南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (6): 1149-1158.doi: 10.12122/j.issn.1673-4254.2024.06.16
• • 上一篇
何欣容( ), 熊斯丽(
), 熊斯丽( ), 朱真如, 孙景苑, 曹传辉(
), 朱真如, 孙景苑, 曹传辉( ), 王惠(
), 王惠( )
)
收稿日期:2024-03-07
出版日期:2024-06-20
发布日期:2024-07-01
通讯作者:
曹传辉,王惠
E-mail:theahexr@163.com;179980987@qq.com;huichuancao@163.com;635137884@qq.com
作者简介:何欣容,在读硕士研究生,E-mail: theahexr@163.com基金资助:
Xinrong HE( ), Sili XIONG(
), Sili XIONG( ), Zhenru ZHU, Jingyuan SUN, Chuanhui CAO(
), Zhenru ZHU, Jingyuan SUN, Chuanhui CAO( ), Hui WANG(
), Hui WANG( )
)
Received:2024-03-07
Online:2024-06-20
Published:2024-07-01
Contact:
Chuanhui CAO, Hui WANG
E-mail:theahexr@163.com;179980987@qq.com;huichuancao@163.com;635137884@qq.com
Supported by:摘要:
目的 探索泛素结合酶2T(UBE2T)对肝细胞癌放疗敏感性的影响及机制。 方法 采用空白对照载体或过表达UBE2T慢病毒载体转染小鼠Hepa1-6肝癌细胞建立对照组(LV-Control)和过表达组(LV-UBE2T),qPCR以及Western blotting检测上述细胞UBE2T表达情况;对两组细胞进行射线照射(IR)处理,克隆形成实验检测UBE2T过表达对Hepa1-6肝癌细胞放疗敏感性影响;分别在裸鼠和C57BL/6小鼠皮下注射上述细胞建立肝癌皮下荷瘤小鼠模型,对皮下瘤予IR处理,建立LV-Control组、LV-Control+IR组、LV-UBE2T组和LV-UBE2T+IR组,5~6只/组,观察皮下瘤生长速度及体积。通过CIBERSORT算法分析肝癌免疫细胞浸润情况与UBE2T表达量的关系。流式细胞术检测上述4组小鼠的肝癌中CD4+T细胞以及调节性T细胞(Tregs)浸润情况。比色法测定细胞培养上清液中葡萄糖及乳酸的含量;癌症和肿瘤基因图谱(TCGA)的公共数据分析肝癌UBE2T表达量与糖酵解水平和Tregs浸润关系。Western blotting检测UBE2T表达与糖酵解相关蛋白HK1、LDHA表达相关关系。体外共培养模型联合流式细胞术以及qPCR验证UBE2T过表达肝癌与Tregs关系。 结果 qPCR、Western blotting结果显示过表达组中UBE2T表达显著升高(P<0.0001)。克隆形成实验、裸鼠肝癌皮下瘤实验显示UBE2T过表达导致肝细胞癌放疗抵抗(P<0.05),UBE2T导致的放疗抵抗在C57BL/6小鼠肝癌皮下瘤模型上更显著(P<0.01)。CIBERSORT分析提示UBE2T高表达组肝癌中树突状细胞(P<0.01)、滤泡辅助性T细胞(P<0.001)、M2型巨噬细胞(P<0.01)、单核细胞(P<0.05)、总体淋巴细胞(P<0.05)以及Tregs(P<0.0001)浸润比例上调。流式细胞术显示过表达UBE2T小鼠肝癌免疫微环境中Tregs数量上调(P<0.05),IR导致UBE2T组CD4+T细胞以及Tregs浸润增加(P<0.01或P<0.001)。与对照组细胞培养上清液相比,过表达UBE2T组的培养上清液葡萄糖浓度降低(P<0.05),乳酸浓度上调(P<0.01)。GSEA分析提示UBE2T高表达肝癌与糖酵解水平(P<0.001)、Tregs浸润水平呈正相关(P<0.001)。Western blotting显示糖酵解相关蛋白HK1、LDHA表达水平与UBE2T表达水平相关。体外共培养模型显示UBE2T过表达肝癌使Tregs细胞内乳酸含量上调(P<0.001),增殖能力增加(P<0.05)以及免疫抑制功能上调(Il-10,P<0.05;TGF-β,P<0.001)。 结论 UBE2T介导的肝癌细胞放疗抵抗可能与肝癌细胞糖酵解水平提高介导的免疫微环境中Tregs富集相关。
何欣容, 熊斯丽, 朱真如, 孙景苑, 曹传辉, 王惠. UBE2T通过调节性T细胞诱导肝细胞癌的放疗抵抗[J]. 南方医科大学学报, 2024, 44(6): 1149-1158.
Xinrong HE, Sili XIONG, Zhenru ZHU, Jingyuan SUN, Chuanhui CAO, Hui WANG. Overexpression of ubiquitin-conjugating enzyme 2T induces radiotherapy resistance in hepatocellular carcinoma by enriching regulatory T cells in the tumor microenvironment[J]. Journal of Southern Medical University, 2024, 44(6): 1149-1158.
| Primer | Sequence (5'→3') |
|---|---|
Il-10-F Il-10-R | GTCCTTTCACTTGCCCTCATC CAAACYGGTCACAGCTTTCGA |
表1 引物序列
Tab.1 Primers used for RT-qPCR
| Primer | Sequence (5'→3') |
|---|---|
Il-10-F Il-10-R | GTCCTTTCACTTGCCCTCATC CAAACYGGTCACAGCTTTCGA |
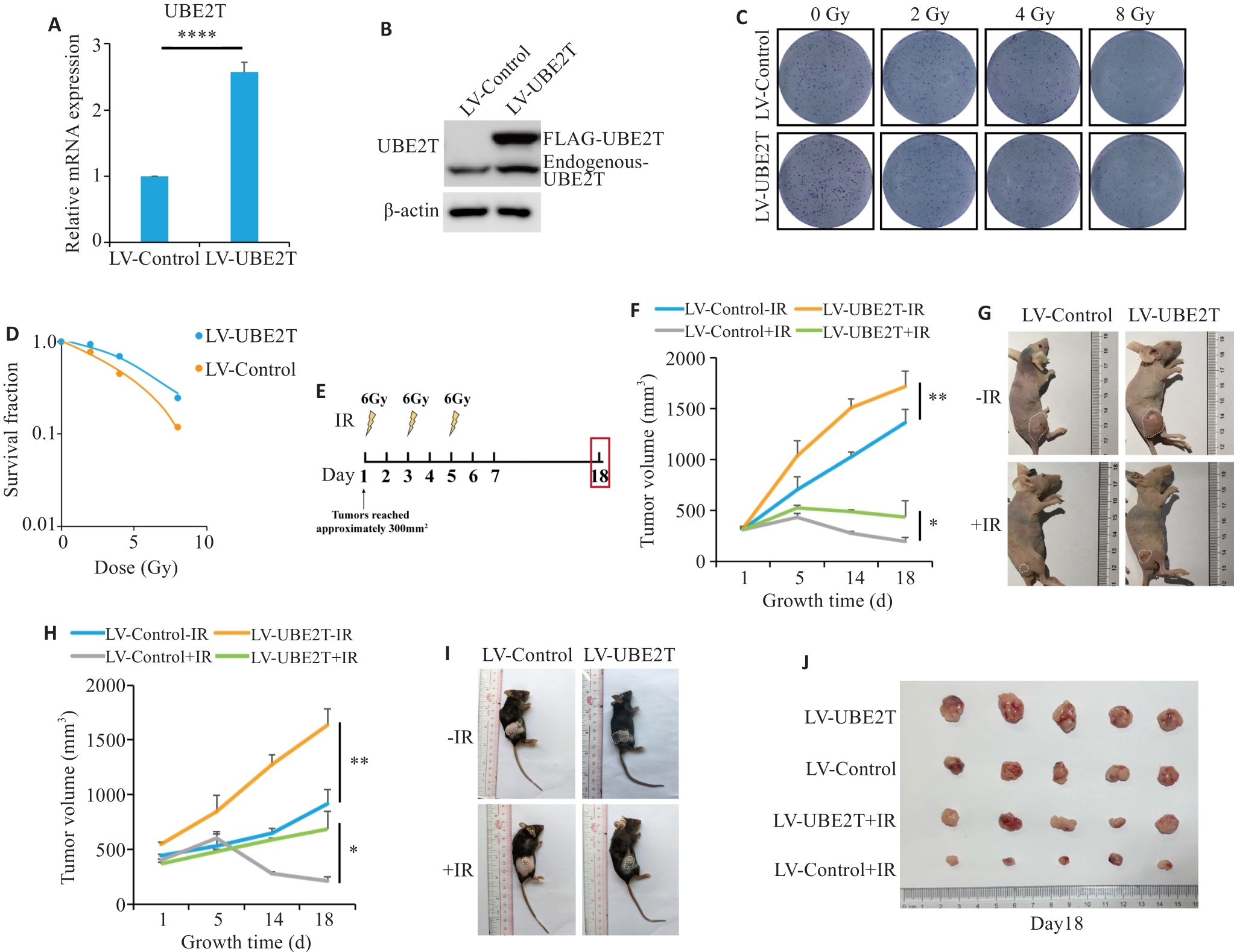
图1 过表达UBE2T可导致肝癌放疗抵抗
Fig.1 Overexpression of UBE2T results in radiotherapy resistance in HCC. A: Relative mRNA level of Ube2t adjusted to Actin. B: Western blotting of UBE2T protein in LV-Control and LV-UBE2T cells. C, D: Colony formation assays of Hepa 1-6 cells with stable UBE2T overexpression. E: Treatment schedules of ionizing radiation (IR) and endpoint for recording. F, G: Tumor growth curves of the nude mice (F, n=5 or 6) and the dissected xenografts from the mice (G). in each group. H-J: Tumor growth curves of C57BL/6 mice (H, n=5 or 6), the tumor-bearing mice (I), and the dissected xenografts (J). In (F) and (H), statistical analyses were performed using a mixed-effects model followed by Tukey's multiple comparison test. In (A), student's t-test was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ****P<0.0001.
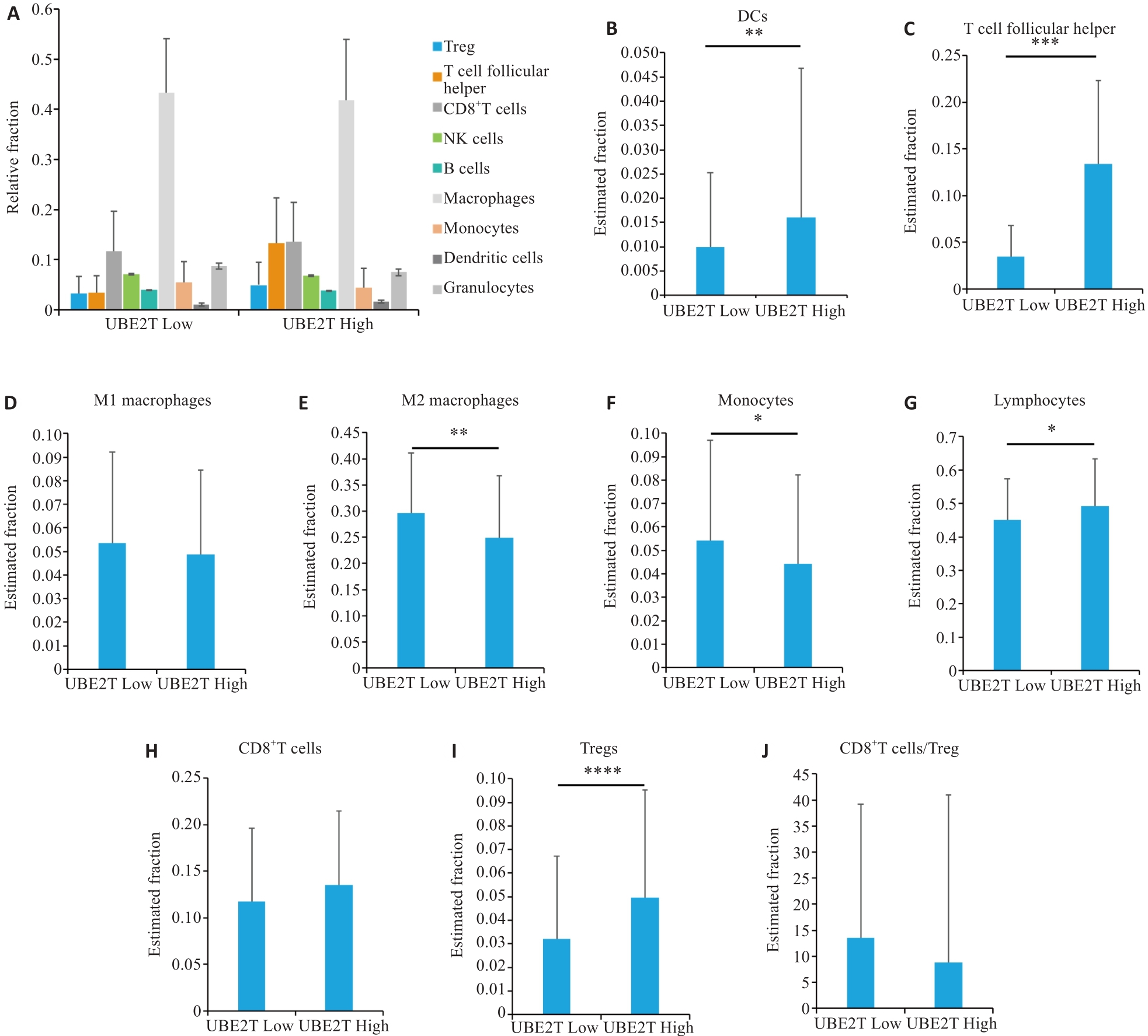
图2 CIBERSORT肝癌免疫微环境分析
Fig.2 Immunological microenvironmental analysis of HCC by CIBERSORT. A: Percentages of different cell types in the two groups. B-J: Estimated fractions of DCs (B), T follicular helper cells (C), M1 macrophages (D), M2 macrophages (E), monocytes (F), lymphocytes (G), CD8+ T cells (H), Tregs (I), and CD8+T cells/Tregs (J) in the two groups. Student's t-test was used for comparisons. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. DCs: Dendritic cells, Tregs: Regulatory T cells.
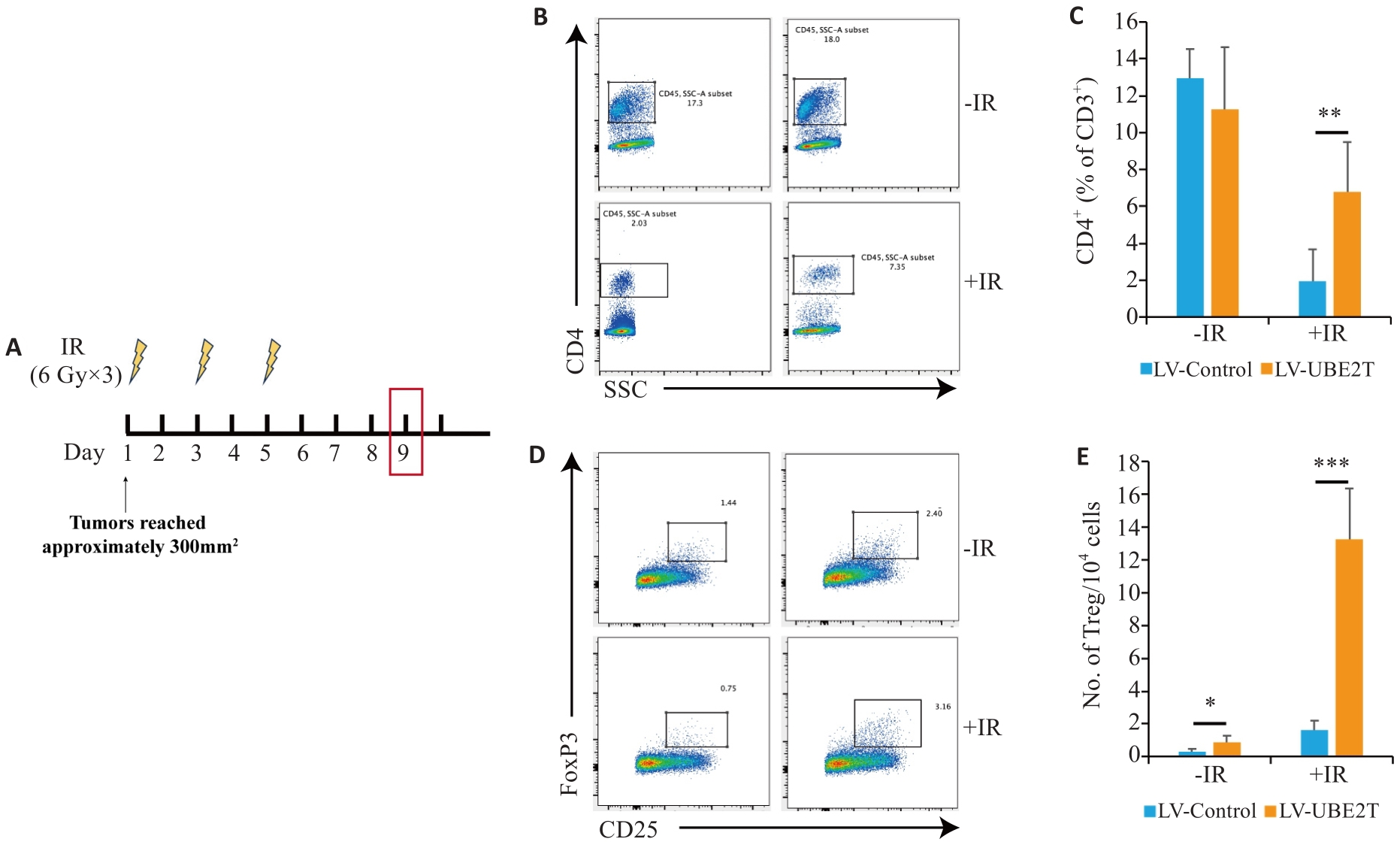
图3 过表达UBE2T肝癌中Tregs增多
Fig.3 The number of Tregs is increased in HCC overexpressing UBE2T. A: Treatment schedules of IR and endpoint for analysis. B, C: Representative contour plots (B) and quantitation (C) of CD4+/CD3+ ratios (n=6-8). D, E: Representative contour plots (D) and quantitation (E) of the number of TIL Tregs (CD25+ Foxp3+) per 104 cells (n=6-8). Student's t-test was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001.
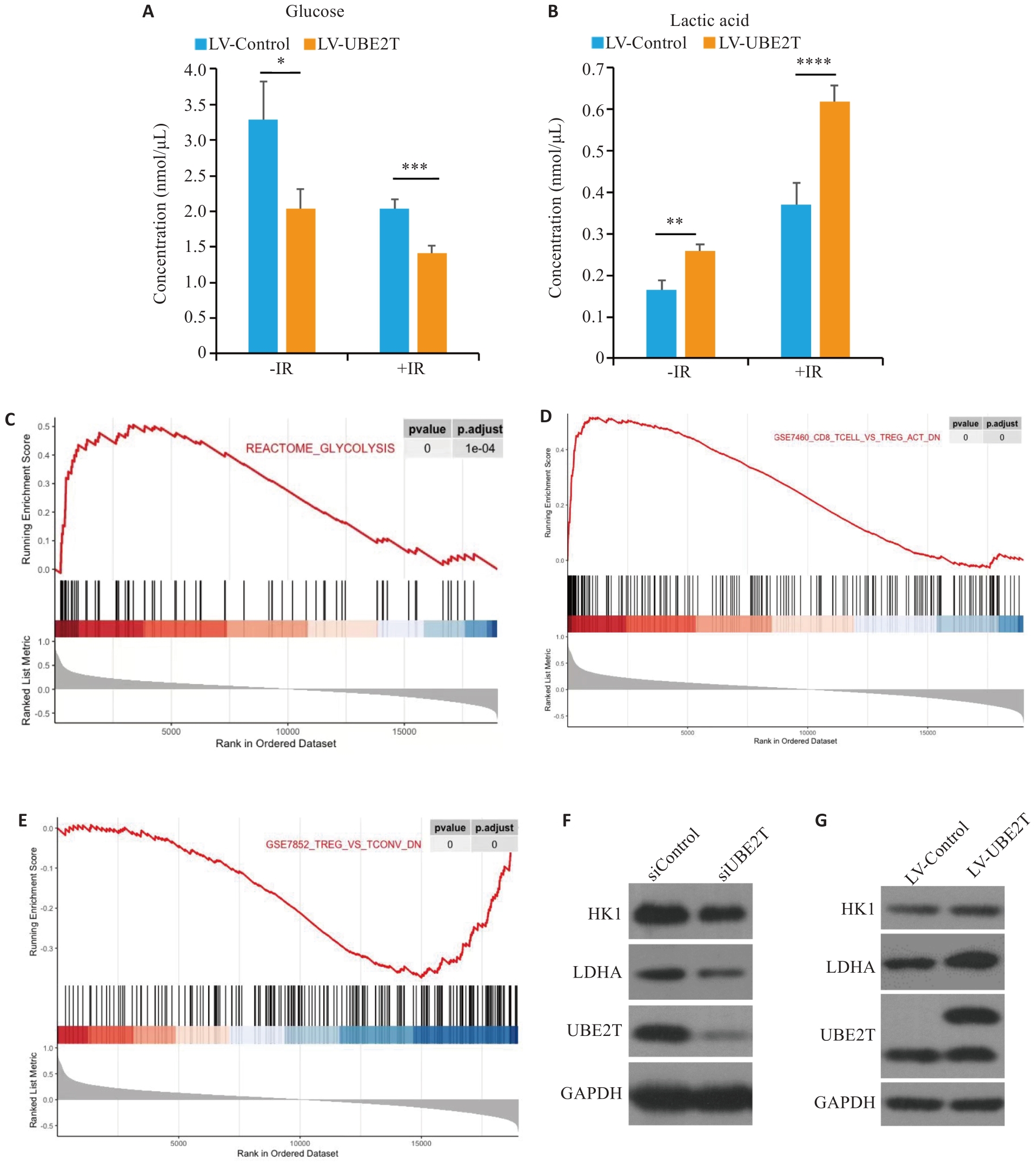
图4 UBE2T表达量对肝癌糖酵解水平的影响及其与调控肝癌免疫微环境的关系
Fig.4 Effect of UBE2T expression on glycolysis levels in HCC and its regulatory effect on immune microenvironment. A, B: Concentration of glucose (A) and lactic acid (B) in the two groups with or without IR. C-E: Results of GSEA are plotted to visualize the correlation between the expression of UBE2T and the gene signatures of glycolysis and Tregs infiltration in the TCGA cohort. Student's t-test was used for comparisons. F, G: Western blotting of HK1, LDHA, and UBE2T protein in siControl, siUNE2T, LV-Control and LV-UBE2T cells. *P<0.05, **P<0.01, ***P<0.001.
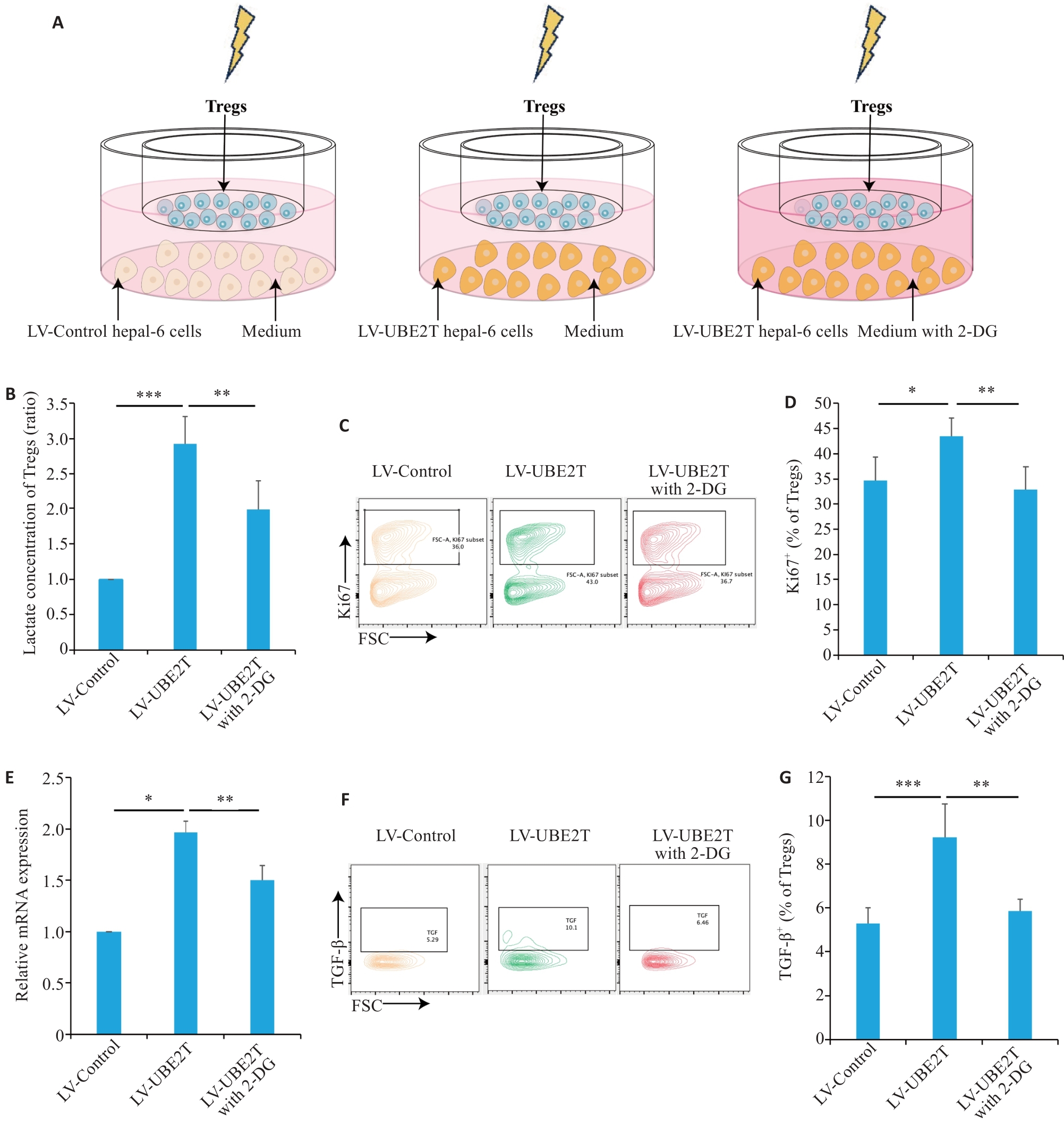
图5 UBE2T过表达肝癌分泌乳酸增多扩增激活Tregs
Fig.5 HCC overexpressing UBE2T promoted lactate secretion and activated Tregs.A: Schematic diagram of the co-culture model. B: Concentrations of Tregs in different co-culture groups (n=5). C, D: Representative contour plots (C) and quantitation (D) of Ki67+ ratios (n=6). E: Relative mRNA level of IL-10 adjusted to Actin in different co-culture groups (n=3). F, G: Representative contour plots (F) and quantitation (G) of the TGF‑β+ ratios (n=5). One-way ANOVA was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. |
| 2 | Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7: 6. |
| 3 | Chen LC, Lin HY, Hung SK, et al. Role of modern radiotherapy in managing patients with hepatocellular carcinoma[J]. World J Gastroenterol, 2021, 27(20): 2434-57. |
| 4 | Kim TH, Koh YH, Kim BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial[J]. J Hepatol, 2021, 74(3): 603-12. |
| 5 | Shi CY, Li Y, Geng L, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: a randomised controlled trial[J]. Eur J Cancer, 2022, 166: 176-84. |
| 6 | Byun HK, Kim HJ, Im YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma[J]. Radiother Oncol, 2019, 133: 1-8. |
| 7 | Bang A, Dawson LA. Radiotherapy for HCC: ready for prime time?[J]. JHEP Rep, 2019, 1(2): 131-7. |
| 8 | Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer[J]. Nat Rev Clin Oncol, 2015, 12(9): 527-40. |
| 9 | Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies[J]. Trends Cancer, 2022, 8(1): 9-20. |
| 10 | Koukourakis MI, Giatromanolaki A. Tumor draining lymph nodes, immune response, and radiotherapy: towards a revisal of therapeutic principles[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(3): 188704. |
| 11 | McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy[J]. Nat Rev Cancer, 2020, 20(4): 203-17. |
| 12 | Yum S, Li MH, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS[J]. Cell Res, 2020, 30(8): 639-48. |
| 13 | Li JY, Zhao Y, Gong S, et al. TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models[J]. Nat Commun, 2023, 14(1): 865. |
| 14 | Jiang XY, Ma Y, Wang T, et al. Targeting UBE2T potentiates gemcitabine efficacy in PancreaticCancer by regulating pyrimidine metabolism and replication stress[J]. Gastroenterology, 2023, 164(7): 1232-47. |
| 15 | Yu ZY, Jiang XY, Qin L, et al. A novel UBE2T inhibitor suppresses Wnt/β‑catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination[J]. Oncogene, 2021, 40(5): 1027-42. |
| 16 | Zhu ZR, Cao CH, Zhang DY, et al. UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism[J]. Cell Death Dis, 2022, 13(2): 154. |
| 17 | Sun JY, Zhu ZR, Li WW, et al. UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation[J]. J Exp Clin Cancer Res, 2020, 39(1): 222. |
| 18 | Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles[J]. Nat Methods, 2015, 12(5): 453-7. |
| 19 | Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77(6): 1598-606. |
| 20 | Qiao L, Dong C, Ma BL. UBE2T promotes proliferation, invasion and glycolysis of breast cancer cells by regualting the PI3K/AKT signaling pathway[J]. J Recept Signal Transduct Res, 2022, 42(2): 151-9. |
| 21 | Wang Y, Leng H, Chen H, et al. Knockdown of UBE2T inhibits osteosarcoma cell proliferation, migration, and invasion by suppressing the PI3K/akt signaling pathway[J]. Oncol Res, 2016, 24(5): 361-9. |
| 22 | Cao K, Ling XD, Jiang XY, et al. Pan-canceranalysis of UBE2T with a focus on prognostic and immunological roles in lung adenocarcinoma[J]. Respir Res, 2022, 23(1): 306. |
| 23 | Huang W, Huang HY, Xiao YZ, et al. UBE2T is upregulated, predicts poor prognosis, and promotes cell proliferation and invasion by promoting epithelial-mesenchymal transition via inhibiting autophagy in an AKT/mTOR dependent manner in ovarian cancer[J]. Cell Cycle, 2022, 21(8): 780-91. |
| 24 | Allen C, Her S, Jaffray DA. Radiotherapy for cancer: present and future[J]. Adv Drug Deliv Rev, 2017, 109: 1-2. |
| 25 | Citrin DE. Recent developments in radiotherapy[J]. N Engl J Med, 2017, 377(22): 2200-1. |
| 26 | Ahmad SS, Duke S, Jena R, et al. Advances in radiotherapy[J]. BMJ, 2012, 345(dec04 1): e7765. |
| 27 | Ohri N, Dawson LA, Krishnan S, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study[J]. J Natl Cancer Inst, 2016, 108(9): djw133. |
| 28 | Fang Y, Zhan YZ, Xie YW, et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC[J]. Hepatology, 2022, 75(6): 1386-401. |
| 29 | Hong WF, Zhang Y, Wang SW, et al. RECQL4 inhibits radiation-induced tumor immune awakening via suppressing the cGAS-STING pathway in hepatocellular carcinoma[J]. Adv Sci, 2024, 11(16): e2308009. |
| 30 | Yin H, Wang XY, Zhang X, et al. UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation[J]. Cancer Lett, 2020, 494: 121-31. |
| 31 | Zhang YH, Hu JY, Ji K, et al. CD39 inhibition and VISTA blockade may overcome radiotherapy resistance by targeting exhausted CD8+ T cells and immunosuppressive myeloid cells[J]. Cell Rep Med, 2023, 4(8): 101151. |
| 32 | Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment[J]. Nat Immunol, 2013, 14(10): 1014-22. |
| 33 | Song M, Yeku OO, Rafiq S, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages[J]. Nat Commun, 2020, 11(1): 6298. |
| 34 | Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma[J]. Cancer Discov, 2019, 9(8): 1124-41. |
| 35 | Dikiy S, Rudensky AY. Principles of regulatory Tcell function[J]. Immunity, 2023, 56(2): 240-55. |
| 36 | Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development[J]. Annu Rev Immunol, 2020, 38: 421-53. |
| 37 | Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer[J]. Immunity, 2019, 50(2): 302-16. |
| 38 | Kang JH, Zappasodi R. Modulating Treg stability to improve cancer immunotherapy[J]. Trends Cancer, 2023, 9(11): 911-27. |
| 39 | Shan F, Somasundaram A, Bruno TC, et al. Therapeutic targeting of regulatory T cells in cancer[J]. Trends Cancer, 2022, 8(11): 944-61. |
| 40 | Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression-implications for anticancer therapy[J]. Nat Rev Clin Oncol, 2019, 16(6): 356-71. |
| 41 | Persa E, Balogh A, Sáfrány G, et al. The effect of ionizing radiation on regulatory T cells in health and disease[J]. Cancer Lett, 2015, 368(2): 252-61. |
| 42 | 郭 康, 刘晓敏, 戴 聪, 等. UBE2T基因在乳腺癌中的差异表达及其与免疫细胞浸润之间的相关性研究: 基于生物信息学分析的结果[J]. 临床医学研究与实践, 2020, 5(34): 5-7, 15. DOI: 10.19347/j.cnki.2096-1413.202034002 |
| 43 | 于运亮, 李 婷, 王莉莉, 等. UBE2C和UBE2T与胰腺癌患者预后及免疫细胞浸润的关系[J]. 山东医药, 2021, 61(28): 13-8. DOI: 10.3969/j.issn.1002-266X.2021.28.004 |
| 44 | Pouysségur J, Marchiq I, Parks SK, et al. 'Warburg effect' controls tumor growth, bacterial, viral infections and immunity-Genetic deconstruction and therapeutic perspectives[J]. Semin Cancer Biol, 2022, 86(Pt 2): 334-46. |
| 45 | Kang H, Kim B, Park J, et al. The Warburg effect on radioresistance: survival beyond growth[J]. Biochim Biophys Acta Rev Cancer, 2023, 1878(6): 188988. |
| 46 | Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells[J]. Mol Aspects Med, 2010, 31(1): 60-74. |
| 47 | Apostolova P, Pearce EL. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment[J]. Trends Immunol, 2022, 43(12): 969-77. |
| 48 | Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment[J]. Science, 2015, 348(6230): 74-80. |
| 49 | Park J, Hsueh PC, Li ZY, et al. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity[J]. Immunity, 2023, 56(1): 32-42. |
| 50 | Watson MJ, Vignali PDA, Mullett SJ, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid[J]. Nature, 2021, 591(7851): 645-51. |
| 51 | Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments[J]. Cell Metab, 2017, 25(6): 1282-93.e7. |
| 52 | Li N, Kang YQ, Wang LL, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment[J]. Proc Natl Acad Sci U S A, 2020, 117(33): 20159-70. |
| [1] | 刘鹏程, 娄丽娟, 刘霞, 王建, 姜颖. M2巨噬细胞特征基因风险评分能准确预测HBV相关肝细胞癌患者的预后[J]. 南方医科大学学报, 2024, 44(5): 827-840. |
| [2] | 钟伟雄, 梁芳蓉, 杨蕊梦, 甄 鑫. 基于多期动态增强CT影像组学特征和多分类器分层融合模型预测肝细胞癌的微血管侵犯[J]. 南方医科大学学报, 2024, 44(2): 260-269. |
| [3] | 朱 权, 黄柏胜, 位磊艳, 罗奇志. 过表达LncRNA MEG3通过促进铁死亡增强肝癌细胞对顺铂的化疗敏感性[J]. 南方医科大学学报, 2024, 44(1): 17-24. |
| [4] | 何艳娟, 李卓颖, 申 琳, 石丁华, 李申堂. 心脏祖细胞来源的外泌体可减轻心肌梗死小鼠的心肌损伤:基于mTOR途径诱导Treg细胞分化[J]. 南方医科大学学报, 2023, 43(9): 1644-1650. |
| [5] | 曹丹萍, 蔡 娟, 李艳娜, 董润雨, 王智雄, 左学良. TMEM64在肝癌组织中高表达并促进肝癌细胞的增殖和侵袭[J]. 南方医科大学学报, 2023, 43(8): 1345-1355. |
| [6] | 兰 玉, 王凯风, 蓝智贤, 周何琪, 孙 剑. 脱醇红酒抑制肝细胞癌的发生和发展:基于诱导细胞周期的阻滞和凋亡[J]. 南方医科大学学报, 2023, 43(8): 1297-1305. |
| [7] | 巩 高, 曹 石, 肖 慧, 方威扬, 阙与清, 刘子蔚, 陈超敏. 深度注意力机制结合临床特征预测肝细胞癌微血管浸润[J]. 南方医科大学学报, 2023, 43(5): 839-851. |
| [8] | 苏莉莉, 梁晚晴, 吕振宇, 韩 啸. PLXNA1在肝癌中高表达并影响患者的生存预后及其免疫微环境[J]. 南方医科大学学报, 2023, 43(11): 1909-1918. |
| [9] | 张 铃, 赵春雨, 许瑶瑶, 陈炎森, 蔡志雄, 林浩伟, 蔡巧燕. 环状RNA hsa_circ_0006834可作为肝细胞癌患者预后的潜在生物标志物[J]. 南方医科大学学报, 2023, 43(11): 1850-1856. |
| [10] | 林 杰, 区活辉, 王卫东, 马 靖, 张伟杰, 刘清波. 过表达CLEC5A基因抑制肝细胞癌增殖和转移并逆转上皮-间质转化[J]. 南方医科大学学报, 2023, 43(1): 85-91. |
| [11] | 张换换, 陈 卓, 赵想弟, 霍 强, 程 秀. 紫草素通过抑制PKM2/PHD3/HIF-1α通路诱导肝癌细胞凋亡[J]. 南方医科大学学报, 2023, 43(1): 92-98. |
| [12] | 王 宁, 王一晗, 姜朋涛, 吕明华, 胡志芳, 徐 曦. DNAM-1通过IL-2/STAT-5通路调节Ⅰ型调节性T细胞的增殖和功能[J]. 南方医科大学学报, 2022, 42(9): 1288-1295. |
| [13] | 宁诗雨, 何春梅, 郭泽皓, 张 豪, 莫之婧. VIPR1启动子甲基化促进转录因子AP-2α下调VIPR1的表达并促进肝细胞癌的生长[J]. 南方医科大学学报, 2022, 42(7): 957-965. |
| [14] | 杨雪佳, 李玉杰, 吴登强, 马义丽, 周素芳. 肝细胞癌进展过程中的关键基因ATP1B3和ENAH的筛选与鉴定:基于数据挖掘和临床验证[J]. 南方医科大学学报, 2022, 42(6): 815-823. |
| [15] | 李 超, 陈双江, 姜业臻. 过表达miR-607通过下调TRPC5表达抑制肝细胞癌的生长和转移[J]. 南方医科大学学报, 2022, 42(11): 1587-1593. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||