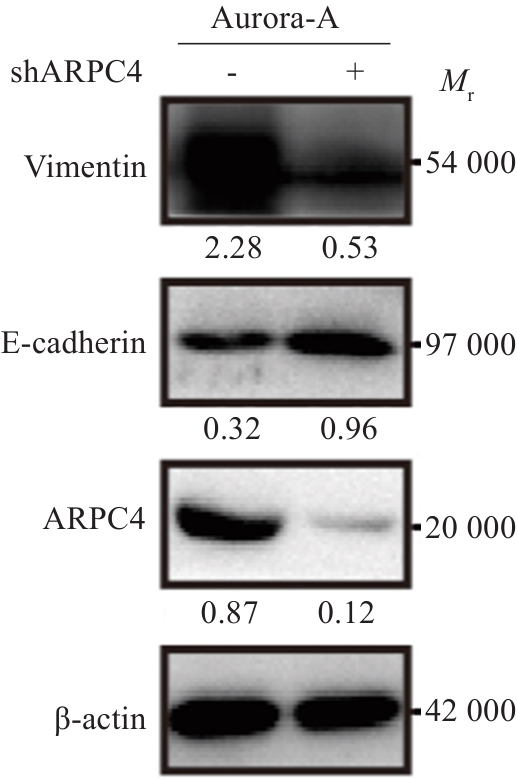
图5 敲低ARPC4拮抗Aurora-A过表达诱导的宫颈癌细胞EMT
Fig.5 ARPC4 knockdown antagonizes the effect of Aurora-A overexpression for promoting epithelial-mesenchymal transition of HeLa cells.
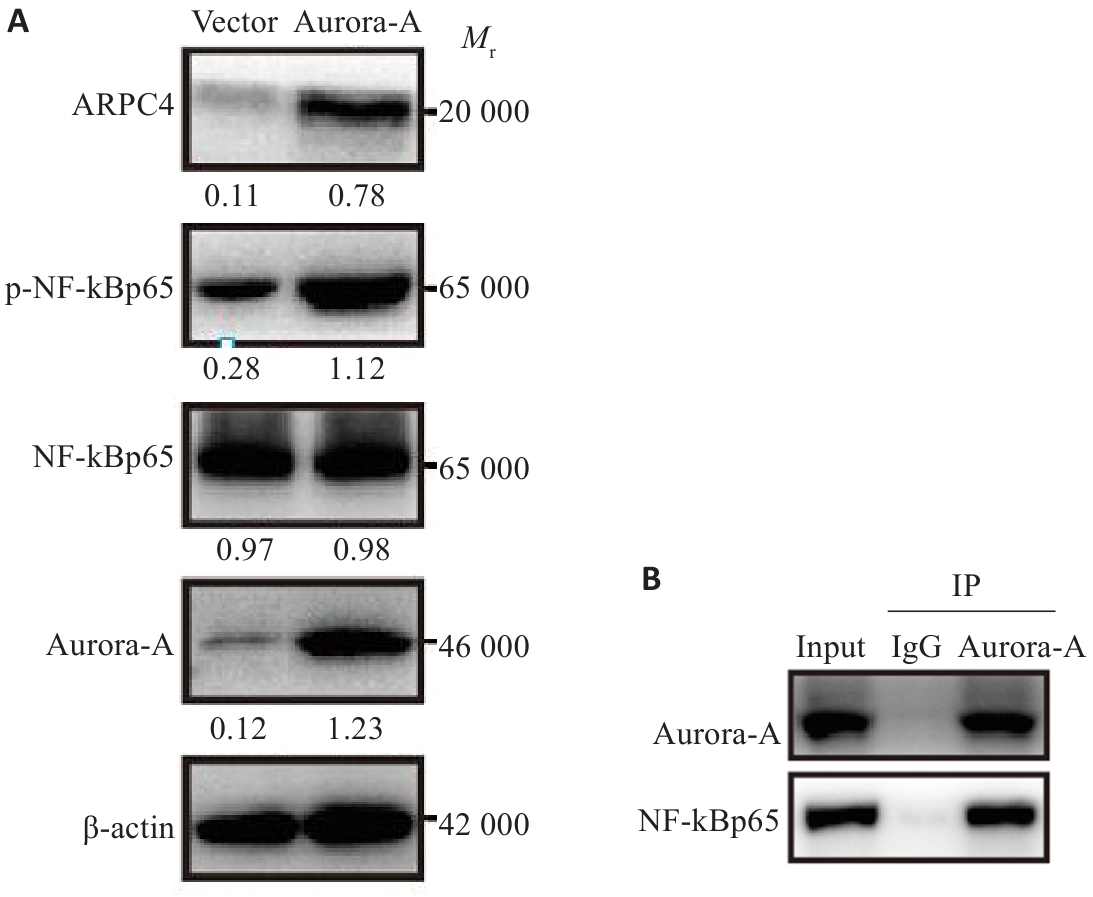
图6 过表达Aurora-A对NF-κBp65-ARPC4信号通路的影响
Fig.6 Effect of Aurora-A overexpression on NF-κBp65-ARPC4 signaling pathway in HeLa cells. A: Western blotting for detecting NF-κBp65-ARPC4 signaling pathway proteins in HeLa cells. B: Co-immunoprecipitation result showing interaction between Aurora-A and NF-κBp65.
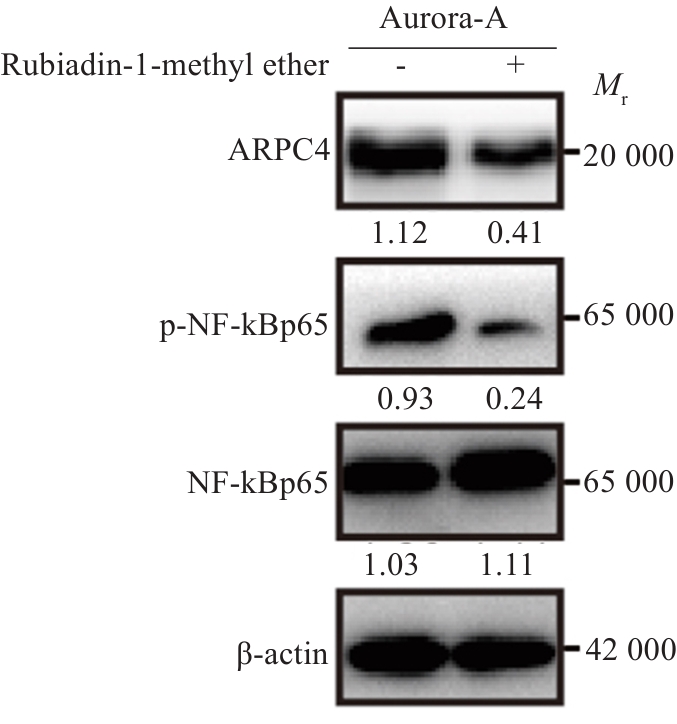
图7 Aurora-A通过激活NF-κBp65信号通路上调ARPC4表达
Fig.7 Aurora-A upregulates ARPC4 expression by activating the NF‑κBp65 signaling pathway. The changes in ARPC4, NF‑κBp65, and p-NF‑κBp65 expressions were detected using Western blotting.
| 1 | Ma Z, Zou XX, Yan ZH, et al. Preliminary analysis of cervical cancer immunotherapy[J]. Am J Clin Oncol, 2022, 45(11): 486-90. |
| 2 | Bejar FG, Oaknin A, Williamson C, et al. Novel therapies in gynecologic cancer[J]. Am Soc Clin Oncol Educ Book, 2022, 42: 1-17. |
| 3 | Kumar L, Harish P, Malik PS, et al. Chemotherapy and targeted therapy in the management of cervical cancer[J]. Curr Probl Cancer, 2018, 42(2): 120-8. |
| 4 | Su XJ, Wang SY, Huo YX, et al. Short interfering RNA-mediated silencing of actin-related protein 2/3 complex subunit 4 inhibits the migration of SW620 human colorectal cancer cells[J]. Oncol Lett, 2018, 15(3): 2847-54. |
| 5 | Yokotsuka M, Iwaya K, Saito T, et al. Overexpression of HER2 signaling to WAVE2-Arp2/3 complex activates MMP-independent migration in breast cancer[J]. Breast Cancer Res Treat, 2011, 126(2): 311-8. |
| 6 | Moriya Y, Nohata N, Kinoshita T, et al. Tumor suppressive microRNA-133a regulates novel molecular networks in lung squamous cell carcinoma[J]. J Hum Genet, 2012, 57(1): 38-45. |
| 7 | Kishore AH, Vedamurthy BM, Mantelingu K, et al. Specific small-molecule activator of aurora kinase A induces autophosphorylation in a cell-free system[J]. J Med Chem, 2008, 51(4): 792-7. |
| 8 | Coumar MS, Chu CY, Lin CW, et al. Fast-forwarding hit to lead: aurora and epidermal growth factor receptor kinase inhibitor lead identification[J]. J Med Chem, 2010, 53(13): 4980-8. |
| 9 | Borisa AC, Bhatt HG. A comprehensive review on aurora kinase: small molecule inhibitors and clinical trial studies[J]. Eur J Med Chem, 2017, 140: 1-19. |
| 10 | Lin XR, Xiang XS, Hao LP, et al. The role of Aurora-a in human cancers and future therapeutics[J]. Am J Cancer Res, 2020, 10(9): 2705-29. |
| 11 | Cheng AX, Zhang P, Wang B, et al. Aurora-a mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect[J]. Nat Commun, 2019, 10(1): 5566. |
| 12 | Sun HZ, Wang HS, Wang X, et al. Aurora-A/SOX8/FOXK1 signaling axis promotes chemoresistance via suppression of cell senescence and induction of glucose metabolism in ovarian cancer organoids and cells[J]. Theranostics, 2020, 10(15): 6928-45. |
| 13 | Zhi JT, Hu LF, Qian YY, et al. Targeting Aurora-a inhibits tumor progression and sensitizes thyroid carcinoma to Sorafenib by decreasing PFKFB3-mediated glycolysis[J]. Cell Death Dis, 2023, 14(3): 224. |
| 14 | Park SI, Lin CP, Ren N, et al. Inhibition of aurora A kinase in combination with chemotherapy induces synthetic lethality and overcomes chemoresistance in myc-overexpressing lymphoma[J]. Target Oncol, 2019, 14(5): 563-75. |
| 15 | Sun HZ, Wang Y, Wang ZL, et al. Aurora-a controls cancer cell radio- and chemoresistance via ATM/Chk2-mediated DNA repair networks[J]. Biochim Biophys Acta, 2014, 1843(5): 934-44. |
| 16 | Wang XB, Huang J, Liu FL, et al. Aurora A kinase inhibition compromises its antitumor efficacy by elevating PD-L1 expression[J]. J Clin Invest, 2023, 133(9): e161929. |
| 17 | Sun SL, Zhou W, Li XX, et al. Nuclear Aurora kinase A triggers programmed death-ligand 1-mediated immune suppression by activating MYC transcription in triple-negative breast cancer[J]. Cancer Commun, 2021, 41(9): 851-66. |
| 18 | Damodaran AP, Vaufrey L, Gavard O, et al. Aurora A kinase is a priori ty pharmaceutical target for the treatment of cancers[J]. Trends Pharmacol Sci, 2017, 38(8): 687-700. |
| 19 | 王恺越, 周怀君. Aurora A激酶与妇科肿瘤[J]. 国际肿瘤学杂志, 2014, 41(2): 117-20. |
| 20 | Zhang K, Wang T, Zhou H, et al. A novel aurora-a inhibitor (MLN8237) synergistically enhances the antitumor activity of sorafenib in hepatocellular carcinoma[J]. Mol Ther Nucleic Acids, 2018, 13: 176-88. |
| 21 | Ding YH, Zhou ZW, Ha CF, et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells[J]. Drug Des Devel Ther, 2015, 9: 425-64. |
| 22 | Bavetsias V, Linardopoulos S. Aurora kinase inhibitors: current status and outlook[J]. Front Oncol, 2015, 5: 278. |
| 23 | O’Connor OA, Özcan M, Jacobsen ED, et al. Randomized phase III study of alisertib or investigator's choice (selected single agent) in patients with relapsed or refractory peripheral T-cell lymphoma[J]. J Clin Oncol, 2019, 37(8): 613-23. |
| 24 | Kanamori S, Kajihara I, Kanazawa-Yamada S, et al. Expression of aurora kinase A expression in dermatofibrosarcoma protuberans[J]. J Dermatol, 2018, 45(4): 507-8. |
| 25 | Lykkesfeldt AE, Iversen BR, Jensen MB, et al. Aurora kinase A as a possible marker for endocrine resistance in early estrogen receptor positive breast cancer[J]. Acta Oncol, 2018, 57(1): 67-73. |
| 26 | Guo MJ, Lu SC, Huang HM, et al. Increased AURKA promotes cell proliferation and predicts poor prognosis in bladder cancer[J]. BMC Syst Biol, 2018, 12(): 118. |
| 27 | 陈远健. Aurora-A激酶的作用及其在结肠癌发生发展中的研究进展[J]. 医学理论与实践, 2023, 36(13): 2190-2, 2181. |
| 28 | Chiu SC, Chen KC, Hsia JY, et al. Overexpression of Aurora-a bypasses cytokinesis through phosphorylation of suppressed in lung cancer[J]. Am J Physiol Cell Physiol, 2019, 317(3): C600-12. |
| 29 | 左彤彤, 周卫萍. 神经母细胞瘤的分子生物学特性和靶向药物临床研究进展[J]. 中国肿瘤临床, 2021, 48(8): 426-31. |
| 30 | Wang ZH, Ma Z, Cao JP. Effects of repeated aurora-a siRNA transfection on Cilia generation and proliferation of SK-MES-1 or A549 cells[J]. Cancer Biother Radiopharm, 2018, 33(3): 110-7. |
| 31 | Heo SK, Noh EK, Jeong YK, et al. Radotinib inhibits mitosis entry in acute myeloid leukemia cells via suppression of Aurora kinase A expression[J]. Tumour Biol, 2019, 41(5): 1010428319848612. |
| 32 | Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy[J]. Nature, 2005, 434(7035): 917-21. |
| 33 | Sankaran S, Crone DE, Palazzo RE, et al. Aurora-a kinase regulates breast cancer associated gene 1 inhibition of centrosome-dependent microtubule nucleation[J]. Cancer Res, 2007, 67(23): 11186-94. |
| 34 | 许文兵. 子宫颈上皮内瘤变和子宫颈鳞癌中ARPC2/3蛋白的表达及意义[J]. 吉林医学, 2013, 34(32): 6674-5. |
| 35 | 董 洁, 胡观丽, 况 蕾, 等. 宫颈鳞癌组织中TPX2和Aurora-A蛋白的表达水平及预后意义[J]. 安徽医药, 2019, 23(10): 2018-21. |
| 36 | 张 鹏. 激酶Aurora A调控肿瘤代谢与氧化应激的机制探究[D]. 合肥: 中国科学技术大学, 2020. |
| 37 | 瞿根义. ARPC4调控细胞骨架对膀胱癌侵袭转移的作用和机制研究[D]. 福州: 福建医科大学, 2017. |
| [1] | 朱胤福, 李怡燃, 王奕, 黄颖而, 龚昆翔, 郝文波, 孙玲玲. 桂枝茯苓丸活性成分常春藤皂苷元通过抑制JAK2/STAT3通路抑制宫颈癌细胞的生长[J]. 南方医科大学学报, 2025, 45(7): 1423-1433. |
| [2] | 谢婷, 王云云, 郭婷, 袁春华. 雷氏大疣蛛多肽毒素组分通过激活促凋亡通路和协同作用抑制癌细胞增殖[J]. 南方医科大学学报, 2025, 45(7): 1460-1470. |
| [3] | 龚秀莹, 侯顺福, 赵苗苗, 王晓娜, 张致涵, 刘清华, 尹崇高, 李洪利. LncRNA SNHG15通过miR-30b-3p调控COX6B1轴促进肺腺癌细胞增殖、迁移和侵袭的分子机制[J]. 南方医科大学学报, 2025, 45(7): 1498-1505. |
| [4] | 李嘉豪, 冼瑞婷, 李荣. 下调ACADM介导的脂毒性抑制雌激素受体阳性乳腺癌细胞的侵袭与转移[J]. 南方医科大学学报, 2025, 45(6): 1163-1173. |
| [5] | 莫艳秀, 舒洋, 莫钰兰, 刘峻彤, 徐欧欧, 邓华菲, 王岐本. 敲除CDC20可明显抑制宫颈癌细胞的增殖及侵袭转移[J]. 南方医科大学学报, 2025, 45(6): 1200-1211. |
| [6] | 翁诺舟, 谭彬, 曾文涛, 古家宇, 翁炼基, 郑克鸿. 过表达RGL1通过激活CDC42/RAC1复合体上调运动型黏着斑组装促进结直肠癌转移[J]. 南方医科大学学报, 2025, 45(5): 1031-1038. |
| [7] | 曾玉梅, 李继科, 黄仲曦, 周毅波. 绒毛样蛋白VILL通过与LMO7蛋白相互作用抑制鼻咽癌细胞的增殖[J]. 南方医科大学学报, 2025, 45(5): 954-961. |
| [8] | 张毅, 沈昱, 万志强, 陶嵩, 柳亚魁, 王栓虎. CDKN3高表达促进胃癌细胞的迁移和侵袭:基于调控p53/NF-κB信号通路和抑制胃癌细胞凋亡[J]. 南方医科大学学报, 2025, 45(4): 853-861. |
| [9] | 庆顺杰, 沈智勇. 过表达己糖激酶2通过激活JAK/STAT途径促进结直肠癌细胞的增殖、迁移和侵袭并调节肿瘤免疫微环境[J]. 南方医科大学学报, 2025, 45(3): 542-553. |
| [10] | 黄晴晴, 张文静, 张小凤, 王炼, 宋雪, 耿志军, 左芦根, 王月月, 李静, 胡建国. 高表达MYO1B促进胃癌细胞增殖、迁移和侵袭并与患者的不良预后有关[J]. 南方医科大学学报, 2025, 45(3): 622-631. |
| [11] | 邹金华, 王惠, 张冬艳. SLC1A5通过促进M2型巨噬细胞极化促进肝癌进展[J]. 南方医科大学学报, 2025, 45(2): 269-284. |
| [12] | 宾禹, 李子雯, 左素微, 孙思诺, 李敏, 宋佳茵, 林旭, 薛刚, 吴靖芳. 载脂蛋白C1高表达通过激活JAK2/STAT3信号通路促进甲状腺乳头状癌细胞的增殖并抑制凋亡[J]. 南方医科大学学报, 2025, 45(2): 359-370. |
| [13] | 曹周芳, 汪元, 王梦娜, 孙玥, 刘菲菲. LINC00837/miR-671-5p/SERPINE2功能轴促进类风湿关节炎成纤维细胞样滑膜细胞的恶性病理学过程[J]. 南方医科大学学报, 2025, 45(2): 371-378. |
| [14] | 褚乔, 王小娜, 续佳颖, 彭荟林, 赵裕琳, 张静, 陆国玉, 王恺. 白头翁皂苷D通过多靶点和多途径抑制三阴性乳腺癌侵袭转移[J]. 南方医科大学学报, 2025, 45(1): 150-161. |
| [15] | 陈孝华, 鲁辉, 王子良, 王炼, 夏勇生, 耿志军, 张小凤, 宋雪, 王月月, 李静, 胡建国, 左芦根. ABI2在胃癌进展和预后中的作用及其调控机制[J]. 南方医科大学学报, 2024, 44(9): 1653-1661. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||