南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (12): 2561-2572.doi: 10.12122/j.issn.1673-4254.2025.12.04
夏金枝1,5( ), 陈悦3,5, 任侣3,5, 李静1,2, 宋雪2,4, 陶露2,4, 胡建国1,2(
), 陈悦3,5, 任侣3,5, 李静1,2, 宋雪2,4, 陶露2,4, 胡建国1,2( )
)
收稿日期:2025-05-16
出版日期:2025-12-20
发布日期:2025-12-22
通讯作者:
胡建国
E-mail:Jinzhixia0511@163.com;jghu9200@bbmu.edu.cn
作者简介:夏金枝,在读硕士研究生,E-mail: Jinzhixia0511@163.com
基金资助:
Jinzhi XIA1,5( ), Yue CHEN3,5, Lü REN3,5, Jing LI1,2, Xue SONG2,4, Lu TAO2,4, Jianguo HU1,2(
), Yue CHEN3,5, Lü REN3,5, Jing LI1,2, Xue SONG2,4, Lu TAO2,4, Jianguo HU1,2( )
)
Received:2025-05-16
Online:2025-12-20
Published:2025-12-22
Contact:
Jianguo HU
E-mail:Jinzhixia0511@163.com;jghu9200@bbmu.edu.cn
Supported by:摘要:
目的 探究咖啡豆醇(Kah)在脊髓损伤后通过抑制小胶质细胞活化介导的炎症反应对运动功能恢复的影响,并阐明其作用机制。 方法 随机将54只8~10周龄的C57BL/6J小鼠分为Sham组(只进行椎板剥离), 脊髓损伤(SCI)组(剥离椎板并撞击脊髓),Kah组(术后每日腹腔注射20 mg/kg的Kah),18只/组。采用BMS评分、足迹分析、游泳实验评估小鼠的运动功能。通过HE、快蓝染色及尼氏染色评估脊髓损伤区域、髓鞘完整性和神经元存活。使用脂多糖刺激BV2细胞建立体外炎症模型及BV2/HT22神经元共培养体系,利用不同浓度Kah干预,通过免疫荧光对体内外活化的小胶质细胞及神经元凋亡的数量进行评估;通过Western blotting检测凋亡相关蛋白(Bax/Bcl-2/cleaved caspase3)及IκBα/NF-κB通路蛋白。通过qRT-PCR 、ELISA检测炎症因子(TNF-α、IL-6、IL-1β)水平。使用NF-κB通路激活剂PMA于体外实验进行干预,分析Kah的作用机制。 结果 Kah治疗改善了SCI小鼠的运动功能;组织学检测显示,Kah组脊髓组织病理性损伤区域较SCI组缩小(P<0.001),同时髓鞘化面积及神经元数目增加(P<0.001);体内外实验显示,Kah通过抑制小胶质细胞的活化并减少其释放炎症因子缓解神经元凋亡;Western blotting结果显示,Kah可降低NF-κB及IκBα的磷酸化水平。体外免疫荧光结果证实,与Kah干预组相比,给予PMA后,活化的BV2细胞及凋亡的神经元增加(P<0.05)。 结论 Kah通过抑制NF-κB通路调控小胶质细胞活化来促进SCI后运动功能的恢复,为临床治疗提供了潜在新策略。
夏金枝, 陈悦, 任侣, 李静, 宋雪, 陶露, 胡建国. 咖啡豆醇通过调控IκBα/NF-κB通路抑制小胶质细胞活化改善脊髓损伤后小鼠的运动功能[J]. 南方医科大学学报, 2025, 45(12): 2561-2572.
Jinzhi XIA, Yue CHEN, Lü REN, Jing LI, Xue SONG, Lu TAO, Jianguo HU. Kahweol improves motor function of mice with spinal cord injury by inhibiting microglial activation via regulating the IκBα/NF-κB pathway[J]. Journal of Southern Medical University, 2025, 45(12): 2561-2572.
| Gene (mice) | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG- |
表1 引物序列
Tab.1 Primer sequences (5' to 3') for RT-qPCR
| Gene (mice) | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG- |
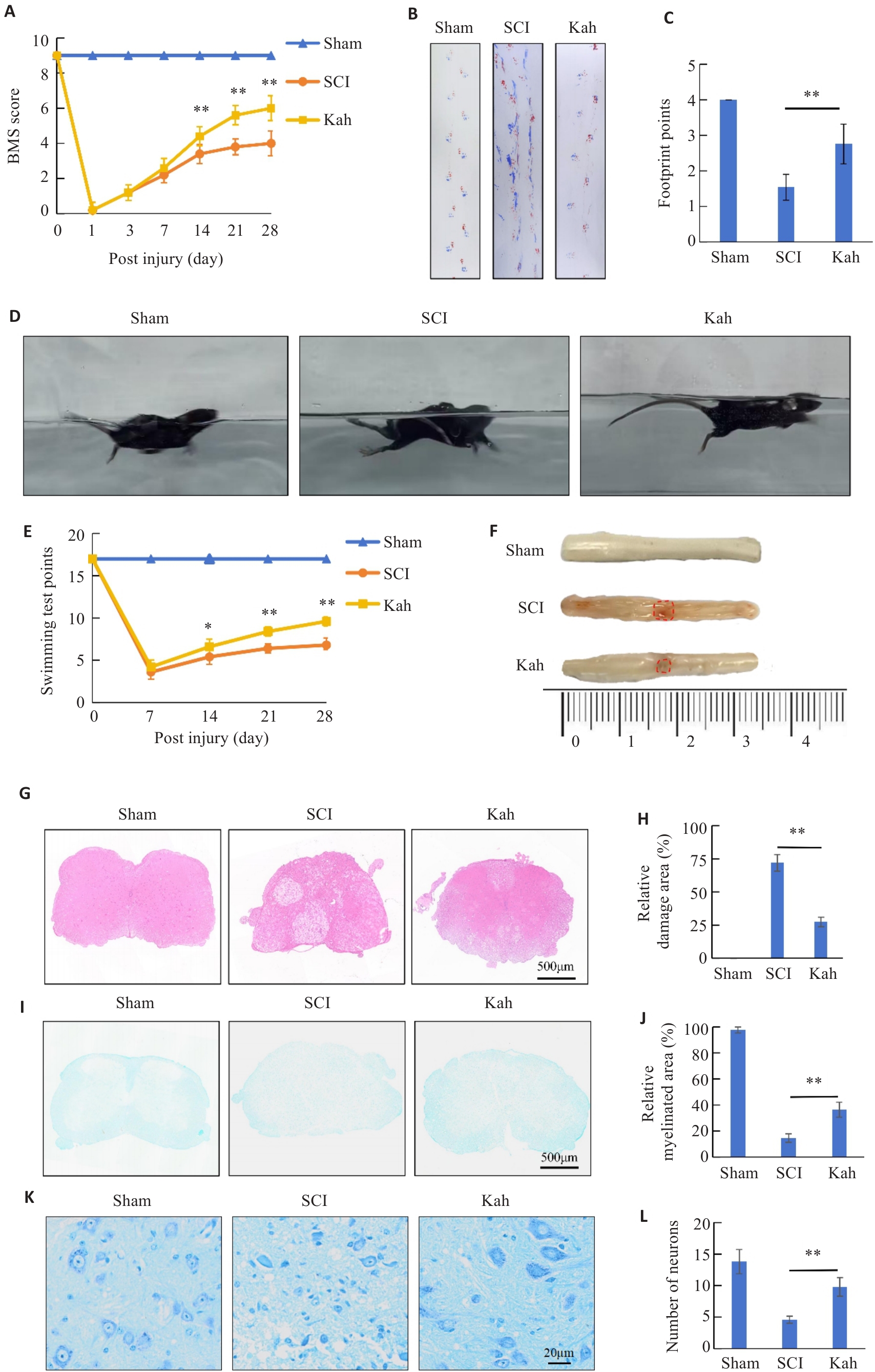
图1 Kah可改善SCI小鼠的运动功能及SCI后脊髓组织的病理损伤
Fig.1 Kahweol (Kah) improves motor function and alleviates spinal cord tissue pathologies of SCI mice. A: BMS Score. B, C: Footprint score. D, E: Swimming experiments. F: Dorsal view of fresh spinal cords on days 7. G: HE staining. H: Quantitative analysis of the lesion areas in the 3 groups. I: LFB staining. J: Quantitative analysis of residual myelination in the 3 groups. K: Nissl staining. L: Quantitative analysis of the number of motor neurons in the 3 groups. n=5 in each group. *P<0.05, **P<0.01.
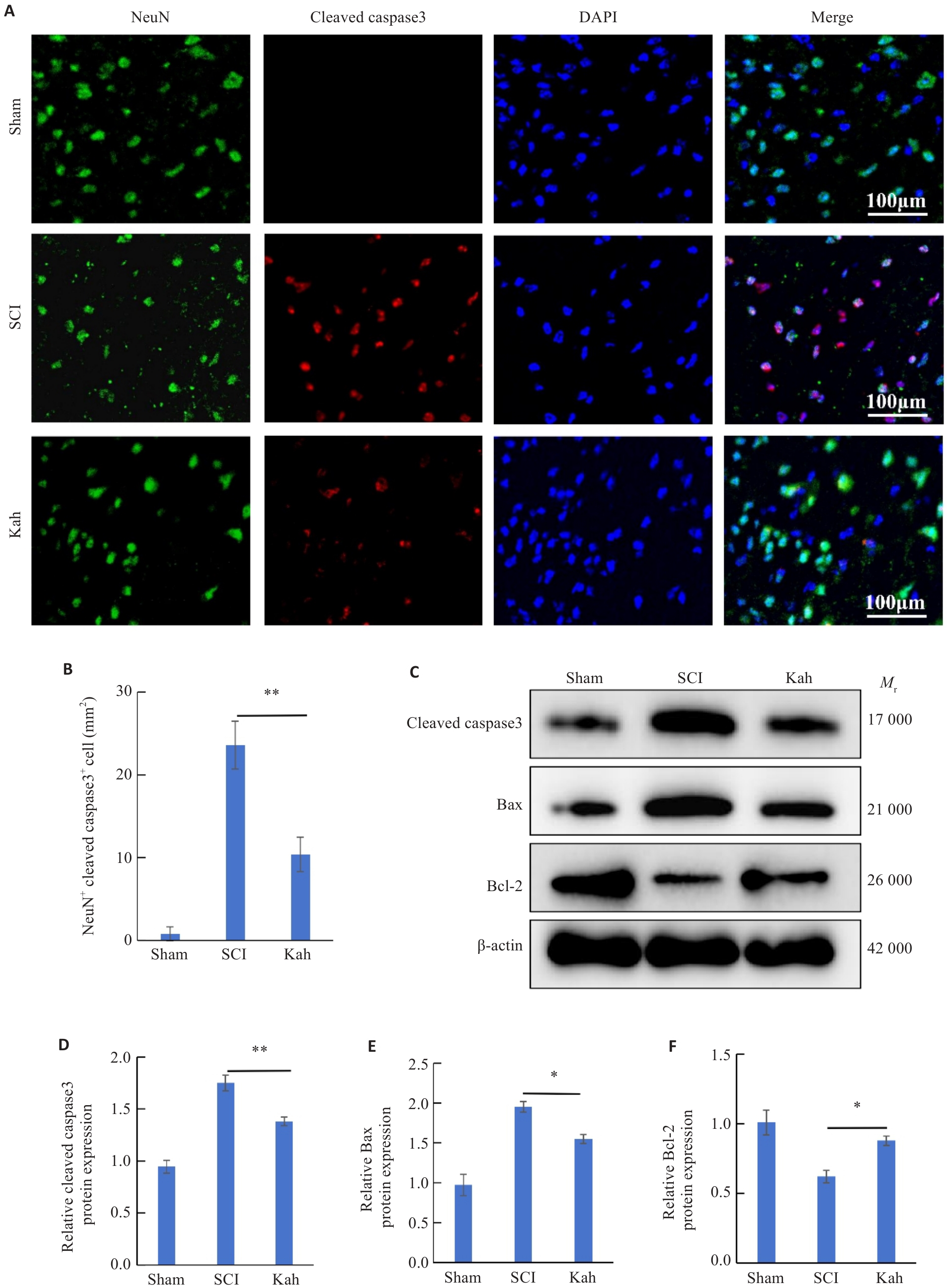
图2 Kah可减轻脊髓损伤后神经元的凋亡
Fig.2 Kah attenuates neuronal apoptosis in the spinal cord of SCI mice. A: Immunofluorescent staining for NeuN and cleaved caspase3. B: Quantification analysis of the number of NeuN+ cleaved caspase3+ cells. C-F: Western blotting and quantitative analysis of protein expression levels. n=3 in each group. *P<0.05, **P<0.01.
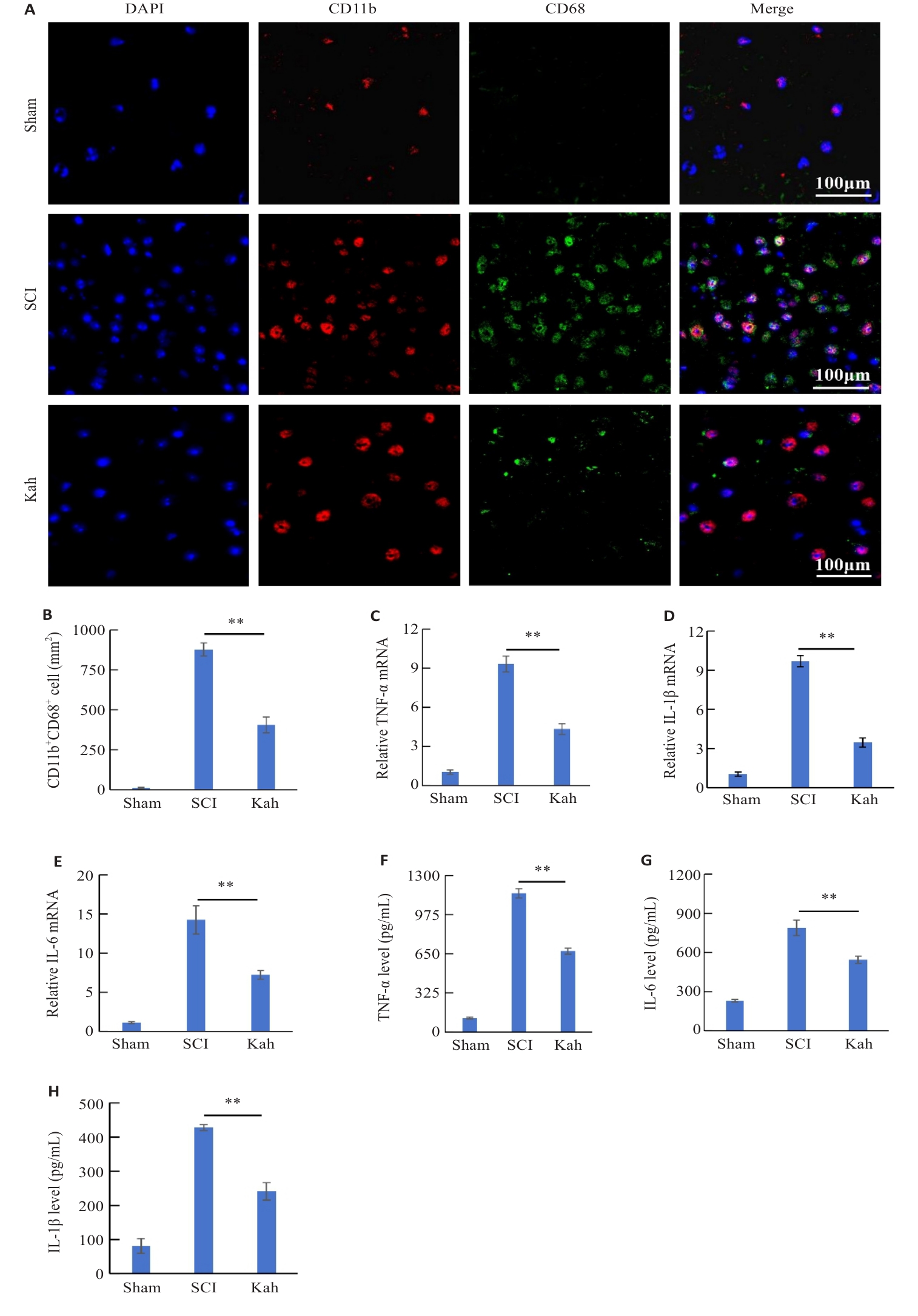
图3 Kah减轻了脊髓损伤后炎症相关因子的表达并抑制小胶质细胞的活化
Fig. 3 Kah reduces the expressions of inflammation-related factors and inhibits microglial activation in the spinal cord of SCI mice. A: Immunofluorescent staining for CD11b and CD68. B: Quantification analysis of the number of the CD11b+ CD68+ cells. C-E: Effect of Kah on mRNA levels of TNF‑α, IL-6 and IL-1β. F-H: Quantification of protein levels of TNF-α, IL-6 and IL-1β. n=3 in each group. **P<0.01.
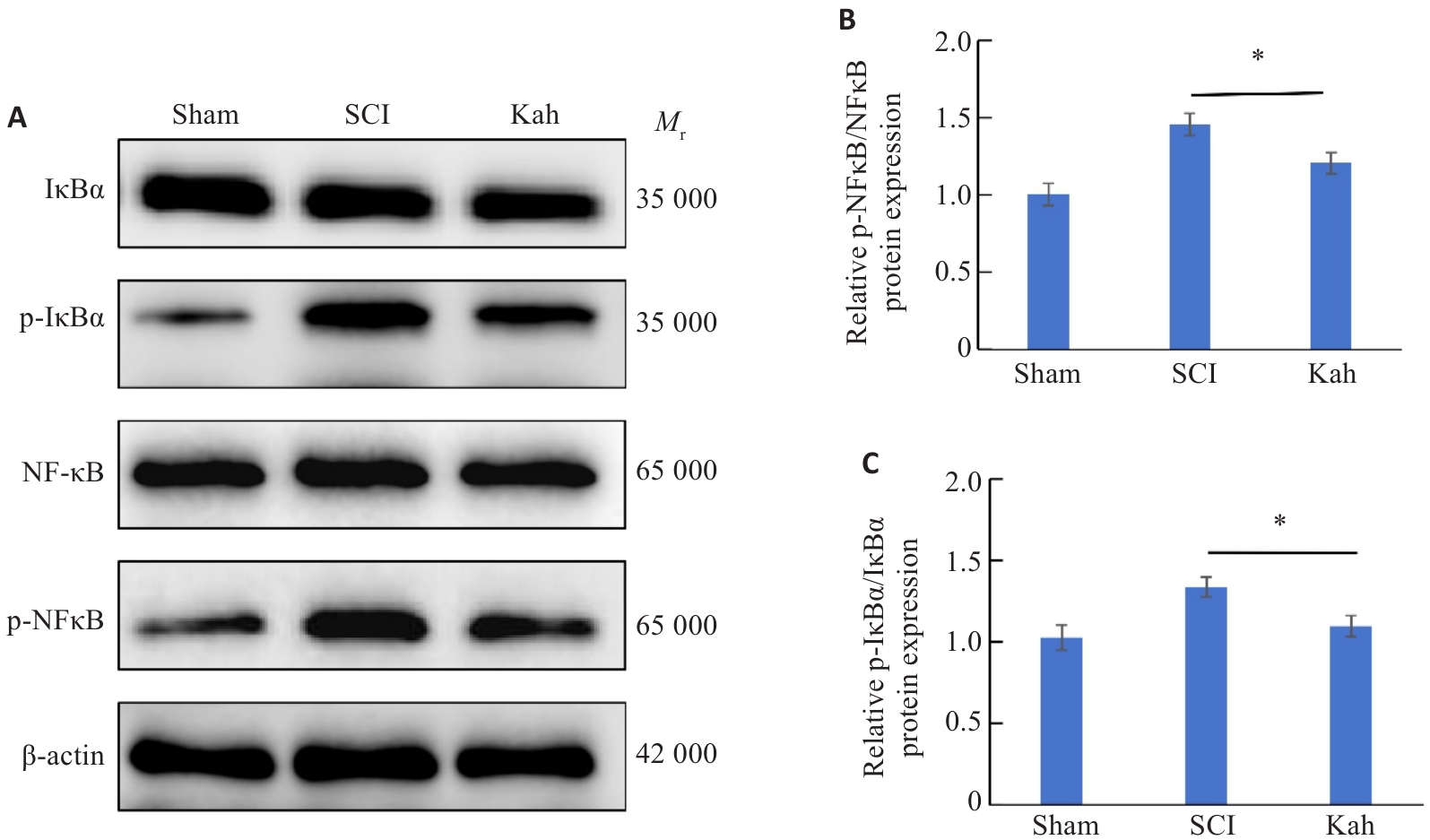
图4 Kah对SCI的保护性作用可能与NF-κB信号通路有关
Fig. 4 The protective effect of Kah in SCI mice may be related to the NF-κB signaling pathway. A-C: Proteins expressions of NF-κB, p-NF-κB, IκBα and p-IκBα in the spinal cord of SCI mice detected by Western blotting and quantitative analysis of protein levels. n=3 in each group. *P<0.05.
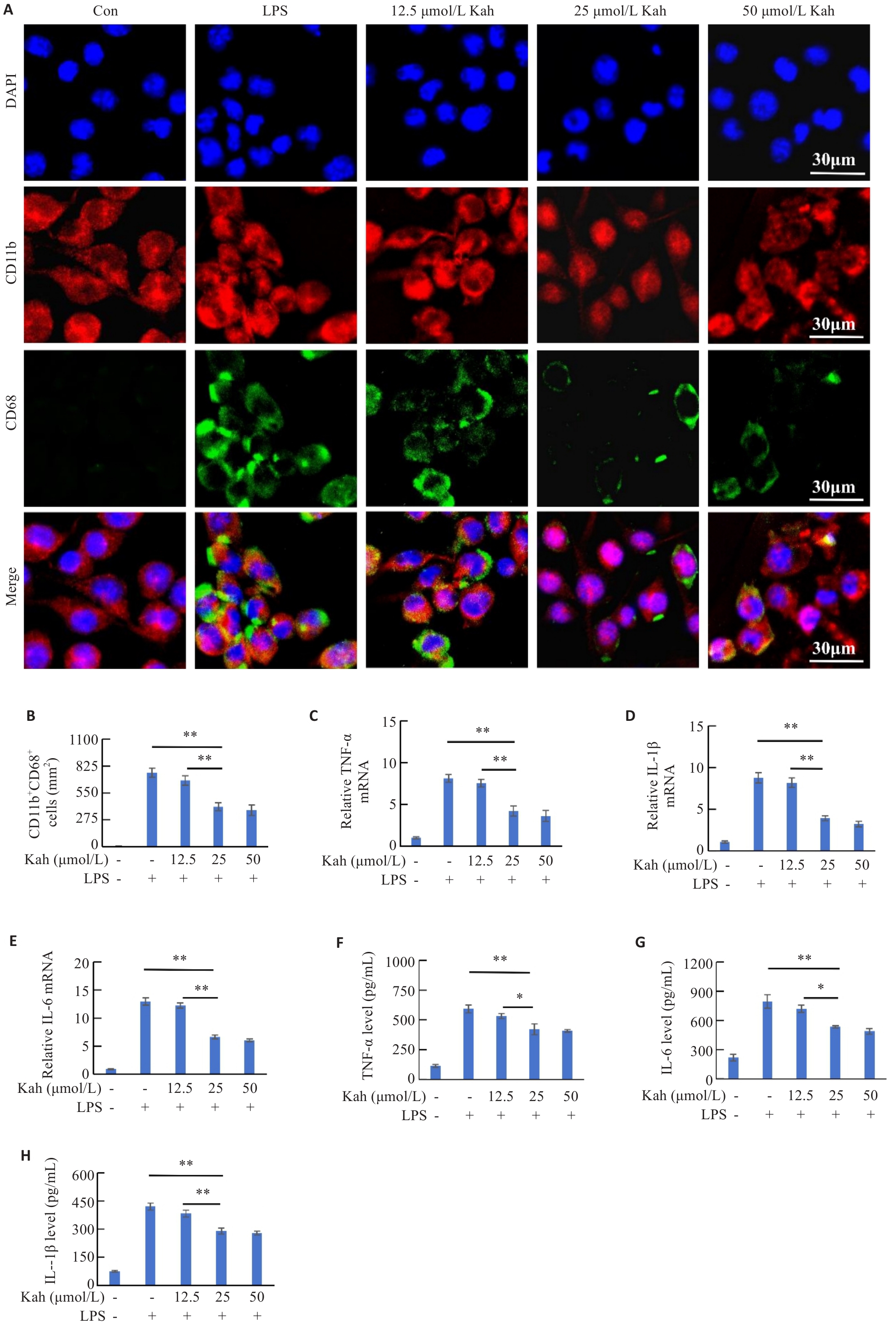
图5 Kah抑制LPS诱导的BV2细胞活化和炎症因子的表达
Fig.5 Kah inhibits LPS-induced microglial activation and expressions of inflammatory cytokines. A: Immunofluorescent staining for CD11b and CD68. B: Quantification analysis of the number of the CD11b+ CD68+ cells. C-E: Effect of Kah on mRNA levels of TNF‑α, IL-6 and IL-1β. F-H: Quantification of protein levels of TNF-α, IL-6 and IL-1β. n=3 in each group. *P<0.05, **P<0.001.
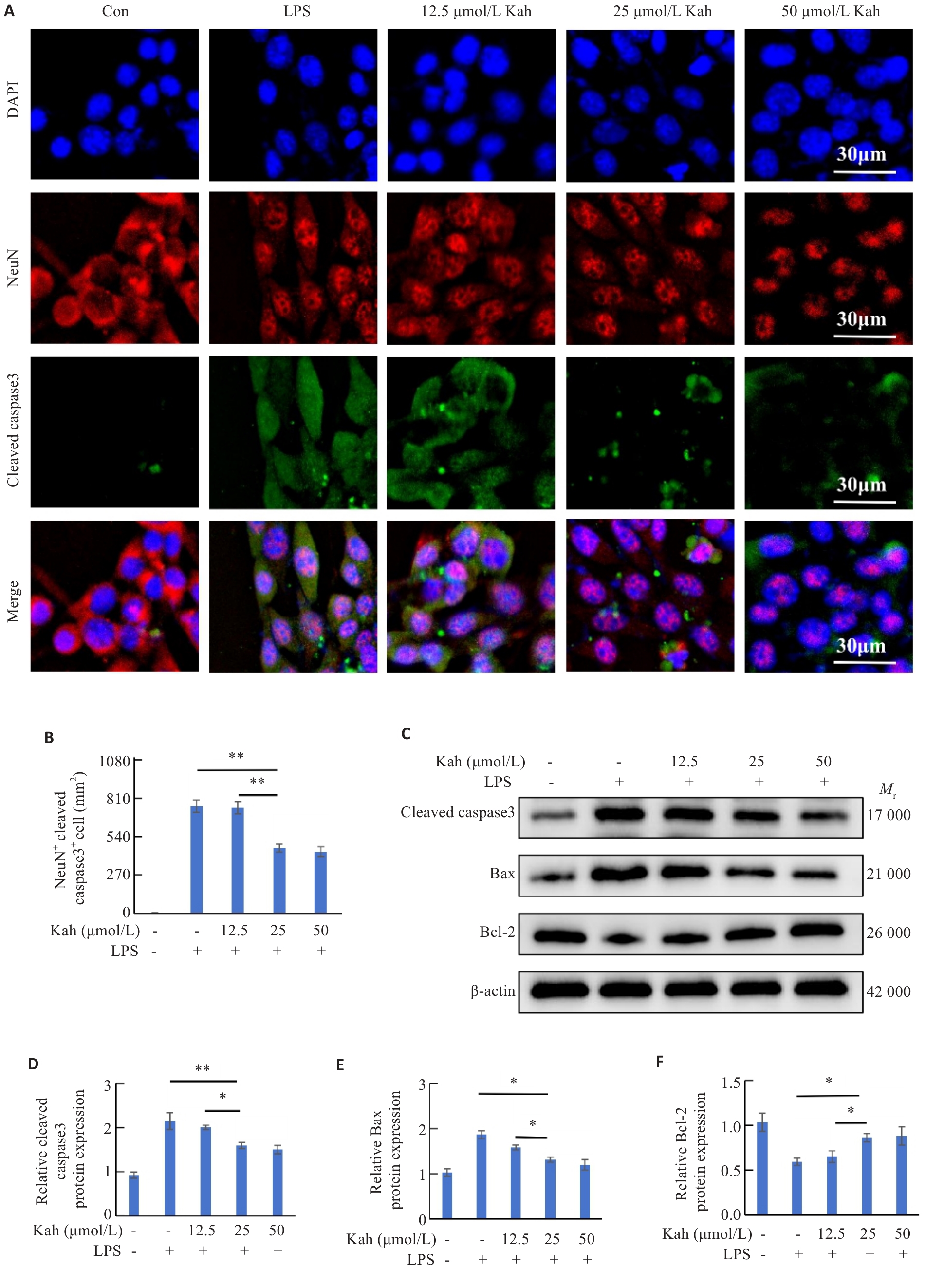
图6 Kah减轻BV2细胞活化引起的神经元凋亡
Fig.6 Kah attenuates neuronal apoptosis caused by microglial activation. A: Immunofluorescent staining for NeuN and cleaved caspase-3. B: Quantification analysis of the number of the NeuN+/cleaved caspase-3+ cells. C-F: Western blotting analysis and quantitative analysis of protein expression levels. n=3 in each group. *P<0.05, **P<0.001.
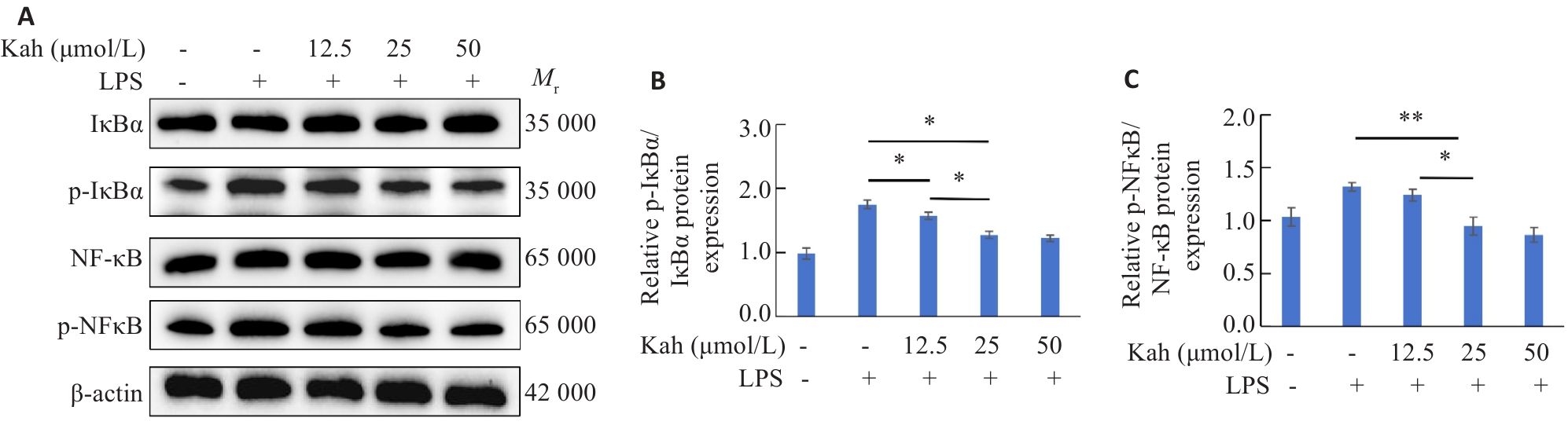
图7 Kah抑制NF-κB信号通路
Fig.7 Kah inhibits the NF-kB signaling pathway. A-C: Proteins expressions of NF-κB, p-NF-κB, IκBα and p-IκBα in BV2 cells detected by Western blotting and quantitative analysis of protein levels. n=3 in each group. *P<0.05, **P<0.001.
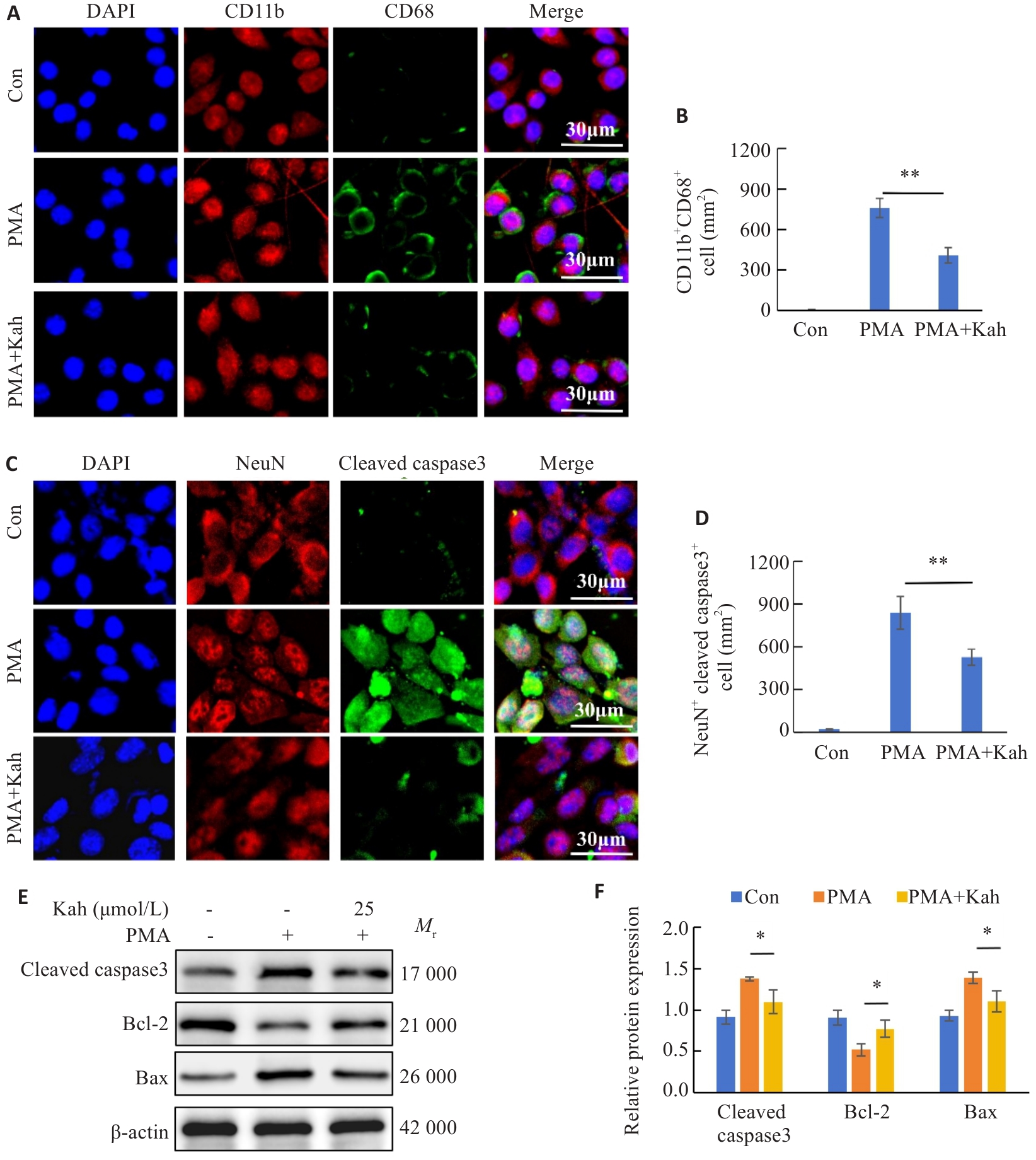
图8 Kah通过抑制NF-κB通路减轻BV2细胞活化及神经元凋亡
Fig.8 Kah attenuates microglial activation and neuronal apoptosis by inhibiting the NF-κB pathway. A: Immunofluorescent staining for CD11b and CD68. B: Quantification analysis of the number of CD11b+CD68+ cells. C: Immunofluorescent staining for NeuN and cleaved caspase3. D: Quantification analysis of the number of the NeuN+ cleaved caspase3+ cells. E, F: Western blotting and quantitative analysis of protein expression levels. n=3 in each group. *P<0.05, **P<0.001.
| [1] | Martynyuk T, Ricard J, Bracchi-Ricard V, et al. Mitigating sTNF/TNFR1 activation on VGluT2+spinal cord interneurons improves immune function after mid-thoracic spinal cord injury[J]. Brain Behav Immun, 2025, 123: 633-43. doi:10.1016/j.bbi.2024.10.021 |
| [2] | Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms[J]. Int J Mol Sci, 2020, 21(20): E7533. doi:10.3390/ijms21207533 |
| [3] | Ma H, Wang C, Han L, et al. Tofacitinib promotes functional recovery after spinal cord injury by regulating microglial polarization via JAK/STAT signaling pathway[J]. Int J Biol Sci, 2023, 19(15): 4865-82. doi:10.7150/ijbs.84564 |
| [4] | Kunnumakkara AB, Shabnam B, Girisa S, et al. Inflammation, NF-κB, and chronic diseases: how are they linked?[J]. Crit Rev Immunol, 2020, 40(1): 1-39. doi:10.1615/critrevimmunol.2020033210 |
| [5] | Zhao HS, Mei XF, Yang DF, et al. Resveratrol inhibits inflammation after spinal cord injury via SIRT-1/NF-κB signaling pathway[J]. Neurosci Lett, 2021, 762: 136151. doi:10.1016/j.neulet.2021.136151 |
| [6] | Song C, Zhang K, Luo C, et al. Inhibiting the NF-κB/DRP1 axis affords neuroprotection after spinal cord injury via inhibiting polarization of pro-inflammatory microglia[J]. Front Biosci: Landmark Ed, 2024, 29(8): 307. doi:10.31083/j.fbl2908307 |
| [7] | Fei M, Li Z, Cao YW, et al. microRNA-182 improves spinal cord injury in mice by modulating apoptosis and the inflammatory response via IKKβ/NF-κB[J]. Lab Investig, 2021, 101(9): 1238-53. doi:10.1038/s41374-021-00606-5 |
| [8] | Wang H, Lin F, Wu Y, et al. Carrier-free nanodrug based on co-assembly of methylprednisolone dimer and rutin for combined treatment of spinal cord injury[J]. ACS Nano, 2023, 17(13): 12176-87. doi:10.1021/acsnano.3c00360 |
| [9] | Baroudi M, Rezk A, Daher M, et al. Management of traumatic spinal cord injury: a current concepts review of contemporary and future treatment[J]. Injury, 2024, 55(6): 111472. doi:10.1016/j.injury.2024.111472 |
| [10] | Socała K, Szopa A, Serefko A, et al. Neuroprotective effects of coffee bioactive compounds: a review[J]. Int J Mol Sci, 2020, 22(1): E107. doi:10.3390/ijms22010107 |
| [11] | Eldesouki S, Qadri R, Abu Helwa R, et al. Recent updates on the functional impact of kahweol and cafestol on cancer[J]. Molecules, 2022, 27(21): 7332. doi:10.3390/molecules27217332 |
| [12] | Seo HY, Kim MK, Lee SH, et al. Kahweol ameliorates the liver inflammation through the inhibition of NF-κB and STAT3 activation in primary kupffer cells and primary hepatocytes[J]. Nutrients, 2018, 10(7): E863. doi:10.3390/nu10070863 |
| [13] | Lee HF, Lin JS, Chang CF. Acute kahweol treatment attenuates traumatic brain injury neuroinflammation and functional deficits[J]. Nutrients, 2019, 11(10): E2301. doi:10.3390/nu11102301 |
| [14] | Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress[J]. FEBS Lett, 2008, 582(17): 2655-62. doi:10.1016/j.febslet.2008.06.045 |
| [15] | Xue MT, Sheng WJ, Song X, et al. Atractylenolide III ameliorates spinal cord injury in rats by modulating microglial/macrophage polarization[J]. CNS Neurosci Ther, 2022, 28(7): 1059-71. doi:10.1111/cns.13839 |
| [16] | Duan FX, Shi YJ, Chen J, et al. Neuroprotective effects of P7C3 against spinal cord injury in rats[J]. Exp Biol Med: Maywood, 2019, 244(18): 1680-7. doi:10.1177/1535370219888620 |
| [17] | Kim JY, Leem J, Kim GM. Kahweol protects against acetaminophen-induced hepatotoxicity in mice through inhibiting oxidative stress, hepatocyte death, and inflammation[J]. Biomed Res Int, 2022, 2022: 8121124. doi:10.1155/2022/8121124 |
| [18] | Kim JY, Jo J, Leem J, et al. Kahweol ameliorates cisplatin-induced acute kidney injury through pleiotropic effects in mice[J]. Biomedicines, 2020, 8(12): E572. doi:10.3390/biomedicines8120572 |
| [19] | Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains[J]. J Neurotrauma, 2006, 23(5): 635-59. doi:10.1089/neu.2006.23.635 |
| [20] | Xu YB, Geng ZJ, Sun Y, et al. Complanatuside A improves functional recovery after spinal cord injury through inhibiting JNK signaling-mediated microglial activation[J]. Eur J Pharmacol, 2024, 965: 176287. doi:10.1016/j.ejphar.2023.176287 |
| [21] | Liu S, Wu Q, Wang LY, et al. Coordination function index: a novel indicator for assessing hindlimb locomotor recovery in spinal cord injury rats based on catwalk gait parameters[J]. Behav Brain Res, 2024, 459: 114765. doi:10.1016/j.bbr.2023.114765 |
| [22] | Jiang D, Gong F, Ge X, et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes[J]. J Nanobiotechnology, 2020, 18(1): 105. doi:10.1186/s12951-020-00665-8 |
| [23] | Lei P, Li Z, Hua Q, et al. Ursolic acid alleviates neuroinflammation after intracerebral hemorrhage by mediating microglial pyroptosis via the NF-κB/NLRP3/GSDMD pathway[J]. Int J Mol Sci, 2023, 24(19): 14771. doi:10.3390/ijms241914771 |
| [24] | Zhang Y, Jia J. Betaine mitigates amyloid‑β‑associated neuroinfl-ammation by suppressing the NLRP3 and NF‑κB signaling pathways in microglial cells[J]. J Alzheimers Dis, 2023, 94(s1): S9-19. doi:10.3233/jad-230064 |
| [25] | Shen T, Park YC, Kim SH, et al. Nuclear factor-kappaB/signal transducers and activators of transcription-1-mediated inflammatory responses in lipopolysaccharide-activated macrophages are a major inhibitory target of kahweol, a coffee diterpene[J]. Biol Pharm Bull, 2010, 33(7): 1159-64. doi:10.1248/bpb.33.1159 |
| [26] | Li G, Fu T, Wang W, et al. Pretreatment with kahweol attenuates sepsis-induced acute lung injury via improving mitochondrial homeostasis in a CaMKKII/AMPK-dependent pathway[J]. Mol Nutr Food Res, 2023, 67(19): e2300083. doi:10.1002/mnfr.202300083 |
| [27] | Liu H, Zhang J, Xu X, et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling[J]. Theranostics, 2021, 11(9): 4187-206. doi:10.7150/thno.49054 |
| [28] | Ryu Y, Ogata T, Nagao M, et al. The swimming test is effective for evaluating spasticity after contusive spinal cord injury[J]. PLoS One, 2017, 12(2): e0171937. doi:10.1371/journal.pone.0171937 |
| [29] | Metz GAS, Merkler D, Dietz V, et al. Efficient testing of motor function in spinal cord injured rats[J]. Brain Res, 2000, 883(2): 165-77. doi:10.1016/s0006-8993(00)02778-5 |
| [30] | Davidson LT, Evans MC. Congenital and acquired spinal cord injury and dysfunction[J]. Pediatr Clin North Am, 2023, 70(3): 461-81. doi:10.1016/j.pcl.2023.01.017 |
| [31] | de Oliveira MR, de Souza ICC, Fürstenau CR. Mitochondrial protection promoted by the coffee diterpene kahweol in methylglyoxal-treated human neuroblastoma SH-SY5Y cells[J]. Neurotox Res, 2020, 37(1): 100-10. doi:10.1007/s12640-019-00107-w |
| [32] | Meng TY, Zhang YF, Huang J, et al. Rubusoside mitigates neuroinflammation and cellular apoptosis in Parkinson's disease, and alters gut microbiota and metabolite composition[J]. Phytomedicine, 2024, 124: 155309. doi:10.1016/j.phymed.2023.155309 |
| [33] | Mitchell JP, Carmody RJ. Chapter two NF-κB and the transcriptional control of inflammation[J]. Int Rev Cell Mol Biol, 2018, 335: 41-84. doi:10.1016/bs.ircmb.2017.07.007 |
| [34] | Wei J, Li T, Lin S, et al. Dihydrotestosterone reduces neuroinflammation in spinal cord injury through NF-κB and MAPK pathway[J]. Cell Mol Biol: Noisy-le-grand, 2024, 70(1): 213-8. doi:10.14715/cmb/2024.70.1.29 |
| [1] | 张淑芬, 黄添容, 杨灿洪, 陈家镒, 吕田明, 张嘉发. 莱菔硫烷通过抑制Aβ42寡聚体激活的U87细胞中MAPK/NF-κB信号通路降低反应性星形胶质细胞介导的SH-SY5Y凋亡[J]. 南方医科大学学报, 2026, 46(1): 191-199. |
| [2] | 汤忠富, 黄传兵, 李明, 程丽丽, 陈君洁, 尚双双, 刘思娣. 芪黄健脾滋肾颗粒通过抑制MyD88/NF-κB通路减轻MRL/lpr小鼠肾损害[J]. 南方医科大学学报, 2025, 45(8): 1625-1632. |
| [3] | 范正媛, 沈子涵, 李亚, 沈婷婷, 李高峰, 李素云. 补肺益肾方对香烟烟雾提取物诱导的人支气管上皮细胞损伤的保护作用及其机制[J]. 南方医科大学学报, 2025, 45(7): 1372-1379. |
| [4] | 夏冰, 彭进, 丁九阳, 王杰, 唐国伟, 刘国杰, 王沄, 万昌武, 乐翠云. ATF3通过NF-κB信号通路调控动脉粥样硬化斑块内的炎症反应[J]. 南方医科大学学报, 2025, 45(6): 1131-1142. |
| [5] | 杨毓甲, 杨丽芳, 吴雅玲, 段兆达, 于春泽, 吴春云, 于建云, 杨力. 大麻二酚经PERK-eIF2α-ATF4-CHOP通路减轻多重脑震荡大鼠的神经元内质网应激和凋亡[J]. 南方医科大学学报, 2025, 45(6): 1240-1250. |
| [6] | 何光侣, 储婉玉, 李妍, 盛鑫, 罗浩, 徐爱萍, 卞命杰, 张环环, 汪萌芽, 郑超. Orexin-A通过调节促离子型谷氨酸受体促进脊髓损伤大鼠运动功能恢复[J]. 南方医科大学学报, 2025, 45(5): 1023-1030. |
| [7] | 梁晓涛, 熊一凡, 刘雪琪, 梁小珊, 朱晓煜, 谢炜. 活血疏风颗粒通过抑制TLR4/NF-κB通路改善慢性偏头痛小鼠的中枢敏化[J]. 南方医科大学学报, 2025, 45(5): 986-994. |
| [8] | 陈悦, 肖林雨, 任侣, 宋雪, 李静, 胡建国. 水晶兰苷通过抑制PI3K/AKT信号通路减少神经元凋亡改善脊髓损伤后小鼠的运动功能[J]. 南方医科大学学报, 2025, 45(4): 774-784. |
| [9] | 张毅, 沈昱, 万志强, 陶嵩, 柳亚魁, 王栓虎. CDKN3高表达促进胃癌细胞的迁移和侵袭:基于调控p53/NF-κB信号通路和抑制胃癌细胞凋亡[J]. 南方医科大学学报, 2025, 45(4): 853-861. |
| [10] | 麦泳欣, 周舒婷, 温蕊嘉, 张锦芳, 詹冬香. 桃叶珊瑚苷通过抑制NF-κb信号通路减轻小鼠的膝骨关节炎[J]. 南方医科大学学报, 2025, 45(10): 2104-2110. |
| [11] | 孙一鸣, 徐昕冉, 卓雪瑞, 蔡慧, 王艳. C1q中和抗体可通过C1q/C3通路改善小鼠的产后抑郁样行为[J]. 南方医科大学学报, 2025, 45(10): 2111-2117. |
| [12] | 钟娜, 王会杰, 赵文英, 孙珍贵, 耿彪. 高表达RNF7增强非小细胞肺癌细胞的PD-1耐药:基于活化NF-kB通路促进CXCL1表达和髓源性抑制细胞的募集[J]. 南方医科大学学报, 2024, 44(9): 1704-1711. |
| [13] | 左涵珺, 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟. 天麻素经PI3K/AKT通路改善新生大鼠缺氧缺血性脑损伤后小胶质细胞介导的炎症反应[J]. 南方医科大学学报, 2024, 44(9): 1712-1719. |
| [14] | 姜一凡, 李小荣, 耿嘉逸, 陈永锋, 唐碧, 康品方. 槲皮素通过抑制HMGB1/RAGE/NF-κB信号通路减轻糖尿病引起的大鼠肾脏损伤[J]. 南方医科大学学报, 2024, 44(9): 1769-1775. |
| [15] | 赵娜, 沈梦迪, 赵睿, 奥迪, 骆泽谭, 张银亮, 徐志东, 范方田, 郑海伦. 血根碱通过调控Nrf2/NF-κB通路缓解小鼠溃疡性结肠炎[J]. 南方医科大学学报, 2024, 44(8): 1467-1475. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||