南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (8): 1718-1731.doi: 10.12122/j.issn.1673-4254.2025.08.16
• • 上一篇
张兆君1( ), 吴琼1, 谢苗苗1, 叶洳吟1, 耿晨晨1, 石纪雯1, 杨清玲1, 王文锐1, 石玉荣2(
), 吴琼1, 谢苗苗1, 叶洳吟1, 耿晨晨1, 石纪雯1, 杨清玲1, 王文锐1, 石玉荣2( )
)
收稿日期:2024-12-24
出版日期:2025-08-20
发布日期:2025-09-05
通讯作者:
石玉荣
E-mail:zzj17318530948@163.com;shiyr@126.com
作者简介:张兆君,在读硕士研究生,E-mail: zzj17318530948@163.com
基金资助:
Zhaojun ZHANG1( ), Qiong WU1, Miaomiao XIE1, Ruyin YE1, Chenchen GENG1, Jiwen SHI1, Qingling YANG1, Wenrui WANG1, Yurong SHI2(
), Qiong WU1, Miaomiao XIE1, Ruyin YE1, Chenchen GENG1, Jiwen SHI1, Qingling YANG1, Wenrui WANG1, Yurong SHI2( )
)
Received:2024-12-24
Online:2025-08-20
Published:2025-09-05
Contact:
Yurong SHI
E-mail:zzj17318530948@163.com;shiyr@126.com
摘要:
目的 研究层状双氢氧化物(LDH)负载si-NEAT1对乳腺癌紫杉醇耐药及肿瘤相关巨噬细胞极化作用的分子机制。 方法 qRT-PCR、Western blotting检测乳腺癌亲本细胞(SKBR3)和紫杉醇耐药乳腺癌细胞(SKBR3-PR)中LncRNA NEAT1、miR-133b和PD-L1的表达;设置实验分组Control、si-NEAT1、miR-133b mimics,qRT-PCR、Western blotting检测SKBR3-PR中MRP、BCRP和PD-L1的表达,划痕、Transwell检测增殖迁移能力,流式细胞术检测细胞凋亡;采用si-NEAT1和miR-133b inhibitor进行Rescue实验;肿瘤细胞上清(CM)与巨噬细胞共培养,设置实验分组PBS、IL-4、PBS+SKBR3 CM、PBS+SKBR3-PR CM、IL-4+PR si-N CM,qRT-PCR检测M2型巨噬细胞标志物Arg-1、CD163、IL-10与miR-133b、PD-L1的表达,免疫荧光检测CD163与CD206;构建LDH@si-NEAT1纳米载体转染肿瘤细胞,设置分组Control、LDH、游离si-NEAT1、LDH@si-NEAT1,qRT-PCR、Western blotting、免疫荧光检测MRP、BCRP和PD-L1的表达,划痕、Transwell实验检测细胞增殖迁移能力,流式细胞术检测细胞凋亡;LDH@si-NEAT1 CM与巨噬细胞共培养,设置实验分组PBS、IL-4、IL-4+PR@ CM,qRT-PCR、免疫荧光检测Arg-1、CD163、IL-10及miR-133b和PD-L1的表达。 结果 相较于亲本乳腺癌细胞,紫杉醇耐药乳腺癌细胞中NEAT1表达上调、miR-133b下调和PD-L1上调(P<0.05);si-NEAT1和 miR-133b mimics处理分别抑制了紫杉醇耐药乳腺癌细胞的活性,促进了细胞凋亡(P<0.01),MRP、BCRP表达下调(P<0.05);敲低NEAT1,miR-133b表达上调,PD-L1表达下调(P<0.01),MRP、BCRP表达下调(P<0.001);巨噬细胞M2型极化标志物Arg-1、CD163、IL-10在SKBR3-PR CM与巨噬细胞共培养后表达上调(P<0.05),而si-NEAT1 CM与巨噬细胞共培养后下调(P<0.05);LDH@si-NEAT1作用肿瘤细胞,迁移侵袭能力下调,凋亡增加(P<0.01),MRP和BCRP以及PD-L1蛋白的表达降低(P<0.05),LDH@ si-NEAT1CM作用巨噬细胞Arg-1、CD163、IL-10表达下调,miR-133b上调,PD-L1下调(P<0.01)。 结论 LDH@si-NEAT1通过靶向miR-133b/PD-L1轴,调控乳腺癌细胞紫杉醇耐药与巨噬细胞极化。
张兆君, 吴琼, 谢苗苗, 叶洳吟, 耿晨晨, 石纪雯, 杨清玲, 王文锐, 石玉荣. 层状双氢氧化物负载si-NEAT1通过miR-133b/PD-L1轴调控乳腺癌紫杉醇耐药及巨噬细胞极化[J]. 南方医科大学学报, 2025, 45(8): 1718-1731.
Zhaojun ZHANG, Qiong WU, Miaomiao XIE, Ruyin YE, Chenchen GENG, Jiwen SHI, Qingling YANG, Wenrui WANG, Yurong SHI. Layered double hydroxide-loaded si-NEAT1 regulates paclitaxel resistance and tumor-associated macrophage polarization in breast cancer by targeting miR-133b/PD-L1[J]. Journal of Southern Medical University, 2025, 45(8): 1718-1731.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | CAGCCTCAAGATCATCAGCA | TGTGGTCATGAGTCCTTCCA |
| NEAT1 | CTTCCTCCCTTTAACTTATCCATTCAC | CTCTTCCTCCACCATTACCAACAATAC |
| miR-133b | TCCCCTTCAACCAGCTAA | Universal primer |
| PD-L1 | GTAAGACCACCACCACCAATTC | AGTTGTTGTGTTGATTCTCAGTGTG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| Arg-1 | GGACCTGCCCTTTGCTGACATC | TCTTCTTGACTTCTGCCACCTTGC |
| CD163 | ACAATGAAGATGCTGGCGTGAC | ACAATGAAGATGCTGGCGTGAC |
| IL-10 | ACCAAGACCCAGACATCAAGGC | AGGCATTCTTCACCTGCTCCAC |
| CD68 | CCCACCTGCTTCTCTCATTCCC | TTGTACTCCACCGCCATGTAGC |
表1 PCR引物序列表
Tab.1 PCR primer sequences
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | CAGCCTCAAGATCATCAGCA | TGTGGTCATGAGTCCTTCCA |
| NEAT1 | CTTCCTCCCTTTAACTTATCCATTCAC | CTCTTCCTCCACCATTACCAACAATAC |
| miR-133b | TCCCCTTCAACCAGCTAA | Universal primer |
| PD-L1 | GTAAGACCACCACCACCAATTC | AGTTGTTGTGTTGATTCTCAGTGTG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| Arg-1 | GGACCTGCCCTTTGCTGACATC | TCTTCTTGACTTCTGCCACCTTGC |
| CD163 | ACAATGAAGATGCTGGCGTGAC | ACAATGAAGATGCTGGCGTGAC |
| IL-10 | ACCAAGACCCAGACATCAAGGC | AGGCATTCTTCACCTGCTCCAC |
| CD68 | CCCACCTGCTTCTCTCATTCCC | TTGTACTCCACCGCCATGTAGC |

图1 LncRNA NEAT1调控乳腺癌紫杉醇耐药
Fig.1 LncRNA NEAT1 regulates paclitaxel resistance of breast cancer cells. A, B: Western blotting and immunofluorescence detection of expression of drug resistance-related proteins in resistant breast cancer cells (scale bar=50 μm). C: qRT-PCR technology for detecting the expression of NEAT1. D: Western blotting for detecting drug resistance of the cells. E: Flow cytometry for analyzing apoptosis rate of the resistant breast cancer cells. F, G: Scratch and Transwell assays for assessing the migration and invasion capabilities of resistant cells (scale bar=200 μm). Scratch and Transwell assays for assessing migration and invasion capabilities of resistant breast cancer cells. *P<0.05, **P<0.01, ***P<0.001.
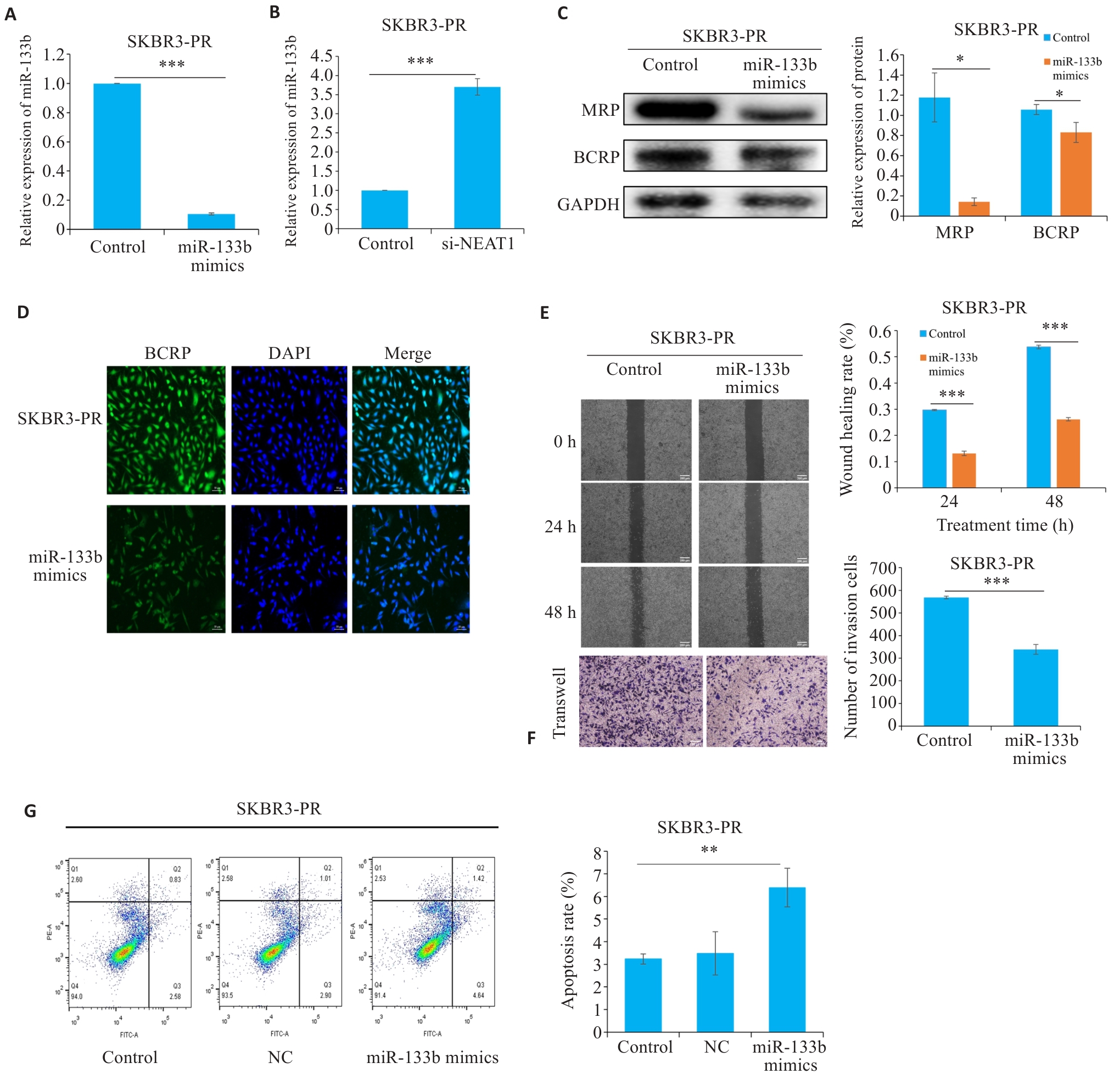
图2 LncRNA NEAT1通过调控miR-133b影响乳腺癌细胞耐药
Fig.2 LncRNA NEAT1 affects drug resistance in breast cancer cells by regulating miR-133b. A, B: qRT-PCR for detecting the expression of miR-133b. C: Western blotting for detecting expressions of drug resistance proteins. D: Immunofluorescence for detecting the expression of resistance proteins. E, F: Scratch and Transwell assays for assessing the migration and invasiveness of resistant cells. G: Flow cytometry for detecting apoptosis rate of resistant cells. *P<0.05, **P<0.01, ***P<0.001.
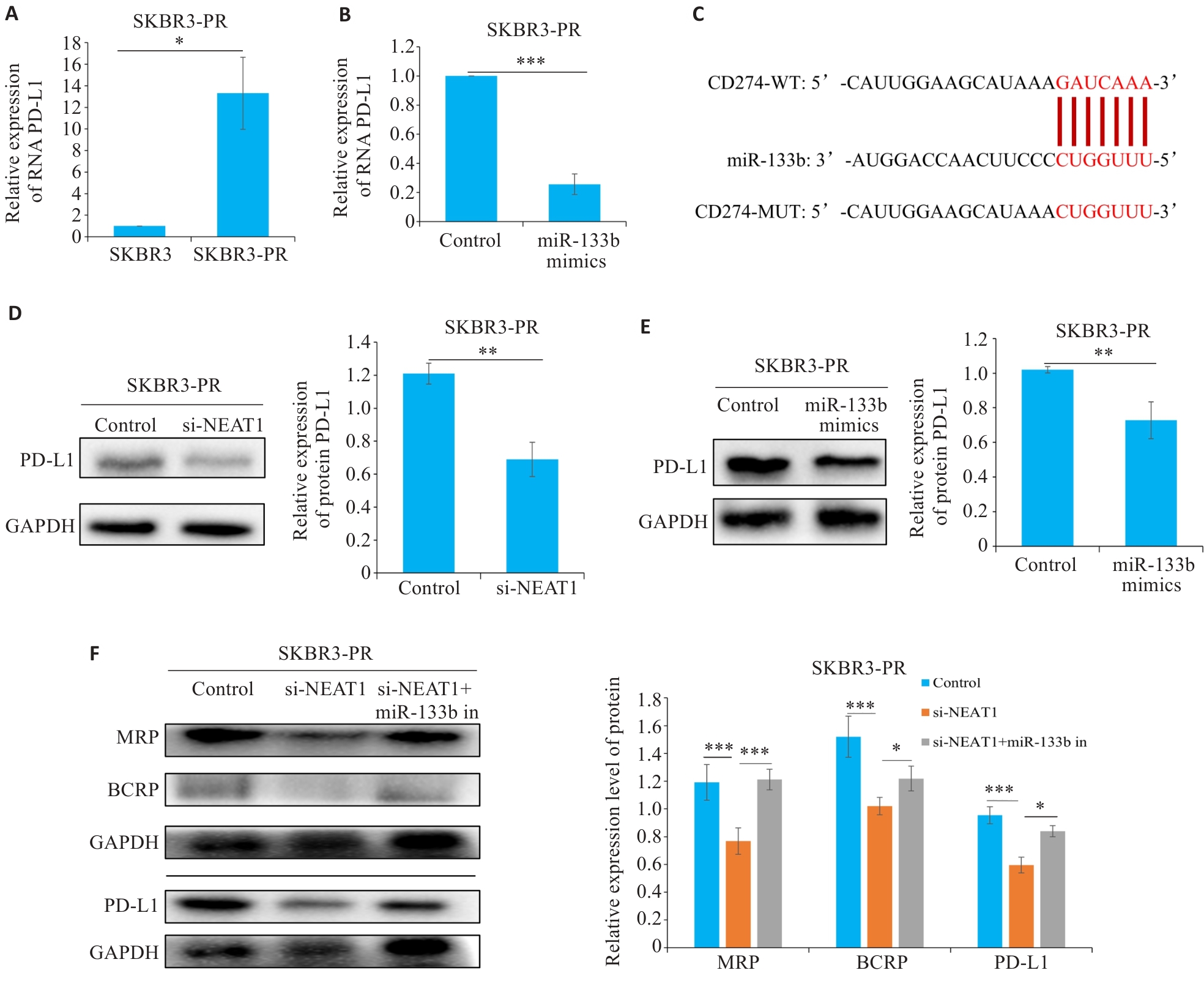
图3 LncRNA NEAT1调控miR-133b靶向PD-L1影响乳腺癌紫杉醇耐药
Fig.3 LncRNA NEAT1 regulates miR-133b to target PD-L1 and affect paclitaxel resistance in breast cancer cells. A, B: qRT-PCR for detecting the expression of PD-L1. C: Bioinformatics analysis reveals a regulatory relationship between miR-133b and PD-L1. D, E: Western blotting for detecting the expression of PD-L1. F: Western blotting for detecting the expressions of MRP, BCRP, and PD-L1 in drug-resistant breast cancer cells. *P<0.05, **P<0.01, ***P<0.001.
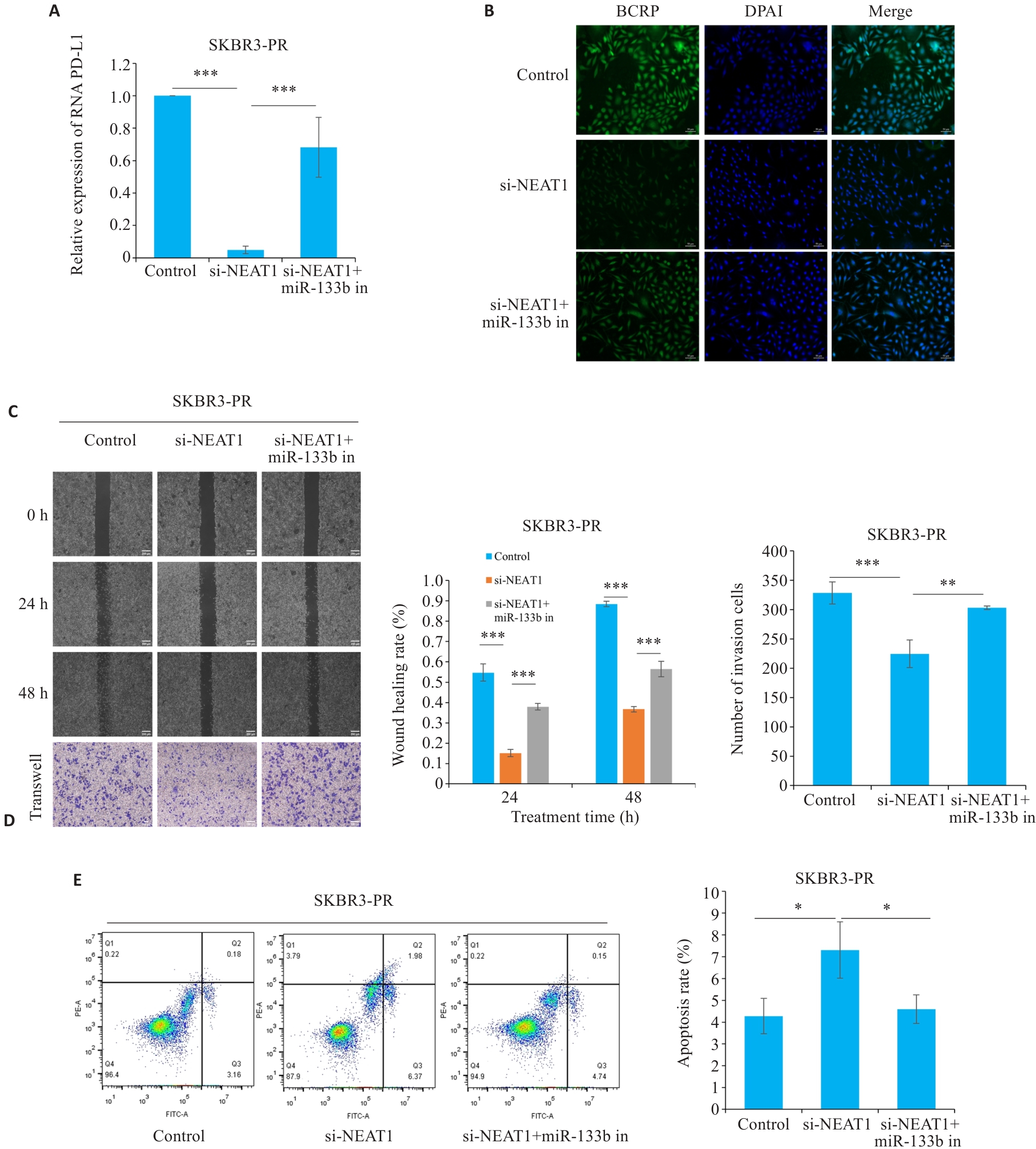
图4 LncRNA NEAT1通过调控miR-133b靶向PD-L1影响乳腺癌紫杉醇耐药
Fig.4 LncRNA NEAT1 regulates miR-133b to target PD-L1 and affect paclitaxel resistance in breast cancer cells. A: qRT-PCR for detecting the expression of PD-L1. B: Immunofluorescence for detecting the expression of BCRP in resistant cells (scale bar=50 μm). C, D: Scratch and Transwell assays for assessing migration and invasion capabilities of resistant cells (scale bar=200 μm). E: Flow cytometry for detecting apoptosis rate of the resistant cells. *P<0.05, **P<0.01, ***P<0.001.
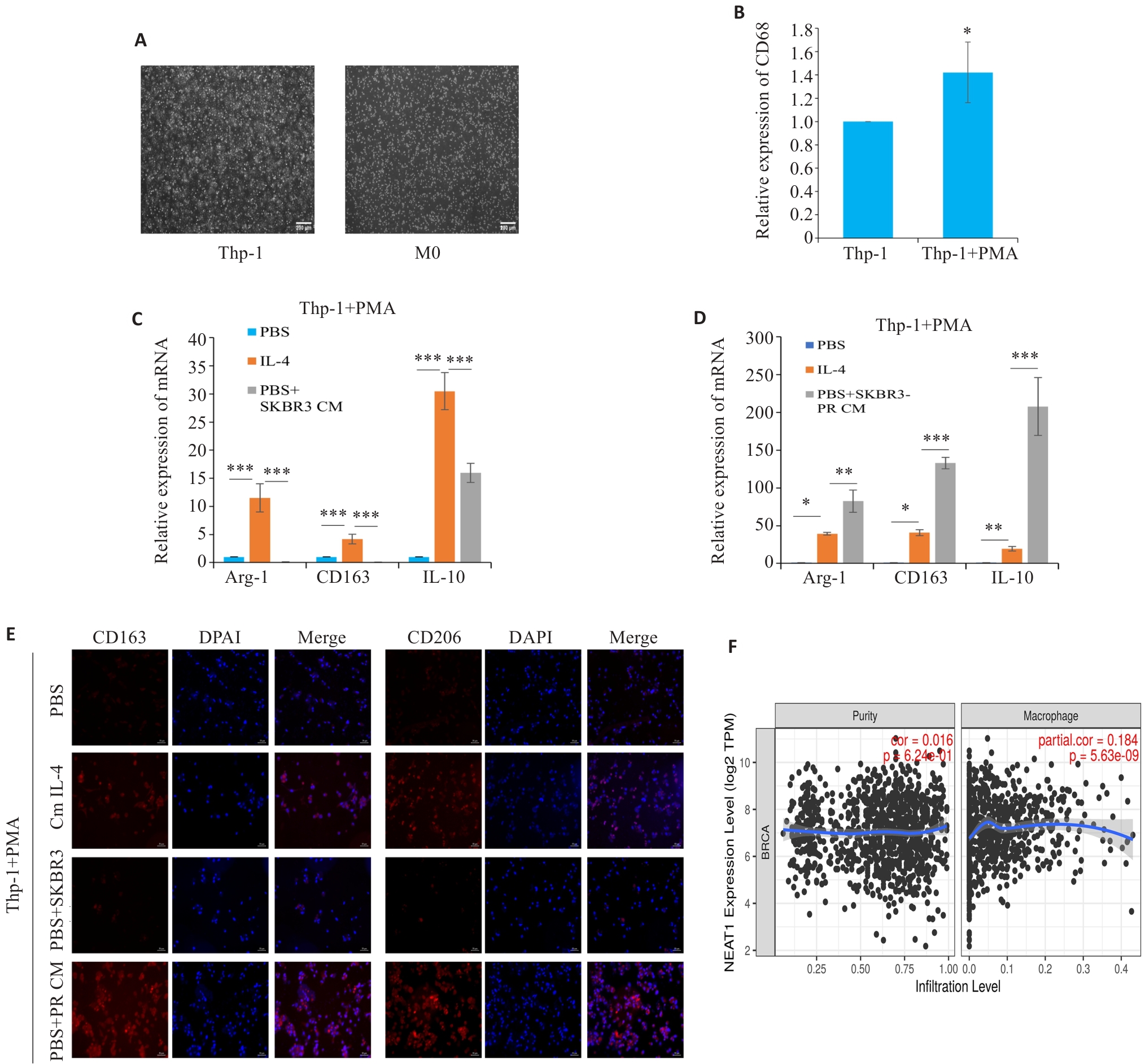
图5 耐药细胞上清调控TAMs 极化
Fig.5 Resistance cell supernatant can regulate the polarization of tumor-associated macrophages. A: Morphology of Thp-1 and M0-type macrophages (scale bar=200 μm ). B: qRT-PCR for detecting the expression of CD68. C, D: qRT-PCR for detecting the expression of M2 macrophage polarization markers Arg-1, CD163, and IL-10. E: Immunofluorescence staining for detecting M2 polarization markers CD163 and CD206 in the macrophages (scale bar=50 μm). F: Timer2.0 website predicts that the expression of NEAT1 is positively correlated with macrophage infiltration. *P<0.05, **P<0.01, ***P<0.001.
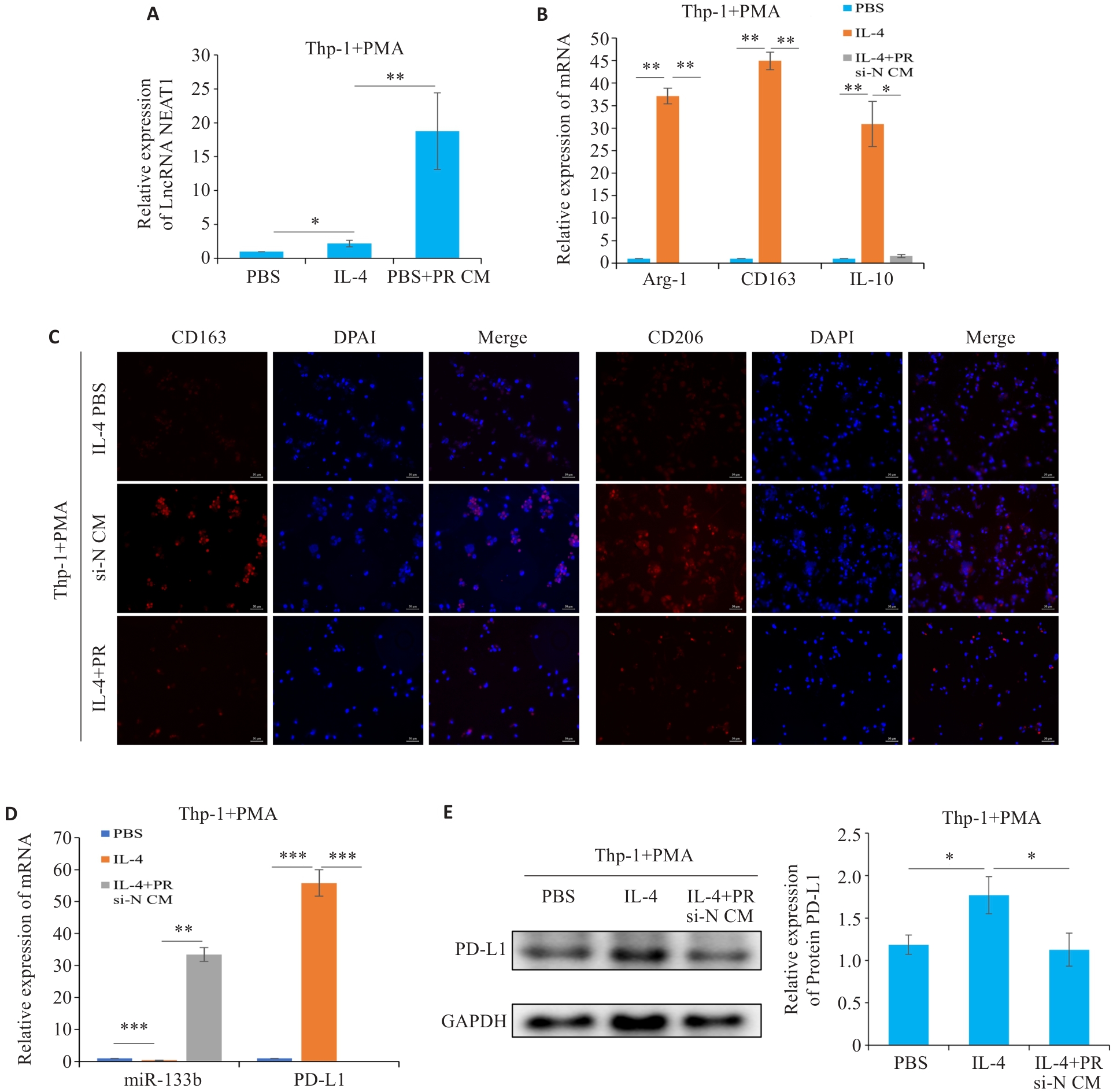
图6 LncRNA NEAT1调控TAMs 极化
Fig.6 LncRNA NEAT1 regulates polarization of tumor-associated macrophages in breast cancer. A, D: qRT-PCR for detecting the expressions of NEAT1, miR-133b, and PD-L1, respectively. B, C: qRT-PCR and Immunofluorescence for detecting M2 macrophage polarization markers (scale bar=50 μm). E: Western blotting for detecting the expression of PD-L1 in the macrophages. *P<0.05, **P<0.01, ***P<0.001.
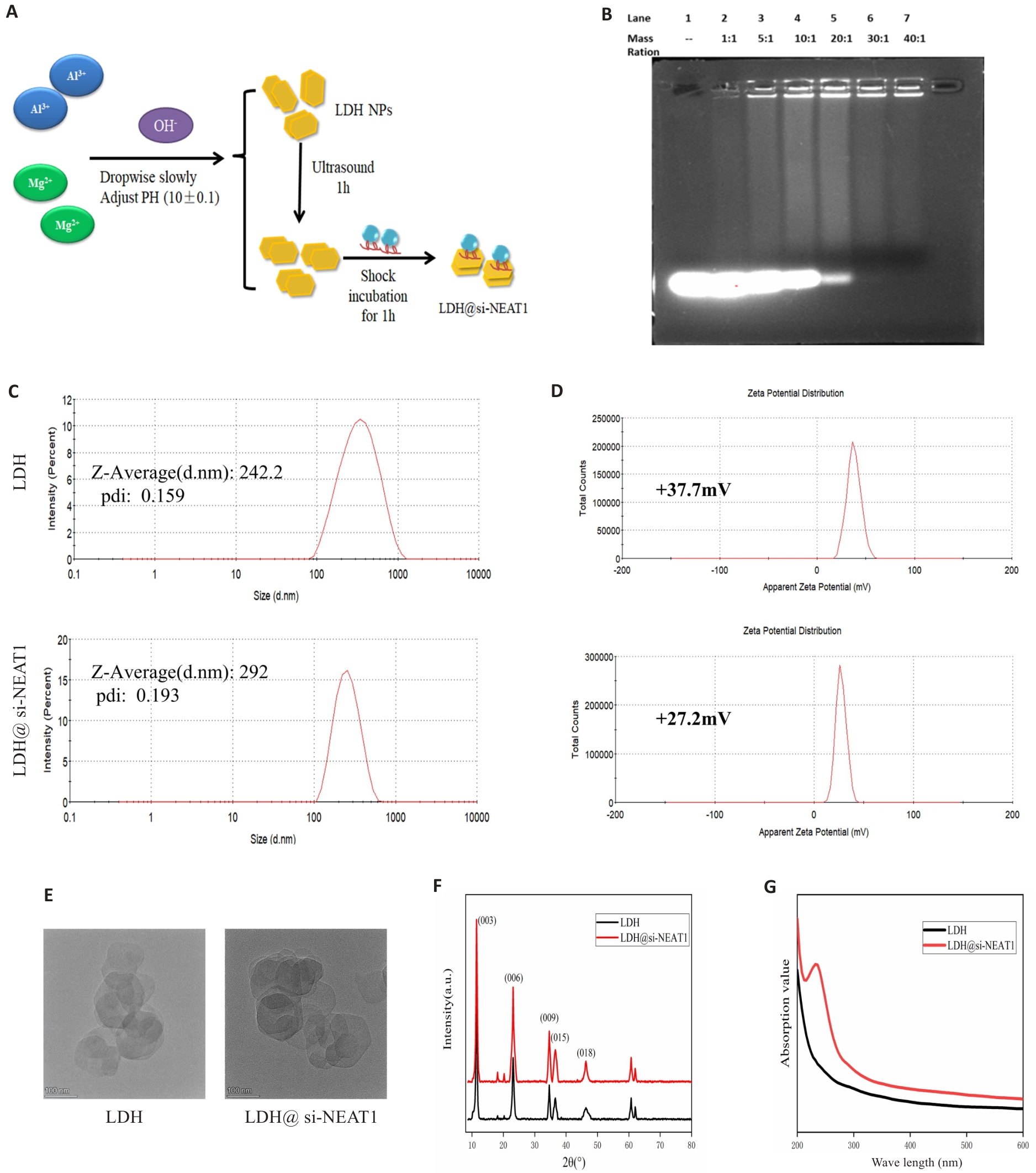
图7 LDH负载si-NEAT1纳米材料的构建
Fig.7 Construction of LDH Nanoparticles Loaded with si-NEAT1. A: Synthesis and loading of LDH. B: Agarose gel electrophoresis to determine the optimal loading ratio of LDH with si-NEAT1. C, D: Malvern particle size analysis for assessing particle size distribution and zeta potential of LDH and LDH@si-NEAT1. E: Transmission electron microscopy images of LDH and LDH@si-NEAT1. F, G: X-ray diffraction and UV-Vis spectra of LDH and LDH@si-NEAT1.
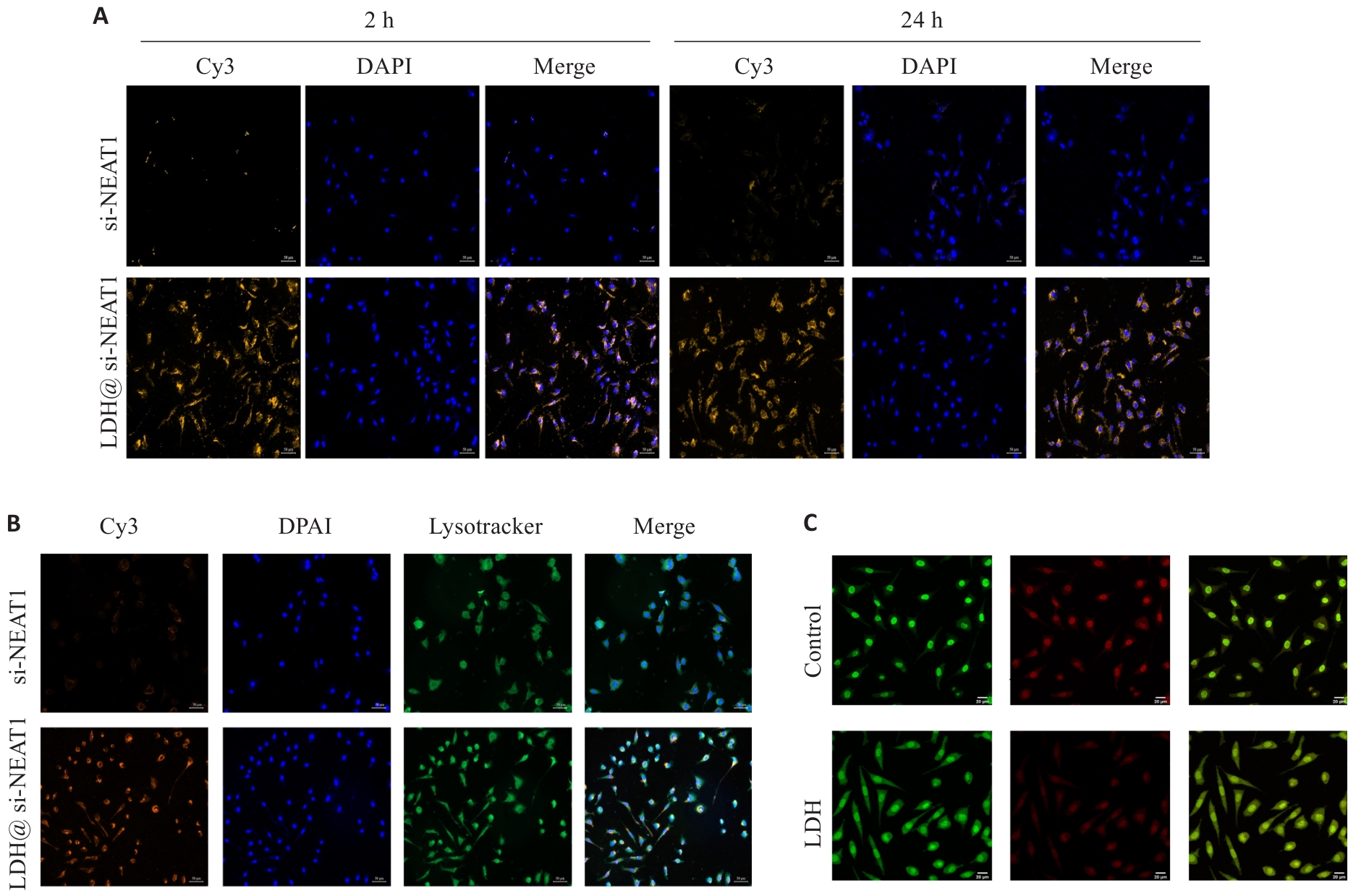
图8 表征LDH@ si-NEAT1实验
Fig.8 LDH@si-NEAT1 characterization experiments. A: Uptake experiment of LDH@si-NEAT1 (Original magnification: ×200). B: Confocal microscopy to detect lysosomal escape of LDH@si-NEAT1 (×200). C: Acridine orange staining to test lysosomal membrane permeability (×600).
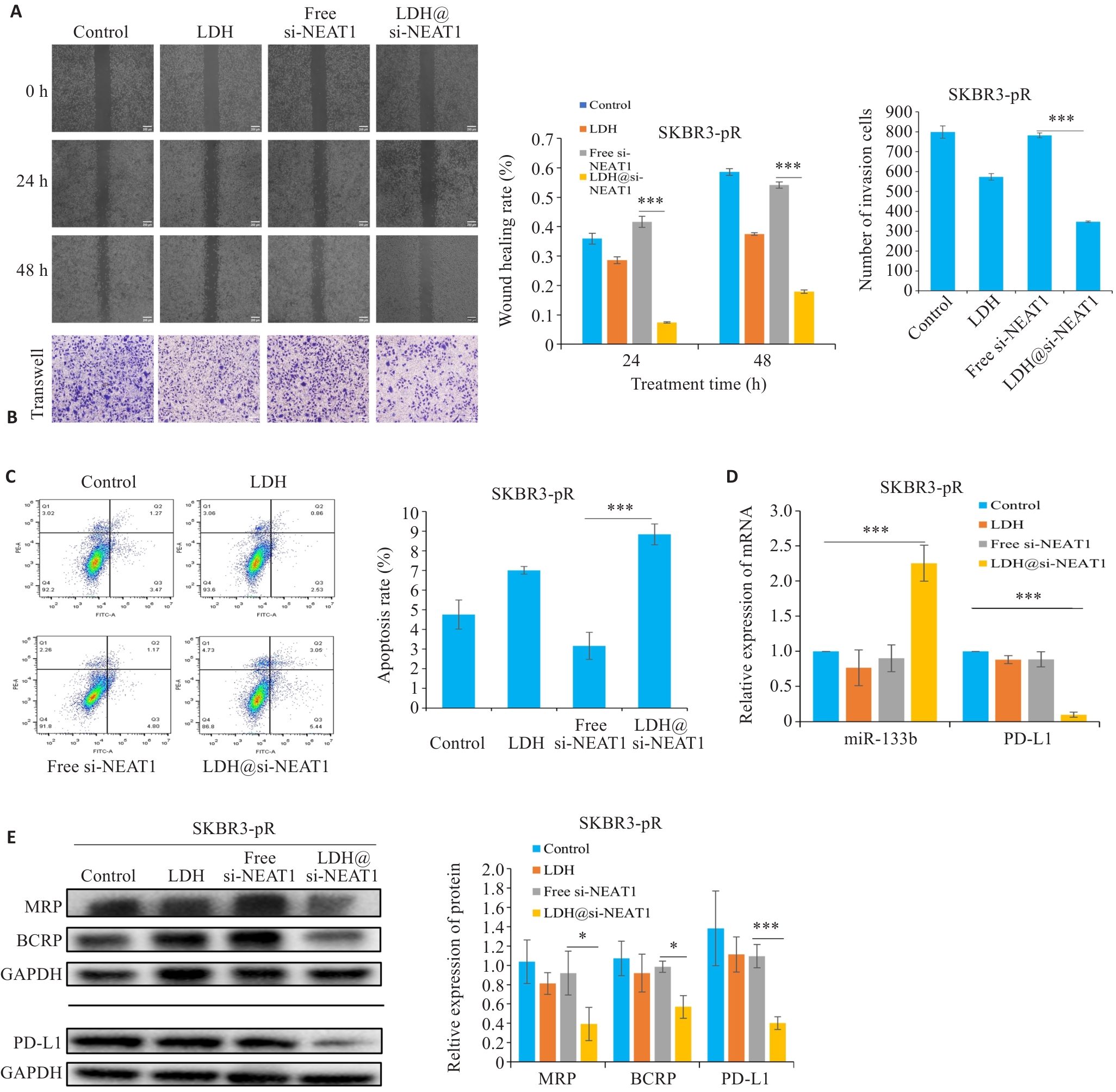
图9 LDH@si-NEAT1调控乳腺癌细胞耐药
Fig.9 LDH@si-NEAT1 regulate paclitaxel resistance in breast cancer cells. A, B: Scratch and Transwell assays to assess the migration and invasion capabilities of drug-resistant cells. C: Flow cytometry for detecting apoptosis rate of drug-resistant cells. D: qRT-PCR for detecting expressions of miR-133b and PD-L1, respectively. E: Western blotting for detecting expressions of drug resistance proteins and PD-L1. *P<0.05, ***P<0.001.
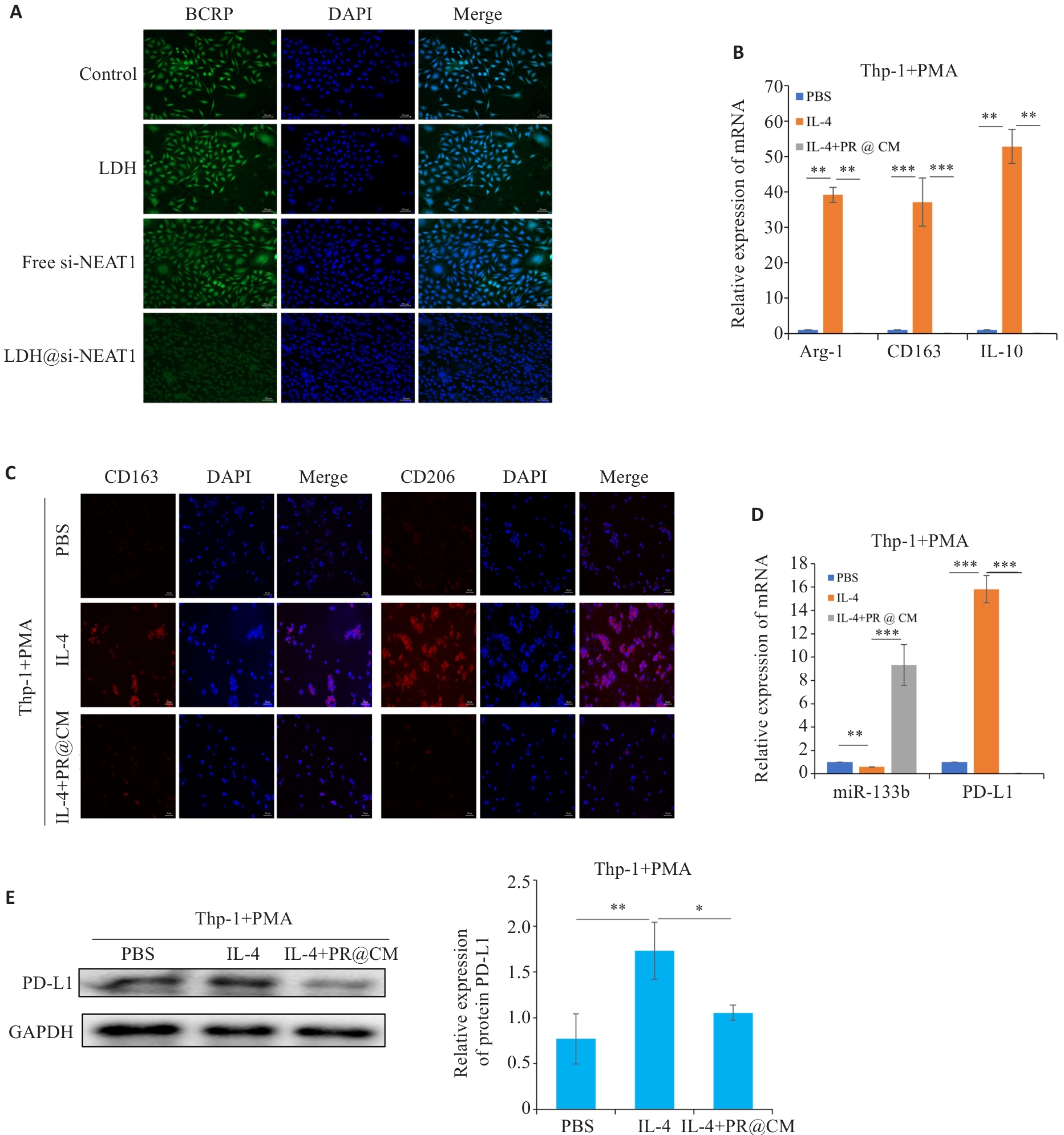
图10 LDH@si-NEAT1调控巨噬细胞极化状态
Fig.10 LDH@si-NEAT1 regulate M2 polarization state. A: Immunofluorescence staining for detecting expressions of drug resistance proteins (scale bar=50 μm). B, C: qRT-PCR and immunofluorescence staining for detecting M2 macrophage polarization markers (scale bar=50 μm). D: qRT-PCR of the expressions of miR-133b and PD-L1. E: Western blotting of the expression of PD-L1 in macrophages. *P<0.05, **P<0.01, ***P<0.001.
| [1] | Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-63. doi:10.3322/caac.21834 |
| [2] | 石英香, 王 婧, 石玉荣, 等. 乳腺癌紫杉醇耐药机制的研究进展[J]. 齐齐哈尔医学院学报, 2022, 43(6): 557-61. |
| [3] | Abu Samaan TM, Samec M, Liskova A, et al. Paclitaxel's mechanistic and clinical effects on breast cancer[J]. Biomolecules, 2019, 9(12): 789. doi:10.3390/biom9120789 |
| [4] | Ghafouri-Fard S, Taheri M. Nuclear enriched abundant transcript 1 (NEAT1): a long non-coding RNA with diverse functions in tumorigenesis[J]. Biomed Pharmacother, 2019, 111: 51-9. doi:10.1016/j.biopha.2018.12.070 |
| [5] | Wang C, Duan YJ, Duan G, et al. Stress induces dynamic, cytotoxicity-antagonizing TDP-43 nuclear bodies via paraspeckle LncRNA NEAT1-mediated liquid-liquid phase separation[J]. Mol Cell, 2020, 79(3): 443-58.e7. doi:10.1016/j.molcel.2020.06.019 |
| [6] | Wei XB, Jiang WQ, Zeng JH, et al. Exosome-derived lncRNA NEAT1 exacerbates sepsis-associated encephalopathy by promoting ferroptosis through regulating miR-9-5p/TFRC and GOT1 axis[J]. Mol Neurobiol, 2022, 59(3): 1954-69. doi:10.1007/s12035-022-02738-1 |
| [7] | Luo X, Wei QL, Jiang XY, et al. CSTF3 contributes to platinum resistance in ovarian cancer through alternative polyadenylation of lncRNA NEAT1 and generating the short isoform NEAT1_1[J]. Cell Death Dis, 2024, 15(6): 432. doi:10.1038/s41419-024-06816-1 |
| [8] | Puccio N, Manzotti G, Mereu E, et al. Combinatorial strategies targeting NEAT1 and AURKA as new potential therapeutic options for multiple myeloma[J]. Haematologica, 2024, 109(12): 4040-55. |
| [9] | Li MY, Yang YH, Xiong LT, et al. Metabolism, metabolites, and macrophages in cancer[J]. J Hematol Oncol, 2023, 16(1): 80. doi:10.1186/s13045-023-01478-6 |
| [10] | Borisenko GG, Matsura T, Liu SX, et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells: existence of a threshold[J]. Arch Biochem Biophys, 2003, 413(1): 41-52. doi:10.1016/s0003-9861(03)00083-3 |
| [11] | Liu DL, Wei YH, Liu YD, et al. The long non-coding RNA NEAT1/miR-224-5p/IL-33 axis modulates macrophage M2a polarization and A1 astrocyte activation[J]. Mol Neurobiol, 2021, 58(9): 4506-19. doi:10.1007/s12035-021-02405-x |
| [12] | 彭 威, 徐 旸, 王文锐. 层状双氢氧化物作为基因药物递送载体的研究进展[J]. 中国医药工业杂志, 2020, 51(10): 1243-53. doi:10.16522/j.cnki.cjph.2020.10.003 |
| [13] | Li L, Soyhan I, Warszawik E, et al. Layered double hydroxides: recent progress and promising perspectives toward biomedical applications[J]. Adv Sci (Weinh), 2024, 11(20): e2306035. doi:10.1002/advs.202306035 |
| [14] | 徐惠敏, 史竞彤, 孙轶华. 肿瘤相关巨噬细胞的极化方式及其对乳腺癌进展的影响[J]. 现代肿瘤医学, 2021, 29(22): 4049-54. doi:10.3969/j.issn.1672-4992.2021.22.037 |
| [15] | Mou SJ, Yang PF, Liu YP, et al. BCLAF1 promotes cell proliferation, invasion and drug-resistance though targeting lncRNA NEAT1 in hepatocellular carcinoma[J]. Life Sci, 2020, 242: 117177. doi:10.1016/j.lfs.2019.117177 |
| [16] | Wei XY, Tao S, Mao HL, et al. Exosomal lncRNA NEAT1 induces paclitaxel resistance in breast cancer cells and promotes cell migration by targeting miR-133b[J]. Gene, 2023, 860: 147230. doi:10.1016/j.gene.2023.147230 |
| [17] | Long F, Li X, Pan JY, et al. The role of lncRNA NEAT1 in human cancer chemoresistance[J]. Cancer Cell Int, 2024, 24(1): 236. doi:10.1186/s12935-024-03426-x |
| [18] | Wang JY, Zhang JH, Liu H, et al. N6-methyladenosine reader hnRNPA2B1 recognizes and stabilizes NEAT1 to confer chemoresistance in gastric cancer[J]. Cancer Commun (Lond), 2024, 44(4): 469-90. doi:10.1002/cac2.12534 |
| [19] | Zhang SJ, Kim EJ, Huang JF, et al. NEAT1 repression by MED12 creates chemosensitivity in p53 wild-type breast cancer cells[J]. FEBS J, 2024, 291(9): 1909-24. doi:10.1111/febs.17097 |
| [20] | Zhen SM, Jia YL, Zhao Y, et al. NEAT1_1 confers gefitinib resistance in lung adenocarcinoma through promoting AKR1C1-mediated ferroptosis defence[J]. Cell Death Discov, 2024, 10(1): 131. doi:10.1038/s41420-024-01892-w |
| [21] | Wang XW, Xu Y, Zhu YC, et al. LncRNA NEAT1 promotes extracellular matrix accumulation and epithelial-to-mesenchymal transition by targeting miR-27b-3p and ZEB1 in diabetic nephropathy[J]. J Cell Physiol, 2019, 234(8): 12926-33. doi:10.1002/jcp.27959 |
| [22] | Lin SP, Wen ZK, Li SX, et al. LncRNA Neat1 promotes the macrophage inflammatory response and acts as a therapeutic target in titanium particle-induced osteolysis[J]. Acta Biomater, 2022, 142: 345-60. doi:10.1016/j.actbio.2022.02.007 |
| [23] | Wei FF, Yan ZY, Zhang XM, et al. LncRNA-NEAT1 inhibits the occurrence and development of pancreatic cancer through spongy miR-146b-5p/traf6[J]. Biotechnol Genet Eng Rev, 2024, 40(2): 1094-112. doi:10.1080/02648725.2023.2192059 |
| [24] | Geng F, Jia WC, Li T, et al. Knockdown of lncRNA NEAT1 suppresses proliferation and migration, and induces apoptosis of cervical cancer cells by regulating the miR-377/FGFR1 axis[J]. Mol Med Rep, 2022, 25(1): 10. doi:10.3892/mmr.2021.12526 |
| [25] | Zhang K, Zhou H, Yan B, et al. TUG1/miR-133b/CXCR4 axis regulates cisplatin resistance in human tongue squamous cell carcinoma[J]. Cancer Cell Int, 2020, 20: 148. doi:10.1186/s12935-020-01224-9 |
| [26] | Li PF, Sun Q, Bai SP, et al. Combination of the cuproptosis inducer disulfiram and anti-PD-L1 abolishes NSCLC resistance by ATP7B to regulate the HIF-1 signaling pathway[J]. Int J Mol Med, 2024, 53(2): 19. doi:10.3892/ijmm.2023.5343 |
| [27] | Rabiee N, Bagherzadeh M, Ghadiri AM, et al. ZnAl nano layered double hydroxides for dual functional CRISPR/Cas9 delivery and enhanced green fluorescence protein biosensor[J]. Sci Rep, 2020, 10(1): 20672. doi:10.1038/s41598-020-77809-1 |
| [28] | Lee J, Seo HS, Park W, et al. Biofunctional layered double hydroxide nanohybrids for cancer therapy[J]. Materials (Basel), 2022, 15(22): 7977. doi:10.3390/ma15227977 |
| [29] | Zhang SW, Pang SY, Pei WH, et al. Layered double hydroxide-loaded miR-30a for the treatment of breast cancer in vitro and in vivo [J]. ACS Omega, 2023, 8(21): 18435-48. doi:10.1021/acsomega.2c07866 |
| [30] | Li L, Qian YJ, Sun LY, et al. Albumin-stabilized layered double hydroxide nanoparticles synergized combination chemotherapy for colorectal cancer treatment[J]. Nanomedicine, 2021, 34: 102369. doi:10.1016/j.nano.2021.102369 |
| [31] | Chang MY, Wang M, Liu B, et al. A cancer nanovaccine based on an FeAl-layered double hydroxide framework for reactive oxygen species-augmented metalloimmunotherapy[J]. ACS Nano, 2024, 18(11): 8143-56. doi:10.1021/acsnano.3c11960 |
| [1] | 马思源, 张博超, 浦春. Circ_0000437通过靶向let-7b-5p/CTPS1轴促进乳腺癌细胞的增殖、侵袭、迁移及上皮间质转化[J]. 南方医科大学学报, 2025, 45(8): 1682-1696. |
| [2] | 李嘉豪, 冼瑞婷, 李荣. 下调ACADM介导的脂毒性抑制雌激素受体阳性乳腺癌细胞的侵袭与转移[J]. 南方医科大学学报, 2025, 45(6): 1163-1173. |
| [3] | 陈镝, 吕莹, 郭怡欣, 张怡荣, 王蕊璇, 周小若, 陈雨欣, 武晓慧. 双氢青蒿素可显著增强阿霉素诱导的三阴性乳腺癌细胞凋亡:基于负向调控STAT3/HIF-1α通路[J]. 南方医科大学学报, 2025, 45(2): 254-260. |
| [4] | 褚乔, 王小娜, 续佳颖, 彭荟林, 赵裕琳, 张静, 陆国玉, 王恺. 白头翁皂苷D通过多靶点和多途径抑制三阴性乳腺癌侵袭转移[J]. 南方医科大学学报, 2025, 45(1): 150-161. |
| [5] | 薛良军, 谈秋瑜, 许静文, 冯璐, 李文锦, 颜亮, 李玉磊. MiR-6838-5p过表达下调DDR1基因表达抑制乳腺癌MCF-7细胞的增殖[J]. 南方医科大学学报, 2024, 44(9): 1677-1684. |
| [6] | 欧阳明子, 崔佳琦, 王慧, 梁正, 皮大锦, 陈利国, 陈前军, 吴迎朝. 开心散通过减轻前额叶皮质铁死亡缓解小鼠的阿霉素化疗性抑郁[J]. 南方医科大学学报, 2024, 44(8): 1441-1449. |
| [7] | 房锦存, 刘立威, 林俊豪, 陈逢生. CDHR2过表达通过抑制PI3K/Akt通路抑制乳腺癌细胞增殖[J]. 南方医科大学学报, 2024, 44(6): 1117-1125. |
| [8] | 崔芝, 马萃娇, 王倩茹, 陈金豪, 严子阳, 杨建林, 吕亚丰, 曹春雨. 表达 TGF-βⅡ受体的腺相关病毒载体抑制小鼠三阴性乳腺癌4T1细胞的增殖和肺转移[J]. 南方医科大学学报, 2024, 44(5): 818-826. |
| [9] | 陈守峰, 张舒超, 樊伟林, 孙 巍, 刘贝贝, 刘建民, 郭园园. 吡非尼酮联合PD-L1抑制剂抑制小鼠异位膀胱肿瘤的生长[J]. 南方医科大学学报, 2024, 44(2): 210-216. |
| [10] | 曾佑琴, 陈思雨, 刘燕, 刘奕彤, 张玲, 夏姣, 吴心语, 魏常友, 冷平. AKBA联合阿霉素抑制三阴性乳腺癌细胞MDA-MB-231的增殖、迁移和裸鼠移植瘤生长[J]. 南方医科大学学报, 2024, 44(12): 2449-2460. |
| [11] | 张晋弘, 刘昕, 刘健. PHPS1通过调控口腔鳞状细胞癌内ROS/SHP-2/AMPK活性促进PD-L1丝氨酸磷酸化进而加速肿瘤凋亡[J]. 南方医科大学学报, 2024, 44(12): 2469-2476. |
| [12] | 徐梦歧, 石宇彤, 刘俊平, 吴敏敏, 张凤梅, 何志强, 唐 敏. JAG1影响单核-巨噬细胞重塑三阴性乳腺癌转移前微环境:基于外泌体中的LncRNA MALAT1[J]. 南方医科大学学报, 2023, 43(9): 1525-1535. |
| [13] | 许家铭, 林 龙, 陈琼慧, 李 兰. Hsa-miR-148a-3p通过下调DUSP1基因促进乳腺癌细胞的恶性行为[J]. 南方医科大学学报, 2023, 43(9): 1515-1524. |
| [14] | 王 丽, 严志锐, 夏耀雄. 抑制RAB27a能够抑制三阴乳腺癌细胞的增殖、侵袭和粘附[J]. 南方医科大学学报, 2023, 43(4): 560-567. |
| [15] | 广东省医疗行业协会乳腺病整形修复管理分会. 乳腺手术的关键操作细节标准:广东省医疗行业协会专家共识[J]. 南方医科大学学报, 2023, 43(10): 1827-1832. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||