南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (12): 2551-2560.doi: 10.12122/j.issn.1673-4254.2025.12.03
• • 上一篇
张芡1( ), 刘博文1, 雷丽1, 王晔1, 张馨月1, 毛樟坤1, 唐鹏2, 张金梅2, 杨佳宜1, 彭彦茜3, 刘泽4(
), 刘博文1, 雷丽1, 王晔1, 张馨月1, 毛樟坤1, 唐鹏2, 张金梅2, 杨佳宜1, 彭彦茜3, 刘泽4( )
)
收稿日期:2025-05-01
出版日期:2025-12-20
发布日期:2025-12-22
通讯作者:
刘泽
E-mail:zhangqian@xnu.edu.cn;582842343@qq.com
作者简介:张 芡,硕士,副教授,E-mail: zhangqian@xnu.edu.cn
基金资助:
Qian ZHANG1( ), Bowen LIU1, Li LEI1, Ye WANG1, Xinyue ZHANG1, Zhangkun MAO1, Peng TANG2, Jinmei ZHANG2, Jiayi YANG1, Yanxi PENG3, Ze LIU4(
), Bowen LIU1, Li LEI1, Ye WANG1, Xinyue ZHANG1, Zhangkun MAO1, Peng TANG2, Jinmei ZHANG2, Jiayi YANG1, Yanxi PENG3, Ze LIU4( )
)
Received:2025-05-01
Online:2025-12-20
Published:2025-12-22
Contact:
Ze LIU
E-mail:zhangqian@xnu.edu.cn;582842343@qq.com
摘要:
目的 探讨丝氨酸蛋白酶抑制剂E1(SERPINE1)对三阴性乳腺癌细胞肿瘤免疫微环境的影响及其与紫杉醇(PTX)耐药性的关系。 方法 采用0~40 µmol/L的PTX处理三阴性乳腺癌细胞系MDA-MB-231,并利用CCK-8法测定PTX对MDA-MB-231细胞的半数抑制浓度(IC50值)。通过低剂量逐步递增的方法,在体外建立PTX耐药模型(MDA-MB-231/PTX)。分别将SERPINE1过表达质粒或SERPINE1 siRNA转染至野生型和耐药型MDA-MB-231细胞株,以实现SERPINE1的过表达或敲低。采用Western blotting检测各组细胞中SERPINE1的表达水平,以评估转染效率。利用Hoechst 33258染色法评估细胞凋亡情况,并通过Western blotting测定凋亡相关的活化天冬氨酸特异性半胱氨酸蛋白酶(cleaved-caspase 3)的表达水平。采用EdU和CCK-8法检测细胞增殖活力。将各组乳腺癌细胞与巨噬细胞共培养后,利用流式细胞术和Western blotting检测巨噬细胞的M1、M2极化水平,并计算M1/M2值。进一步构建裸鼠皮下移植瘤模型,通过监测肿瘤生长及免疫组化染色验证其体内作用。 结果 SERPINE1的过表达抑制MDA-MB-231细胞的凋亡并促进细胞增殖(P<0.01),而敲低SERPINE1则促进MDA-MB-231/PTX细胞的凋亡并抑制细胞增殖(P<0.01)。此外,SERPINE1高表达的乳腺癌细胞有助于巨噬细胞向M2型极化、抑制M1极化、降低M1/M2比值(P<0.01)。体内移植瘤实验同样证实,过表达SERPINE1促进肿瘤生长(P<0.01)。 结论 在MDA-MB-231三阴性乳腺癌细胞中,SERPINE1的过表达促进细胞增殖、抑制细胞凋亡,并增强对PTX的耐药性。SERPINE1参与调控乳腺癌微环境中巨噬细胞的极化状态,促进M2型巨噬细胞的极化。
张芡, 刘博文, 雷丽, 王晔, 张馨月, 毛樟坤, 唐鹏, 张金梅, 杨佳宜, 彭彦茜, 刘泽. 丝氨酸蛋白酶抑制剂E1过表达通过诱导M2型巨噬细胞极化促进三阴性乳腺癌细胞增殖与紫杉醇耐药[J]. 南方医科大学学报, 2025, 45(12): 2551-2560.
Qian ZHANG, Bowen LIU, Li LEI, Ye WANG, Xinyue ZHANG, Zhangkun MAO, Peng TANG, Jinmei ZHANG, Jiayi YANG, Yanxi PENG, Ze LIU. SERPINE1 overexpression promotes proliferation and paclitaxel resistance of triple-negative breast cancer cells by inducing M2 macrophage polarization[J]. Journal of Southern Medical University, 2025, 45(12): 2551-2560.
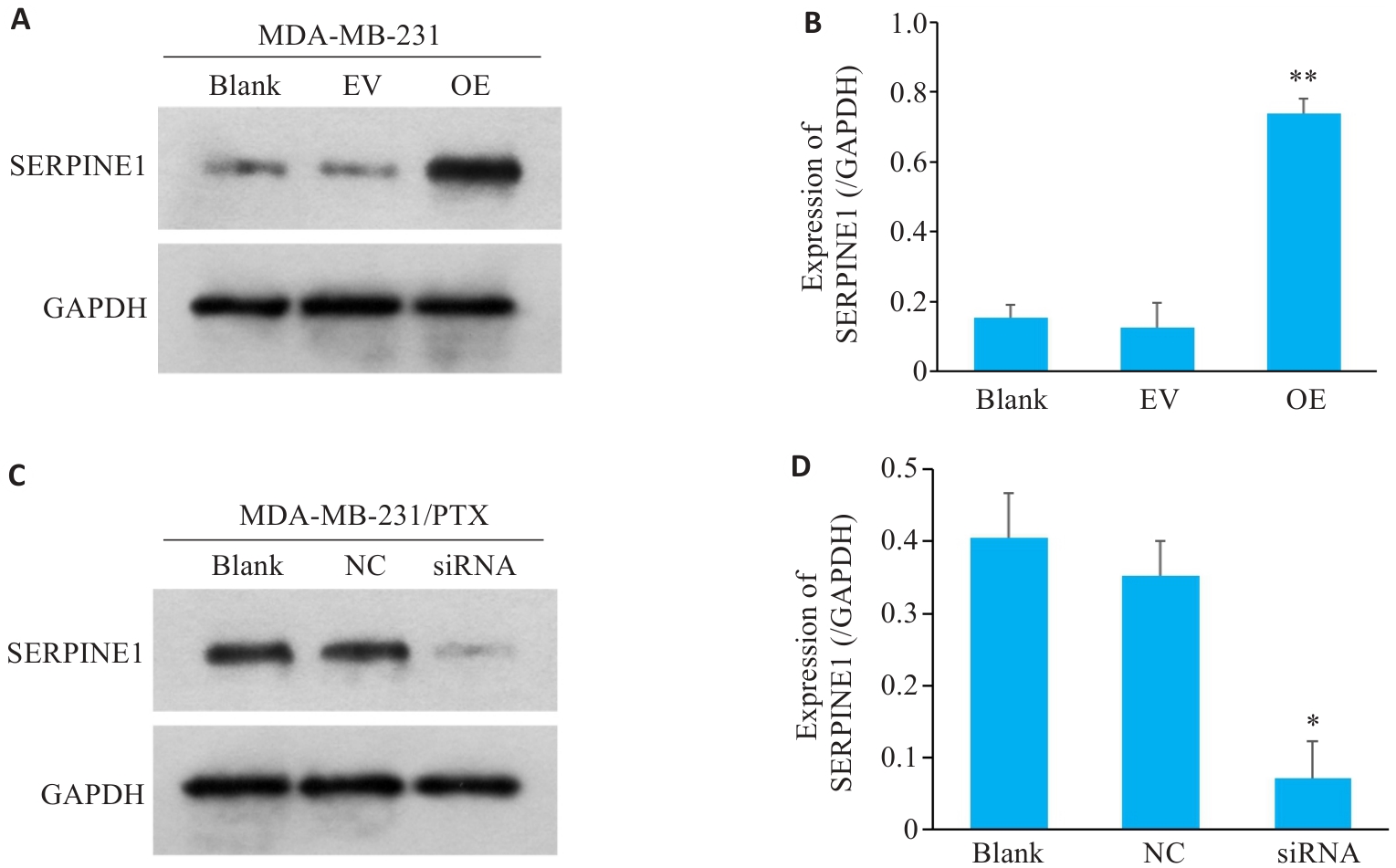
图1 MDA-MB-231细胞和MDA-MB-231/PTX细胞分别进行SERPINE1过表达和敲低干预
Fig.1 SERPINE1 overexpression in MDA-MB-231 cells and SERPINE1 knockdown in MDA-MB-231/PTX cells. A, B: Western blotting for detecting SERPINE1 protein expression in MDA-MB-231 cells transfected with pcDNA4.0-SERPINE1 and vector plasmid (**P<0.01 vs EV group, n=3); C, D: Western blotting for detecting SERPINE1 protein expression in MDA-MB-231/PTX cells transfected with SERPINE1-siRNA and NC-siRNA. GAPDH was used as the loading control (*P<0.05 vs NC group, n=3).
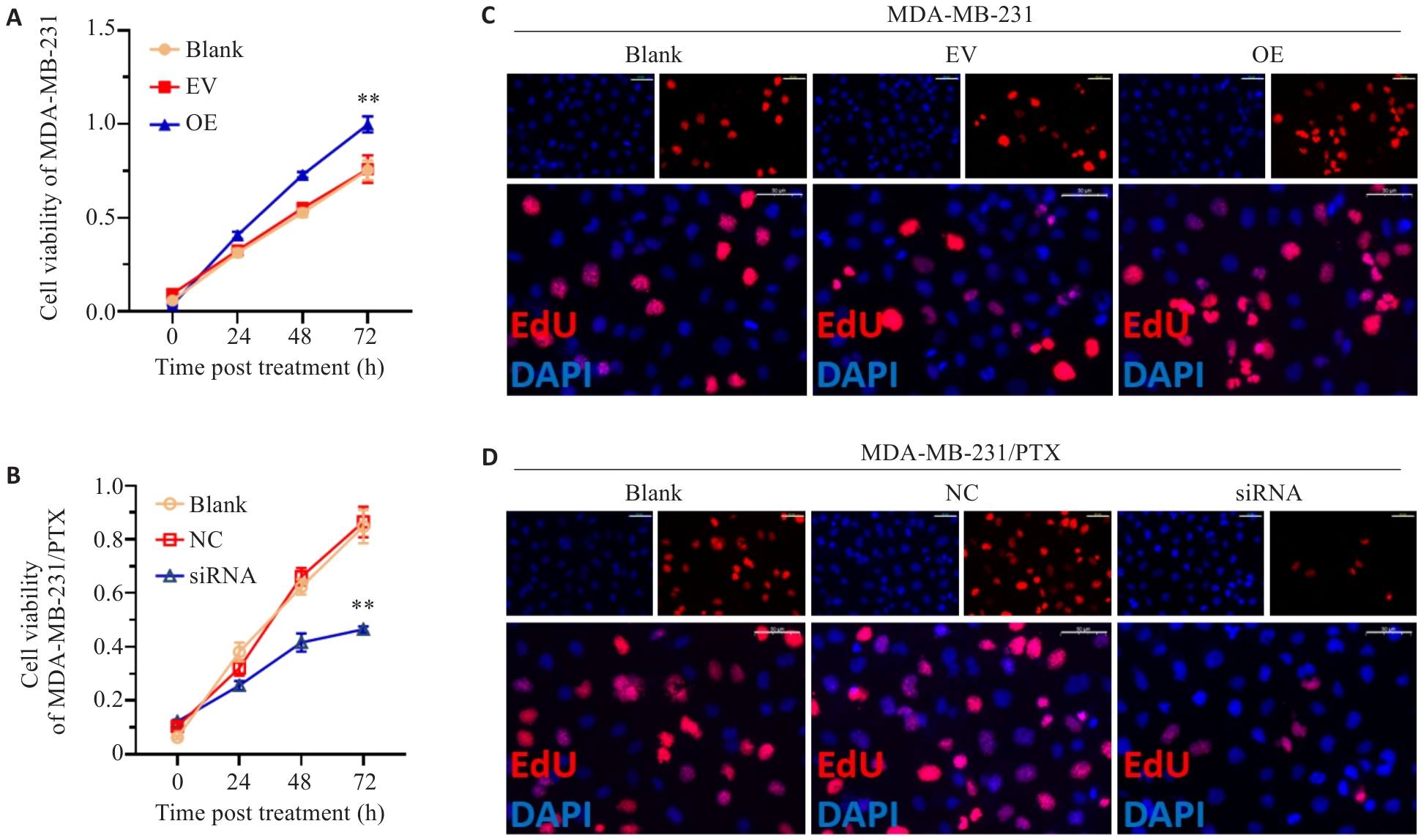
图2 SERPINE1对三阴性乳腺癌细胞增殖活力影响
Fig.2 Proliferative activity of MDA-MB-231 cells with SERPINE1 overexpression and MDA-MB-231/PTX cells with SERPINE1 knockdown. A, B: Results of CCK-8 assay of MDA-MB-231 cells (A) transfected with pcDNA4.0-SERPINE1 or the vector plasmid and MDA-MB-231/PTX cells (B) transfected with SERPINE1-siRNA or NC-siRNA (**P<0.01 vs EV group or NC group). C, D: EdU staining of MDA-MB-231 cells with SERPINE1 overexpression and MDA-MB-231/PTX cells with SERPINE1 knockdown (scale bar=50 μm).
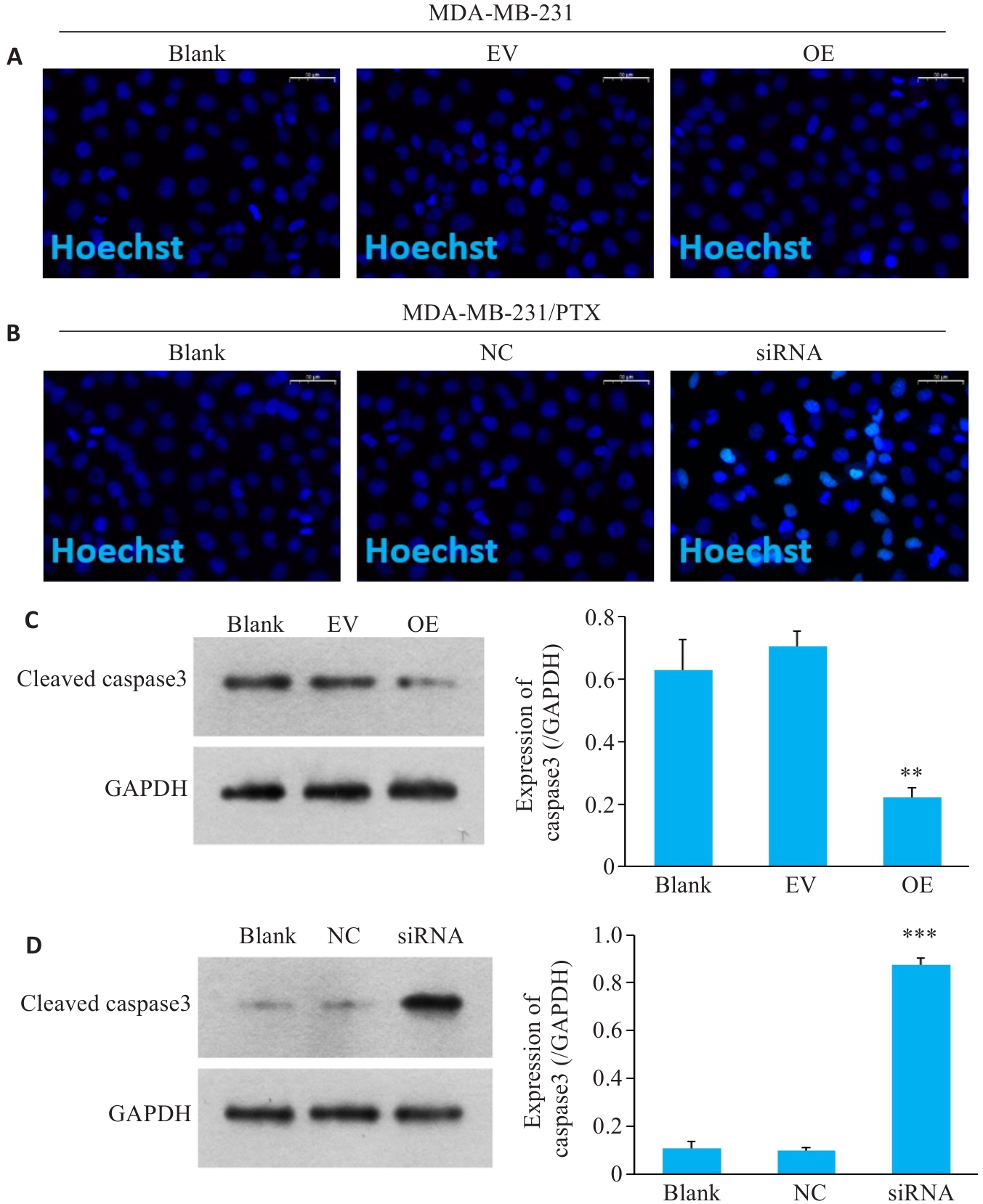
图3 SERPINE1对三阴性乳腺癌细胞凋亡影响
Fig.3 Changes in apoptosis of MDA-MB-231 cells with SERPINE1 overexpression and MDA-MB-231/PTX cells with SERPINE1 knockdown. A, B: DNA damage detected by Hoechst staining in MDA-MB-231 cells with SERPINE1 overexpression and in MDA-MB-231/PTX cells with SERPINE1 knockdown (scale bar=50 μm). C: Western blotting for detecting protein levels of cleaved caspase-3 in MDA-MB-231 cells transfected with pcDNA4.0-SERPINE1 versus the vector plasmid (**P<0.01 vs EV group). D: Western blotting for detecting protein levels of cleaved caspase-3 in MDA-MB-231/PTX cells transfected with SERPINE1-siRNA versus NC-siRNA (***P<0.001 vs NC group, n=3).
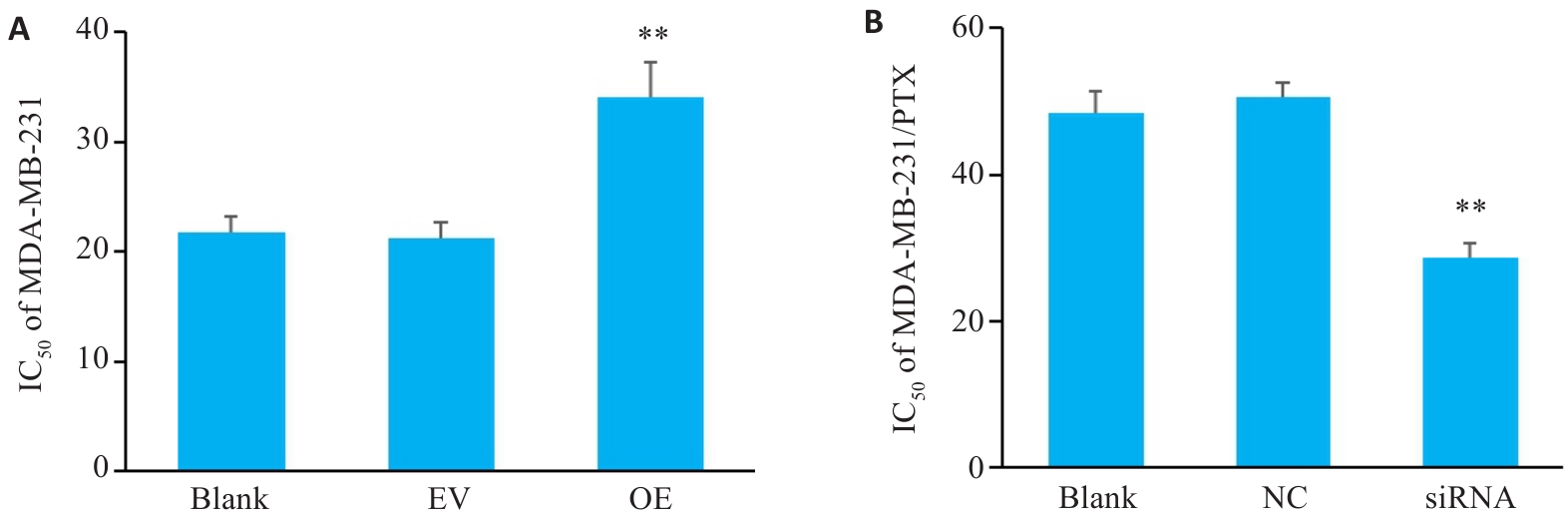
图4 SERPINE1对三阴性乳腺癌细胞PTX耐药性的影响
Fig.4 Changes in paclitaxel resistance of MDA-MB-231 cells with SERPINE1 overexpression and MDA-MB-231/PTX cells with SERPINE1 knockdown. A: CCK-8 assay for determining IC50 value of paclitaxel in MDA-MB-231 cells transfected with pcDNA4.0-SERPINE1 versus the vector plasmid (**P<0.01 vs EV group). B: CCK-8 assay for determining the IC50 value of paclitaxel in MDA-MB-231/PTX cells transfected with SERPINE1-siRNA versus NC-siRNA (**P<0.01 vs NC group, n=3).
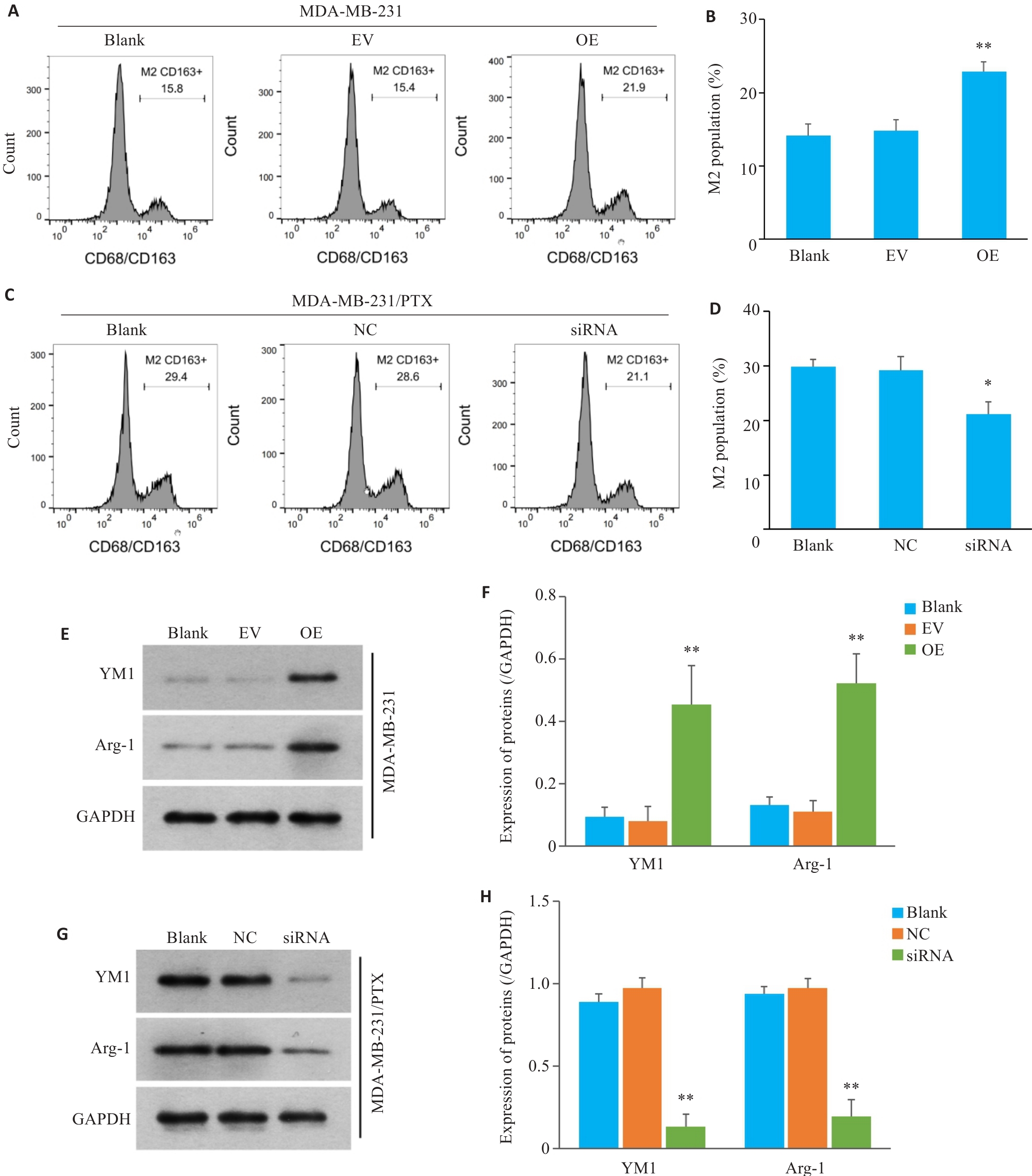
图5 三阴性乳腺癌细胞中SERPINE1对巨噬细胞M2极化影响
Fig.5 Effect of SERPINE1 overexpression in MDA-MB-231 cells and SERPINE1 knockdown in MDA-MB-231/PTX cells on M2 polarization of the co-cultured macrophages. A, B: Flow cytometry for analyzing the proportion of CD163+ macrophages co-cultured with SERPINE1-overexpressing MDA-MB-231 cells (**P<0.01 vs EV group). C, D: Flow cytometry for analyzing the proportion of CD163+ macrophages co-cultured with MDA-MB-231/PTX cells with SERPINE1 knockdown (*P<0.05 vs NC group). E, F: Western blotting for detecting the protein expression of YM1 and Arg-1 in the macrophages from the microenvironment of MDA-MB-231 cells with or without SERPINE1 overexpression (**P<0.01 vs EV group). G, H: Western blotting for detecting the protein expression of YM1 and Arg-1 in macrophages from the microenvironment of MDA-MB-231/PTX cells with or without SERPINE1 knockdown (**P<0.01 vs NC group, n=3).
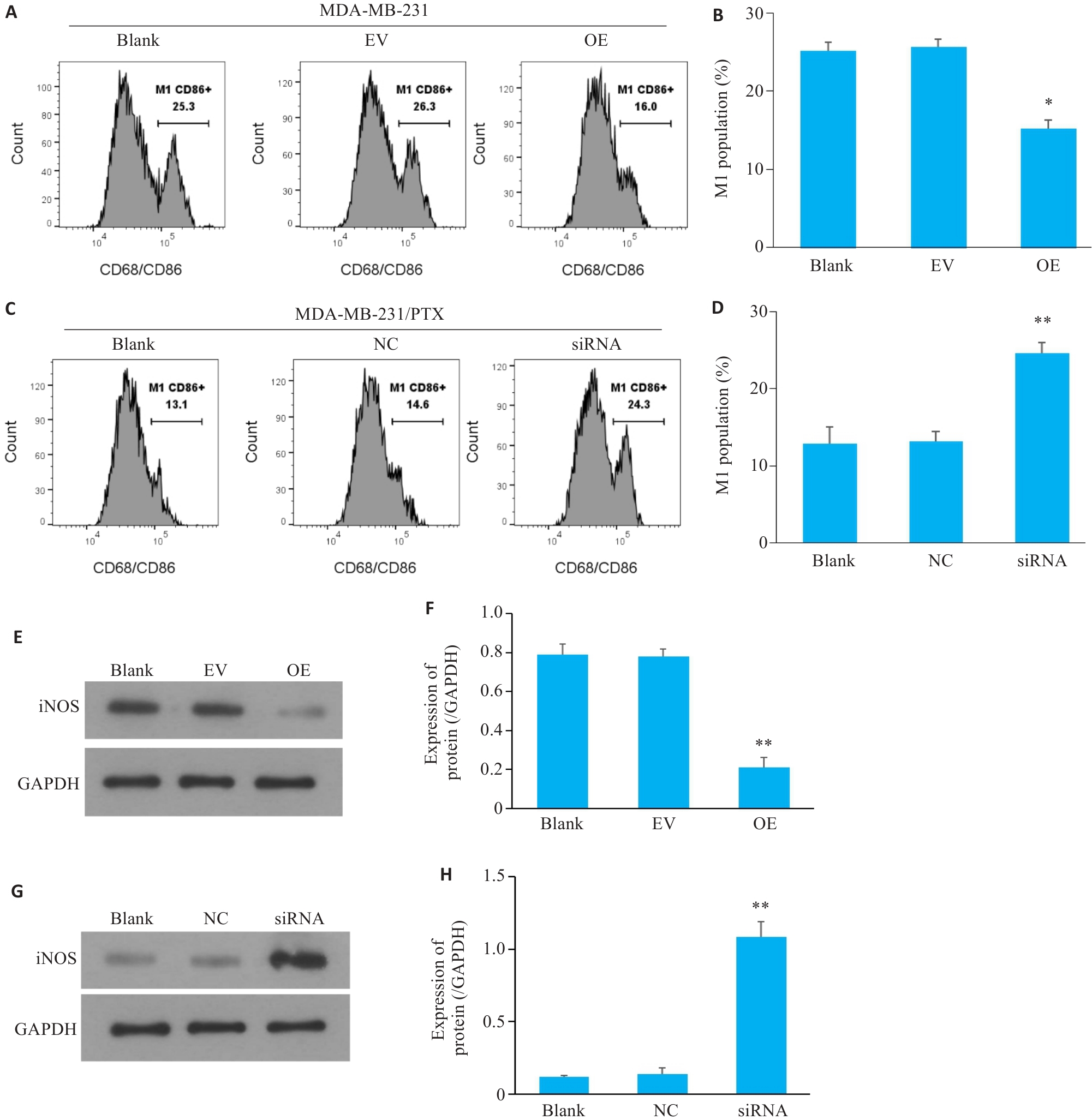
图6 三阴性乳腺癌细胞中SERPINE1对巨噬细胞M1极化影响
Fig.6 M1 polarization of the macrophages co-cultured with MDA-MB-231 cells with SERPINE1 overexpression or MDA-MB-231/PTX cells with SERPINE1 knockdown. A, B: Flow cytometry for analyzing the proportion of CD86+ macrophages co-cultured with MDA-MB-231 cells with SERPINE1 overexpression (*P<0.05 vs EV group). C, D: Flow cytometry for analyzing the proportion of CD86+ macrophages co-cultured with MDA-MB-231/PTX cells with SERPINE1 knockdown (**P<0.01 vs NC group). E, F: Western blotting for detecting the protein expression of iNOS in macrophages from the microenvironment of MDA-MB-231 cells with or without SERPINE1 overexpression (**P<0.01 vs EV group). G, H: Western blotting for detecting the protein expression of iNOS in macrophages from the microenvironment of MDA-MB-231/PTX cells with or without SERPINE1 knockdown (**P<0.01 vs NC group, n=3).
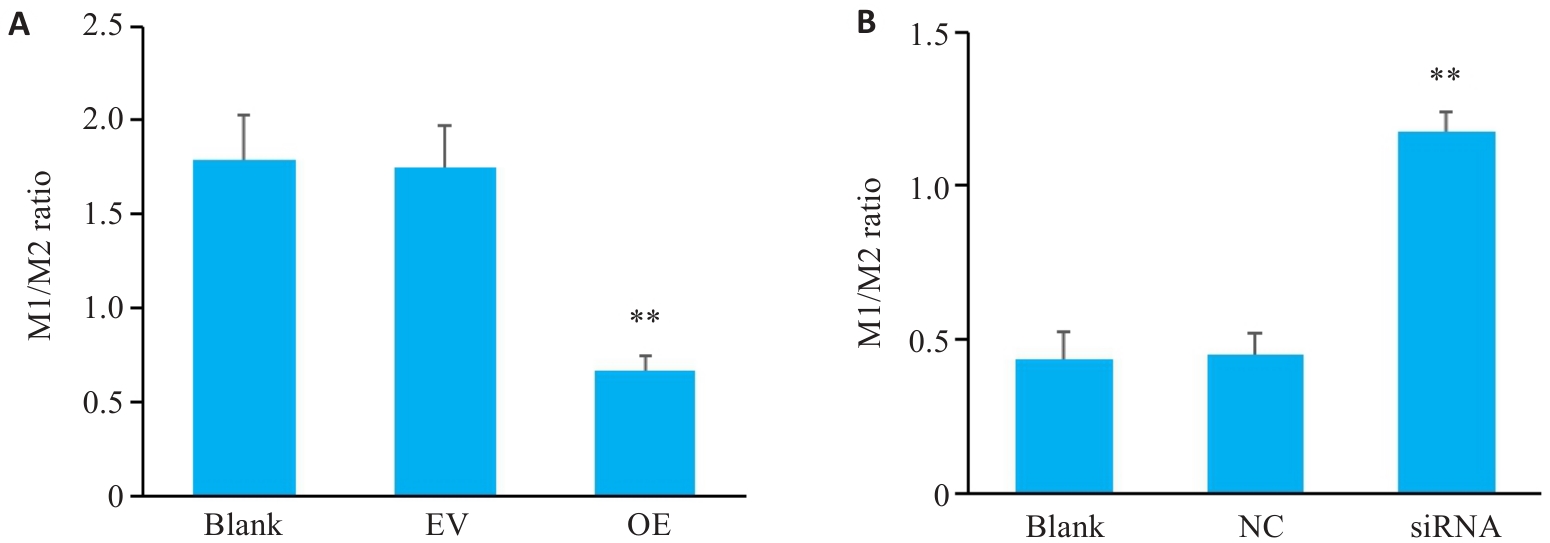
图7 三阴性乳腺癌细胞中SERPINE1对巨噬细胞M1/M2比值影响
Fig.7 M1/M2 ratio of the macrophages co-cultured with MDA-MB-231 cells with SERPINE1 overexpression (A; **P<0.01 vs EV group) and MDA-MB-231/PTX cells with SERPINE1 knockdown (B; **P<0.01 vs blank group, n=3).
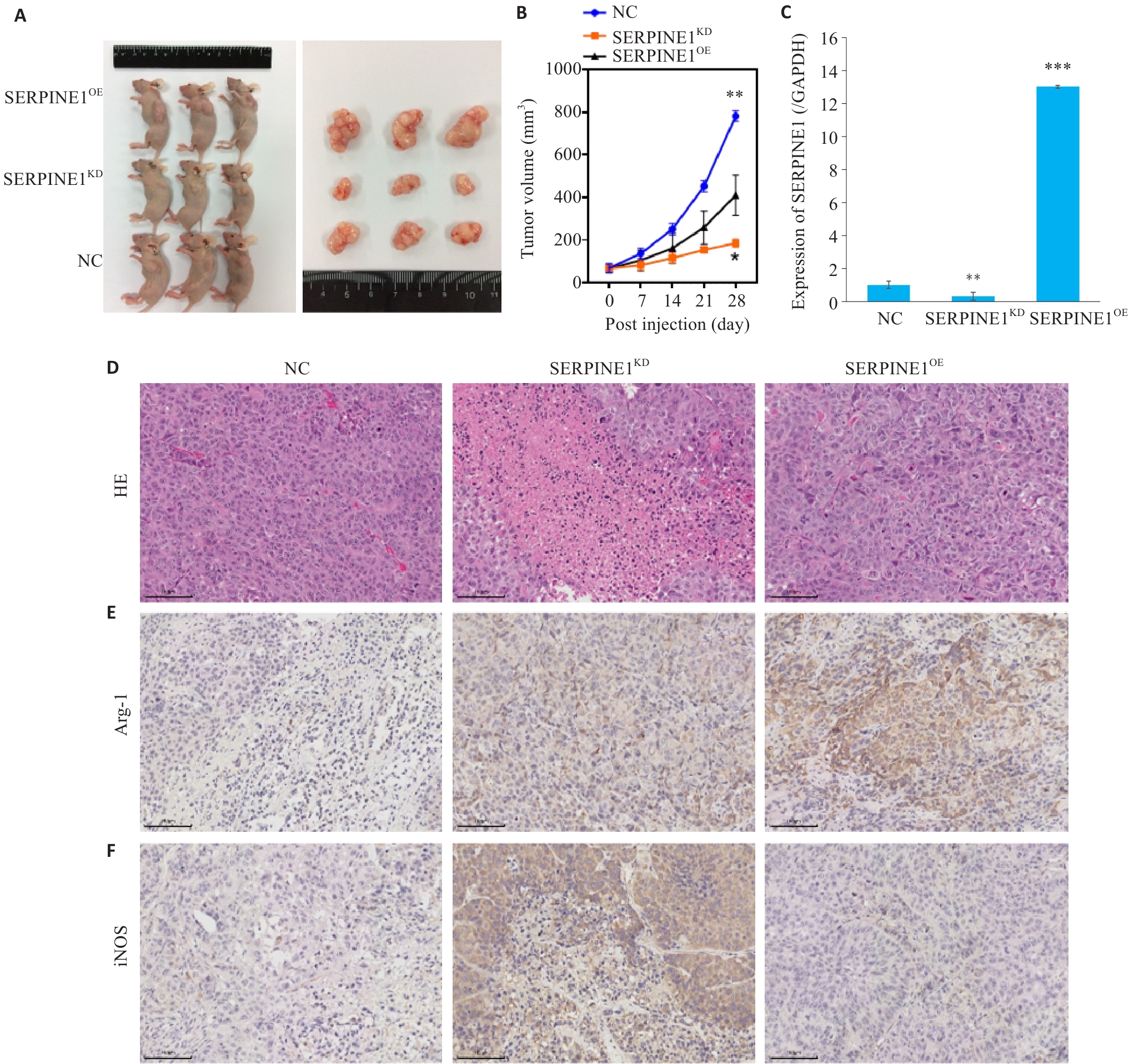
图8 SERPINE1在体内对三阴性乳腺癌生长的影响及对巨噬细胞极化的调控
Fig.8 Regulatory effect of SERPINE1 expression level on growth of triple-negative breast cancer xenografts and macrophage polarization in nude mice. A: Appearance of the tumor-bearing nude mice and the dissected tumors. B: Tumor growth curves over time in each group. C: Protein expression level of SERPINE1 in the xenograft tumor tissues from each group. D: HE staining of xenograft tumor tissues. E: Immunohistochemistry for the M2 macrophage marker Arg-1 in xenograft tumor tissues. F: Immunohistochemistry for the M1 macrophage marker iNOS in xenograft tumor tissues (scale bar=100 μm). *P<0.05, **P<0.01, ***P<0.001 vs NC group. n=6.
| [1] | Li T, Zhang H, Lian M, et al. Global status and attributable risk factors of breast, cervical, ovarian, and uterine cancers from 1990 to 2021[J]. J Hematol Oncol, 2025, 18(1): 5. doi:10.1186/s13045-025-01660-y |
| [2] | Waks AG, Winer EP. Breast cancer treatment: a review[J]. JAMA, 2019, 321(3): 288-300. doi:10.1001/jama.2018.19323 |
| [3] | Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge[J]. Cells, 2019, 8(9): E957. doi:10.3390/cells8090957 |
| [4] | Bai XP, Ni J, Beretov J, et al. Triple-negative breast cancer therapeutic resistance: Where is the Achilles' heel[J]. Cancer Lett, 2021, 497: 100-11. doi:10.1016/j.canlet.2020.10.016 |
| [5] | Lyons TG. Targeted therapies for triple-negative breast cancer[J]. Curr Treat Options Oncol, 2019, 20(11): 82. doi:10.1007/s11864-019-0682-x |
| [6] | Deng M, Wang J, Chen Y, et al. Combination of SF1126 and gefitinib induces apoptosis of triple-negative breast cancer cells through the PI3K/AKT-mTOR pathway[J]. Anticancer Drugs, 2015, 26(4): 422-7. doi:10.1097/cad.0000000000000202 |
| [7] | Beniey M, Hubert A, Haque T, et al. Sequential targeting of PARP with carboplatin inhibits primary tumour growth and distant metastasis in triple-negative breast cancer[J]. Br J Cancer, 2023, 128(10): 1964-75. doi:10.1038/s41416-023-02226-w |
| [8] | Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)[J]. Ann Oncol, 2020, 31(12): 1623-49. |
| [9] | Liao L, Zhang YL, Deng L, et al. Protein phosphatase 1 subunit PPP1R14B stabilizes STMN1 to promote progression and paclitaxel resistance in triple-negative breast cancer[J]. Cancer Res, 2023, 83(3): 471-84. doi:10.1158/0008-5472.can-22-2709 |
| [10] | Li SJ, Wei XH, He JY, et al. Plasminogen activator inhibitor-1 in cancer research[J]. Biomed Pharmacother, 2018, 105: 83-94. doi:10.1016/j.biopha.2018.05.119 |
| [11] | Li Y, Shen L, Tao K, et al. Key roles of p53 signaling pathway-related factors GADD45B and SERPINE1 in the occurrence and development of gastric cancer[J]. Mediators Inflamm, 2023, 2023: 6368893. doi:10.1155/2023/6368893 |
| [12] | Fox SB, Taylor M, Grøndahl-Hansen J, et al. Plasminogen activator inhibitor-1 as a measure of vascular remodelling in breast cancer[J]. J Pathol, 2001, 195(2): 236-43. doi:10.1002/path.931 |
| [13] | Li Y, Yu H, Han X, et al. Analyses of hypoxia-related risk factors and clinical relevance in breast cancer[J]. Front Oncol, 2024, 14: 1350426. doi:10.3389/fonc.2024.1350426 |
| [14] | Tong H, Li K, Zhou M, et al. Coculture of cancer cells with platelets increases their survival and metastasis by activating the TGFβ/Smad/PAI-1 and PI3K/AKT pathways[J]. Int J Biol Sci, 2023, 19(13): 4259-77. doi:10.7150/ijbs.85986 |
| [15] | Xu J, Zhang WW, Tang L, et al. Epithelial-mesenchymal transition induced PAI-1 is associated with prognosis of triple-negative breast cancer patients[J]. Gene, 2018, 670: 7-14. doi:10.1016/j.gene.2018.05.089 |
| [16] | Feng LR, Li GX, Li DB, et al. Cuproptosis-related gene SERPINE1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancer[J]. J Cancer Res Clin Oncol, 2023, 149(12): 10851-65. doi:10.1007/s00432-023-04900-1 |
| [17] | Yagi T, Sawada K, Miyamoto M, et al. Continuous administration of anti-VEGFA antibody upregulates PAI-1 secretion from ovarian cancer cells via miR-143-3p downregulation[J]. Mol Cancer Res, 2023, 21(10): 1093-106. doi:10.1158/1541-7786.mcr-23-0015 |
| [18] | Pan JX, Qu F, Wang FF, et al. Aberrant SERPINE1 DNA methylation is involved in carboplatin induced epithelial-mesenchymal transition in epithelial ovarian cancer[J]. Arch Gynecol Obstet, 2017, 296(6): 1145-52. doi:10.1007/s00404-017-4547-x |
| [19] | Li XD, Dong P, Wei WS, et al. Overexpression of CEP72 promotes bladder urothelial carcinoma cell aggressiveness via epigenetic CREB-mediated induction of SERPINE1[J]. Am J Pathol, 2019, 189(6): 1284-97. doi:10.1016/j.ajpath.2019.02.014 |
| [20] | Thakore VP, Patel KD, Vora HH, et al. Up-regulation of extracellular-matrix and inflammation related genes in oral squamous cell carcinoma[J]. Arch Oral Biol, 2024, 161: 105925. doi:10.1016/j.archoralbio.2024.105925 |
| [21] | Liu Y, Liang YC, Su YJ, et al. Exploring the potential mechanisms of Yi-Yi-Fu-Zi-Bai-Jiang-San therapy on the immune-inflamed pheno-type of colorectal cancer via combined network pharmacology and bioinformatics analyses[J]. Comput Biol Med, 2023, 166: 107432. doi:10.1016/j.compbiomed.2023.107432 |
| [22] | Masuda T, Nakashima T, Namba M, et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts[J]. J Cell Mol Med, 2019, 23(4): 2984-94. doi:10.1111/jcmm.14205 |
| [23] | Zhang Q, Lei L, Jing D. Knockdown of SERPINE1 reverses resistance of triple-negative breast cancer to paclitaxel via suppression of VEGFA[J]. Oncol Rep, 2020, 44(5): 1875-84. |
| [24] | Su YH, Wu YZ, Ann DK, et al. Obesity promotes radioresistance through SERPINE1-mediated aggressiveness and DNA repair of triple-negative breast cancer[J]. Cell Death Dis, 2023, 14(1): 53. doi:10.1038/s41419-023-05576-8 |
| [25] | Placencio VR, DeClerck YA. Plasminogen activator inhibitor-1 in cancer: rationale and insight for future therapeutic testing[J]. Cancer Res, 2015, 75(15): 2969-74. doi:10.1158/0008-5472.can-15-0876 |
| [26] | Xu Z, Cui Y, Li Y, et al. Research progress on nonspecific immune microenvironment in breast cancer[J]. Zhejiang da Xue Xue Bao Yi Xue Ban, 2018, 47(4): 426-34. |
| [27] | Wang S, Pang L, Liu Z, et al. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: a novel therapeutic target[J]. BMC Cancer, 2021, 21(1): 767. doi:10.1186/s12885-021-08536-7 |
| [28] | 李文静, 张 磊, 赖娅娜, 等. 人乳腺癌多西紫杉醇耐药细胞株的建立及特性 [J]. 第三军医大学学报, 2011, 33(21): 3-11. |
| [29] | Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer[J]. BMJ, 2023, 381: e071674. doi:10.1136/bmj-2022-071674 |
| [30] | Qiu XY, Qu Y, Guo BB, et al. Micellar paclitaxel boosts ICD and chemo-immunotherapy of metastatic triple negative breast cancer[J]. J Control Release, 2022, 341: 498-510. doi:10.1016/j.jconrel.2021.12.002 |
| [31] | Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins[J]. BMC Cancer, 2018, 18(1): 41. doi:10.1186/s12885-017-3953-6 |
| [32] | Yang CX, He LY, He PQ, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway[J]. Med Oncol, 2015, 32(2): 14. doi:10.1007/s12032-014-0352-6 |
| [33] | Huang B, Chen ZH, Geng LL, et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways[J]. Cell, 2019, 179(5): 1160-76. e24. doi:10.1016/j.cell.2019.10.027 |
| [34] | Zhang W, Xu J, Fang H, et al. Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling[J]. FASEB J, 2018, 32(1): 276-88. doi:10.1096/fj.201700237rr |
| [35] | Huang X, Wang L, Guo H, et al. Single-cell RNA sequencing reveals SERPINE1-expressing CAFs remodelling tumour microenviro-nment in recurrent osteosarcoma[J]. Clin Transl Med, 2024, 14(1): e1527. doi:10.1002/ctm2.1527 |
| [36] | Chen S, Morine Y, Tokuda K, et al. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway[J]. Int J Oncol, 2021, 59(2): 59. doi:10.3892/ijo.2021.5239 |
| [1] | 张兆君, 吴琼, 谢苗苗, 叶洳吟, 耿晨晨, 石纪雯, 杨清玲, 王文锐, 石玉荣. 层状双氢氧化物负载si-NEAT1通过miR-133b/PD-L1轴调控乳腺癌紫杉醇耐药及巨噬细胞极化[J]. 南方医科大学学报, 2025, 45(8): 1718-1731. |
| [2] | 陈镝, 吕莹, 郭怡欣, 张怡荣, 王蕊璇, 周小若, 陈雨欣, 武晓慧. 双氢青蒿素可显著增强阿霉素诱导的三阴性乳腺癌细胞凋亡:基于负向调控STAT3/HIF-1α通路[J]. 南方医科大学学报, 2025, 45(2): 254-260. |
| [3] | 贺松其, 刘洋, 秦梦晨, 何春雨, 江稳滔, 王一钦, 谭思蕊, 孙海燕, 孙海涛. 中医药调控糖酵解重塑肿瘤免疫微环境的研究进展[J]. 南方医科大学学报, 2025, 45(10): 2277-2284. |
| [4] | 褚乔, 王小娜, 续佳颖, 彭荟林, 赵裕琳, 张静, 陆国玉, 王恺. 白头翁皂苷D通过多靶点和多途径抑制三阴性乳腺癌侵袭转移[J]. 南方医科大学学报, 2025, 45(1): 150-161. |
| [5] | 何欣容, 熊斯丽, 朱真如, 孙景苑, 曹传辉, 王惠. UBE2T通过调节性T细胞诱导肝细胞癌的放疗抵抗[J]. 南方医科大学学报, 2024, 44(6): 1149-1158. |
| [6] | 崔芝, 马萃娇, 王倩茹, 陈金豪, 严子阳, 杨建林, 吕亚丰, 曹春雨. 表达 TGF-βⅡ受体的腺相关病毒载体抑制小鼠三阴性乳腺癌4T1细胞的增殖和肺转移[J]. 南方医科大学学报, 2024, 44(5): 818-826. |
| [7] | 曾佑琴, 陈思雨, 刘燕, 刘奕彤, 张玲, 夏姣, 吴心语, 魏常友, 冷平. AKBA联合阿霉素抑制三阴性乳腺癌细胞MDA-MB-231的增殖、迁移和裸鼠移植瘤生长[J]. 南方医科大学学报, 2024, 44(12): 2449-2460. |
| [8] | 徐梦歧, 石宇彤, 刘俊平, 吴敏敏, 张凤梅, 何志强, 唐 敏. JAG1影响单核-巨噬细胞重塑三阴性乳腺癌转移前微环境:基于外泌体中的LncRNA MALAT1[J]. 南方医科大学学报, 2023, 43(9): 1525-1535. |
| [9] | 王 丽, 严志锐, 夏耀雄. 抑制RAB27a能够抑制三阴乳腺癌细胞的增殖、侵袭和粘附[J]. 南方医科大学学报, 2023, 43(4): 560-567. |
| [10] | 刘俊平, 石宇彤, 吴敏敏, 徐梦岐, 张凤梅, 何志强, 唐 敏. JAG1影响血管生成并促进三阴性乳腺癌细胞的迁移、侵袭和粘附[J]. 南方医科大学学报, 2022, 42(7): 1100-1108. |
| [11] | 方雨潇, 王淑美, 杨 倩, 游松凡, 幸享玲. 柴胡桂枝汤加减方联合卡培他滨抑制三阴性乳腺癌裸鼠皮下移植瘤的生长:基于抑制IL-6/STAT3信号通路[J]. 南方医科大学学报, 2022, 42(6): 905-912. |
| [12] | 葛 钰, 卢林明, 田澍雨, 肖 雨, 谢尚富, 王 琪, 支 慧. 皖南蝮蛇毒抑瘤组分-Ⅰ通过MMP2抑制三阴性乳腺癌细胞体外血管生成拟态[J]. 南方医科大学学报, 2022, 42(3): 438-442. |
| [13] | 赵爱月, 苏云霞, 傅德强. MiR-4772通过调控卵巢癌免疫相关基因改变肿瘤免疫微环境[J]. 南方医科大学学报, 2022, 42(11): 1638-1645. |
| [14] | 王 璐, 赵 琳, 张丽芬, 景 鑫, 张玉姣, 邵 珊, 赵新汉, 罗敏娜. VEGF通过激活ERK/MAPK通路促进三阴性乳腺癌肿瘤干细胞的形成[J]. 南方医科大学学报, 2021, 41(10): 1484-1491. |
| [15] | 刘严友葓,徐虹铃,赖 楠,杨子科,康世均 . 过表达IL-12的恶性黑色素瘤细胞在肿瘤免疫微环境重建过程中抑制T细胞表面PD-1的表达[J]. 南方医科大学学报, 2020, 40(06): 856-863. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||