南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (9): 1712-1719.doi: 10.12122/j.issn.1673-4254.2024.09.11
左涵珺( ), 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟(
), 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟( )
)
收稿日期:2024-01-23
出版日期:2024-09-20
发布日期:2024-09-30
通讯作者:
李娟娟
E-mail:zhj2020320@163.com;lijuanjuan@kmmu.edu.cn
作者简介:左涵珺,在读博士研究生,E-mail: zhj2020320@163.com
基金资助:
Hanjun ZUO( ), Zhaoda DUAN, Zhao WANG, Tao GUO, Jinsha SHI, Haolong SHI, Juanjuan LI(
), Zhaoda DUAN, Zhao WANG, Tao GUO, Jinsha SHI, Haolong SHI, Juanjuan LI( )
)
Received:2024-01-23
Online:2024-09-20
Published:2024-09-30
Contact:
Juanjuan LI
E-mail:zhj2020320@163.com;lijuanjuan@kmmu.edu.cn
Supported by:摘要:
目的 探讨天麻素通过PI3K/AKT信号通路对新生大鼠缺氧缺血性脑损伤(HIBD)后活化小胶质细胞介导的炎症反应的作用机制。 方法 将39只3日龄新生SD大鼠随机分为假手术组(sham,n=9)、缺氧缺血模型组(HIBD,n=15)、天麻素处理组(HIBD+G,n=15)。采用Western blotting检测HIBD后TNF-α、IL-1β、IL-10和TGF-β1蛋白的表达;网络药理学筛选天麻素治疗HIBD的潜在作用靶点;Western blotting检测HIBD和氧糖剥夺(OGD)诱导活化的小胶质细胞中PI3K/AKT信号通路的表达;CCK8检测PI3K/AKT通路特异性抑制剂LY294002对BV-2小胶质细胞的细胞毒性作用;RT-qPCR检测LY294002干预后天麻素对TNF-α和TGF-β1的mRNA水平的影响。 结果 Western blotting显示,与HIBD组相比,天麻素降低缺血侧胼胝体区TNF-α和IL-1β的蛋白表达(P<0.05),促进IL-10和TGF-β1的蛋白表达(P<0.05);网络药理学显示,PI3K/AKT信号通路显著富集,且天麻素与PI3K之间具有较好的结合能力;Western blotting显示,与HIBD组、OGD组相比,天麻素促进PI3K和AKT的磷酸化水平(P<0.05);CCK8结果显示LY294002在0~120 μmol/L浓度范围内对BV-2小胶质细胞没有细胞毒性作用;RT-qPCR结果显示,与对照组相比,OGD组TNF-α的mRNA水平升高,TGF-β1的mRNA水平降低(P<0.05);天麻素干预后降低TNF-α的mRNA水平,升高TGF-β1的mRNA水平(P<0.05);LY294002处理后TNF-α的mRNA水平进一步升高,TGF-β1的mRNA水平进一步降低(P<0.05);而LY294002与天麻素联合用药后TNF-α和TGF-β1的mRNA水平无明显变化。 结论 天麻素能抑制HIBD后活化小胶质细胞介导的炎症反应,其作用机制与PI3K/AKT信号通路有关。
左涵珺, 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟. 天麻素经PI3K/AKT通路改善新生大鼠缺氧缺血性脑损伤后小胶质细胞介导的炎症反应[J]. 南方医科大学学报, 2024, 44(9): 1712-1719.
Hanjun ZUO, Zhaoda DUAN, Zhao WANG, Tao GUO, Jinsha SHI, Haolong SHI, Juanjuan LI. Gastrodin improves microglia-mediated inflammatory response after hypoxic-ischemic brain damage in neonatal rats via PI3K/AKT pathway[J]. Journal of Southern Medical University, 2024, 44(9): 1712-1719.
| Primer | Sequence (5'-3') |
|---|---|
| TGF-β1 F | GCTGAACCAAGGAGACGGAA |
| TGF-β1 R | TCTTCTCTGTGGAGCGTTGAT |
| TNF-α F | ACCCTCACACTCACAAACCA |
| TNF-α R | GGCAGAGAGGAGGTTGACTTT |
| β-actin F | GTGGGAATGGGTCAGAAGGA |
| β-actin R | TACATGGCTGGGGTGTTGAA |
表1 引物序列
Tab.1 Primer sequence for RT-qPCR
| Primer | Sequence (5'-3') |
|---|---|
| TGF-β1 F | GCTGAACCAAGGAGACGGAA |
| TGF-β1 R | TCTTCTCTGTGGAGCGTTGAT |
| TNF-α F | ACCCTCACACTCACAAACCA |
| TNF-α R | GGCAGAGAGGAGGTTGACTTT |
| β-actin F | GTGGGAATGGGTCAGAAGGA |
| β-actin R | TACATGGCTGGGGTGTTGAA |
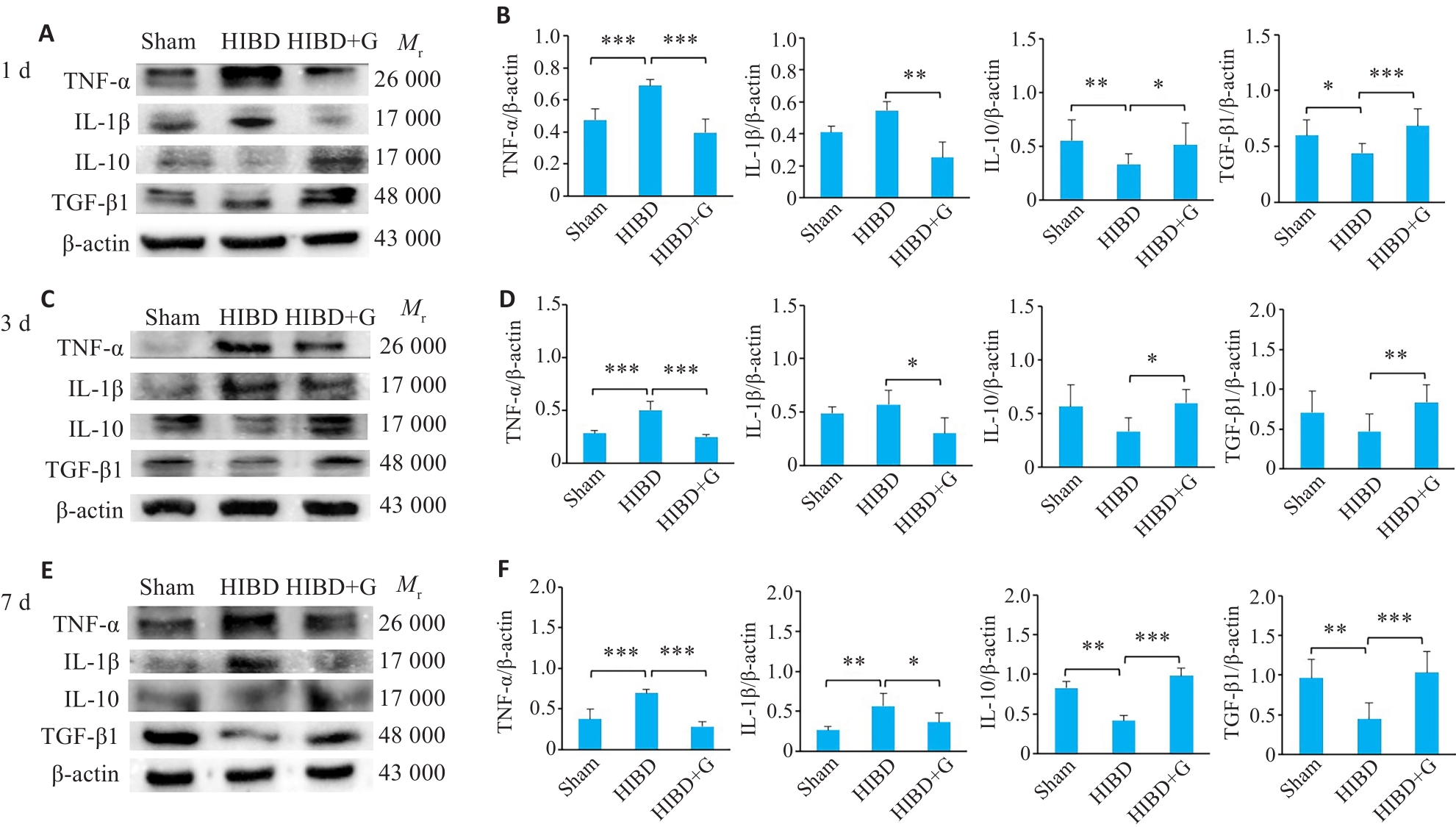
图1 蛋白印迹法检测HIBD后天麻素对炎性因子TNF-α、IL-1β、抗炎因子IL-10和转化生长因子TGF-β1蛋白表达的影响
Fig.1 Effect of gastrodin on expressions of TNF-α, IL-1β, IL-10 and TGF-β1 proteins in the in brain tissue of neonatal rats at 1 day, 3 days, and 7 days after HIBD detected by Western blotting. A, C, E: Protein blots of TNF-α, IL-1β, IL-10, and TGF-β1. B, D, F: Quantification of TNF-α, IL-1β, IL-10, and TGF-β1 expression levels. *P<0.05, **P<0.01, ***P<0.001 (n=3).
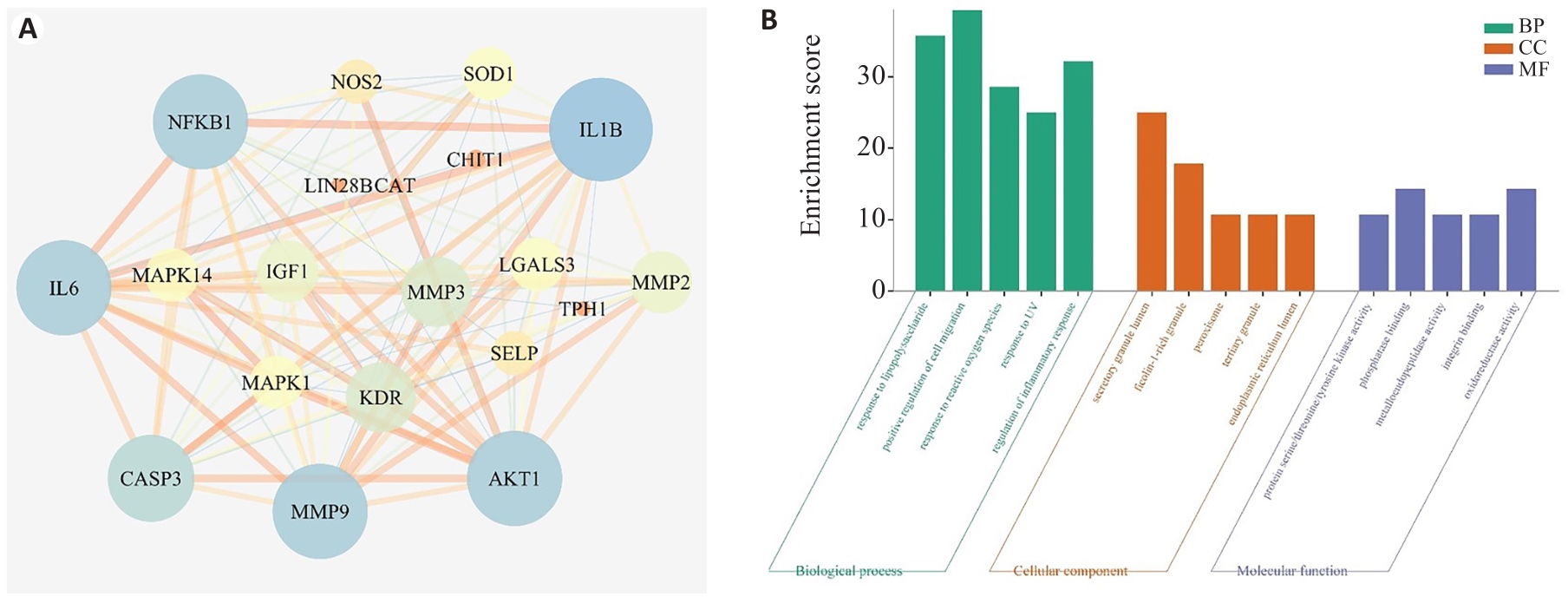
图2 天麻素治疗HIBD潜在核心靶点的PPI网络图和GO富集分析
Fig.2 PPI network diagram and GO enrichment analysis of potential core target of gastrodin treatment of HIBD. A: PPI network diagram. B: GO enrichment analysis.
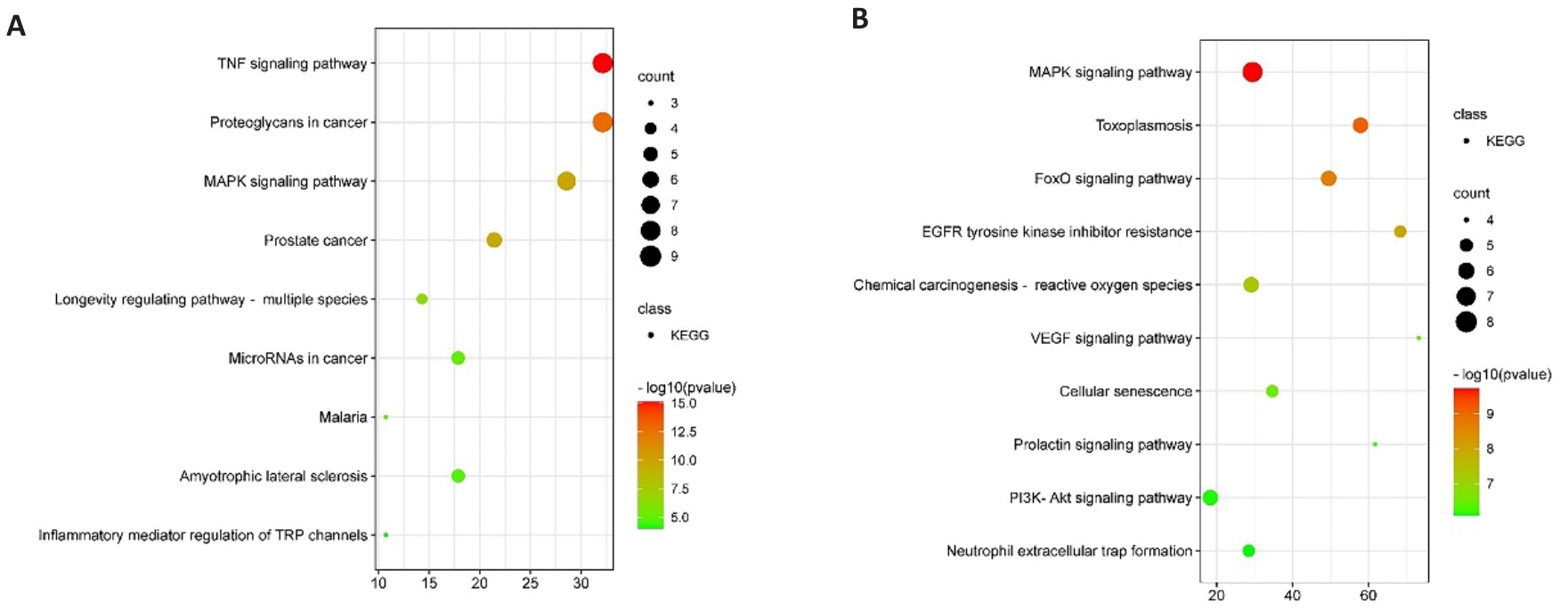
图3 天麻素治疗HIBD潜在核心靶点的KEGG富集分析
Fig.3 KEGG enrichment analysis of potential core targets for gastrodin treatment of HIBD. A, B: KEGG pathway enrichment analysis.
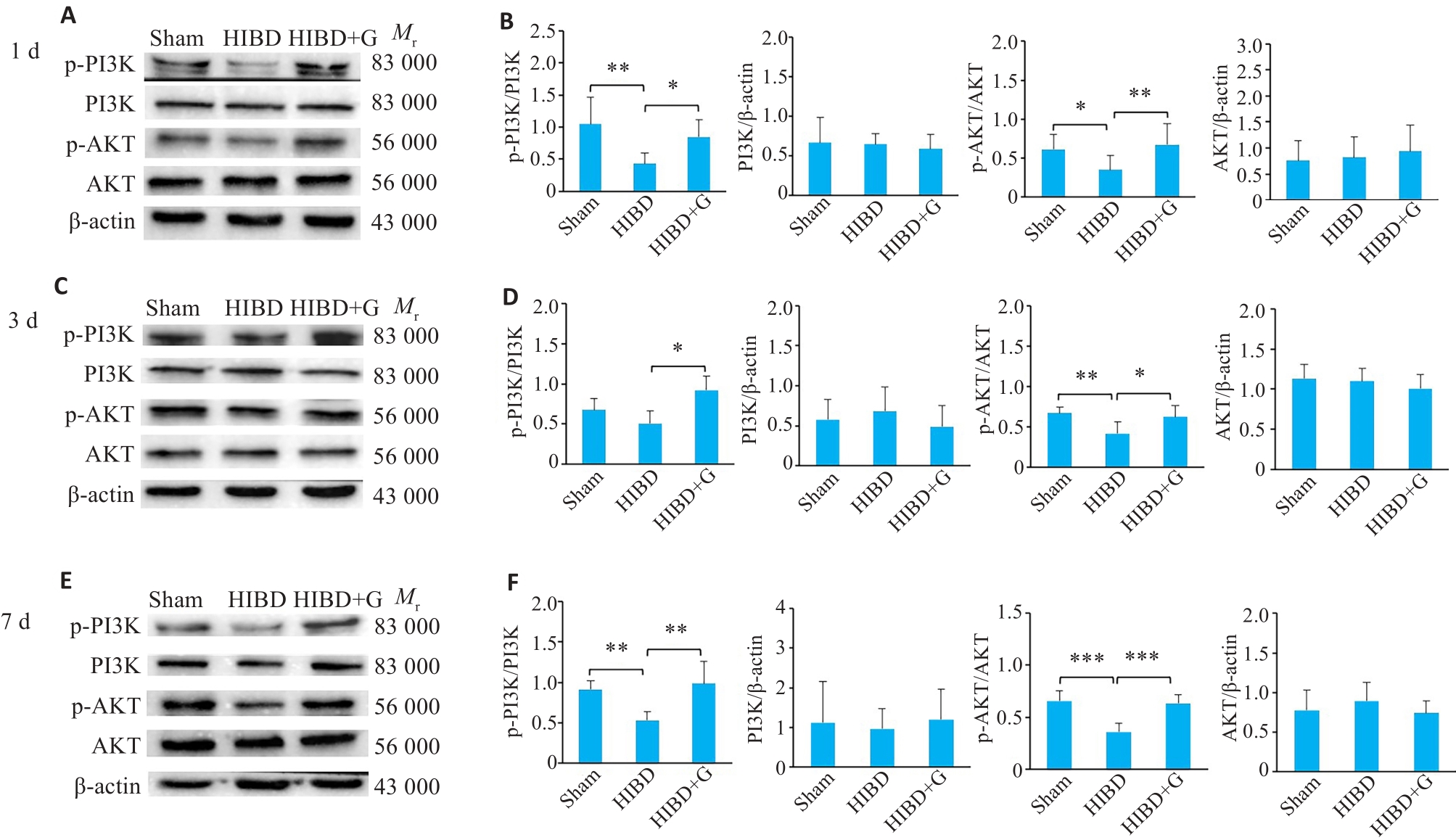
图5 蛋白印迹法检测HIBD后天麻素对活化小胶质细胞中PI3K/AKT信号通路表达的影响
Fig.5 Effect of gastrodin on expressions of PI3K/AKT signaling pathway in activated microglia of neonatal rats at 1 day, 3 days, and 7 days after HIBD detected by Western blotting. A, C, E: Western blots of the related proteins in PI3K/AKT signaling pathway. B, D, F: Quantification of P-PI3K, PI3K, P-AKT, and AKT protein levels. *P<0.05, **P<0.01, ***P<0.001 (n=3).
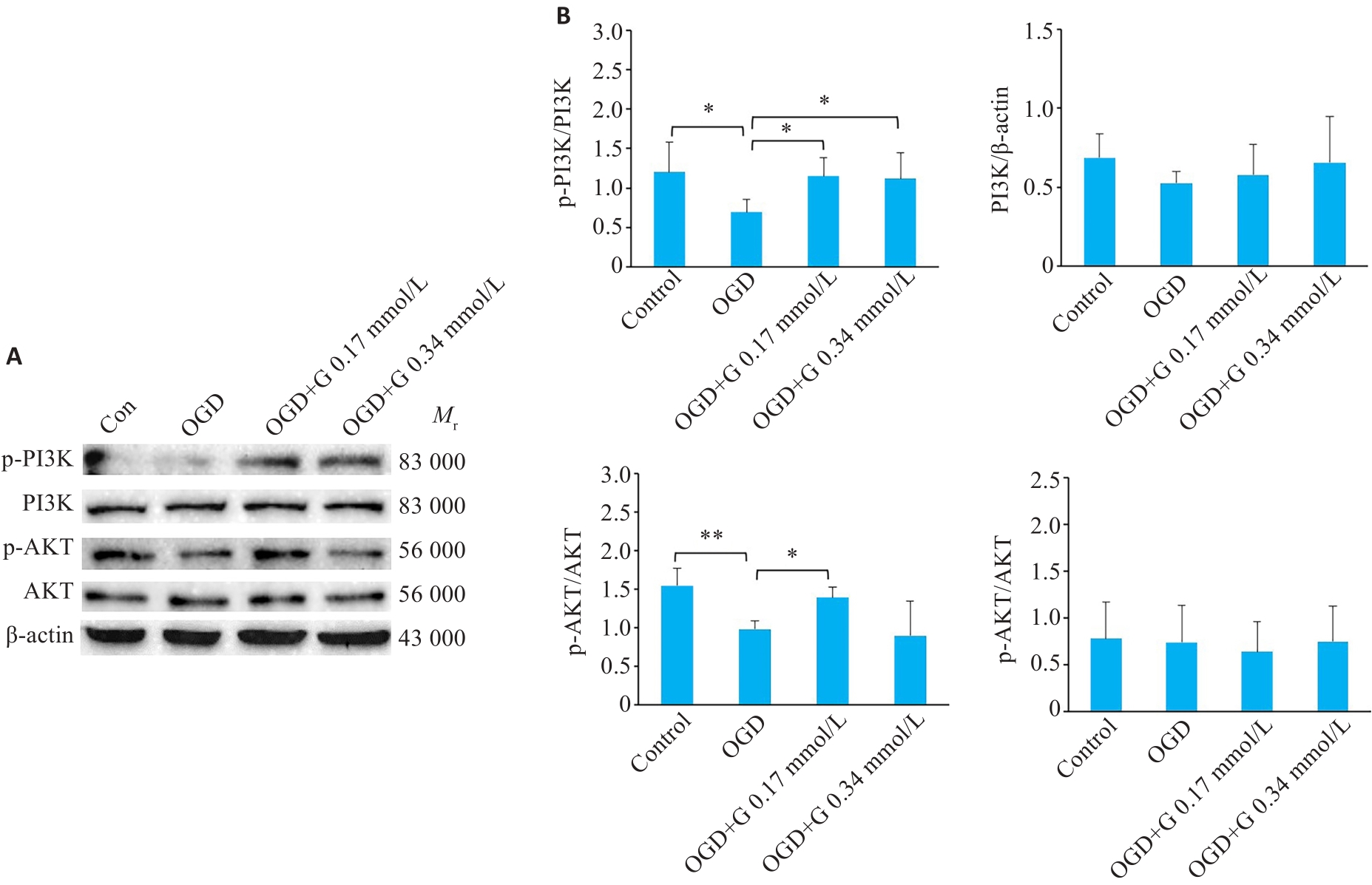
图6 蛋白印迹法检测OGD后天麻素对活化小胶质细胞中PI3K/AKT信号通路表达的影响
Fig.6 Effect of gastrodin on expression of PI3K/AKT signaling pathway in activated microglia BV-2 cells after OGD detected by western blotting. A: Western blots of the related proteins in PI3K/AKT signaling pathway in BV-2 cells. B: Quantification of P-PI3K, PI3K, P-AKT, and AKT protein levels. *P<0.05, **P<0.01 (n=3).
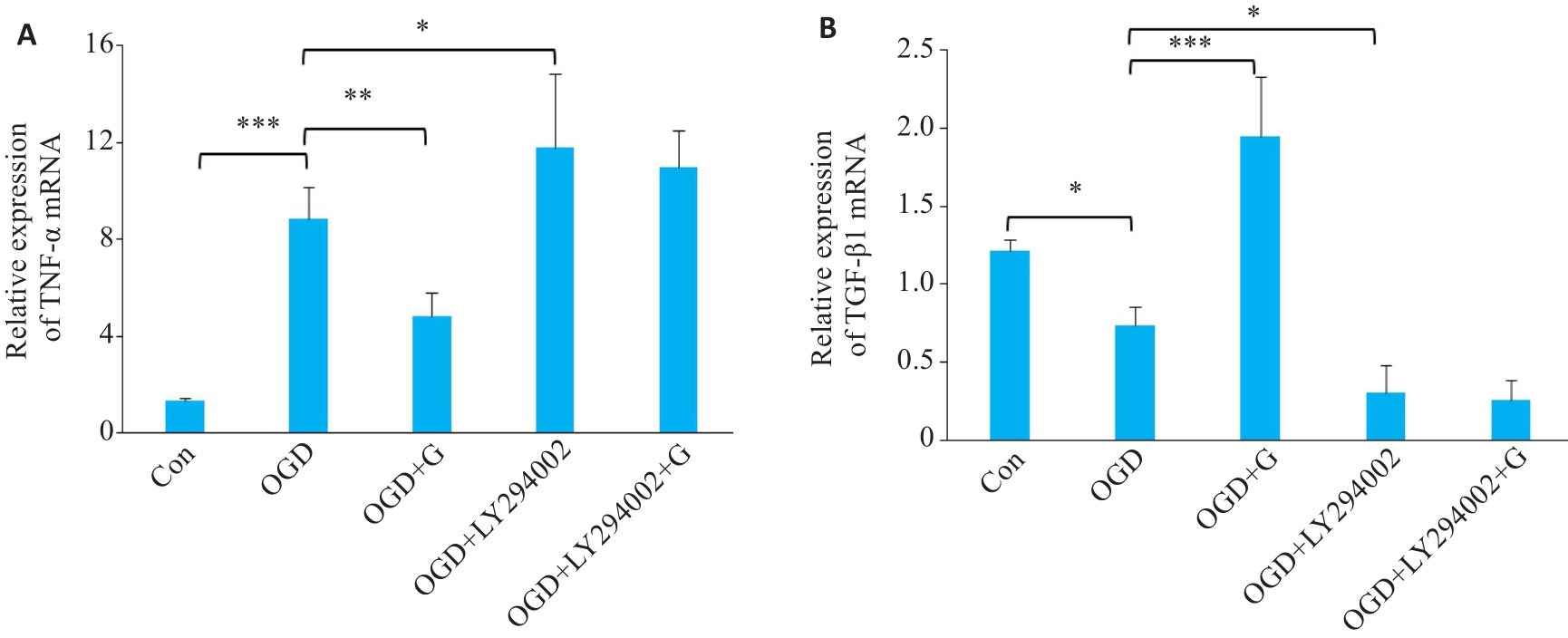
图8 RT-qPCR检测OGD后天麻素通过PI3K/AKT信号通路对活化小胶质细胞中炎症因子TNF-α和转化生长因子TGF-β1 mRNA水平表达变化的影响
Fig.8 Effect of gastrodin and LY294002 on TNF-α (A) and TGF-β1 mRNA (B) expressions in BV-2 cells with OGD detected by RT-qPCR. *P<0.05, **P<0.01, ***P<0.001 (n=3).
| 1 | Le K, Song ZP, Deng J, et al. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation[J]. Inflamm Res, 2020, 69(12): 1201-13. |
| 2 | Lawn JE, Blencowe H, Oza S, et al. Every Newborn: progress, priorities, and potential beyond survival[J]. Lancet, 2014, 384(9938): 189-205. |
| 3 | Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician[J]. JAMA Pediatr, 2015, 169(4): 397-403. |
| 4 | Zalewska T, Jaworska J, Ziemka-Nalecz M. Current and experimental pharmacological approaches in neonatal hypoxic- ischemic encephalopathy[J]. Curr Pharm Des, 2015, 21(11): 1433-9. |
| 5 | Gunn AJ, Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy[J]. Handb Clin Neurol, 2019, 162: 217-37. |
| 6 | Wassink G, Davidson JO, Dhillon SK, et al. Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy[J]. Curr Neurol Neurosci Rep, 2019, 19(2): 2. |
| 7 | Wood T, Osredkar D, Puchades M, et al. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia[J]. Sci Rep, 2016, 6: 23430. |
| 8 | Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial[J]. Pediatrics, 2016, 137(6): e20160191. |
| 9 | Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury[J]. Nat Rev Neurol, 2015, 11(4): 192-208. |
| 10 | Deng WB. Neurobiology of injury to the developing brain[J]. Nat Rev Neurol, 2010, 6(6): 328-36. |
| 11 | Zhou DD, Ji L, Chen YG. TSPO modulates IL-4-induced microglia/macrophage M2 polarization via PPAR‑γ pathway[J]. J Mol Neurosci, 2020, 70(4): 542-9. |
| 12 | Kim HJ, Moon KD, Lee DS, et al. Ethyl ether fraction of Gastrodia elata Blume protects amyloid beta peptide-induced cell death[J]. J Ethnopharmacol, 2003, 84(1): 95-8. |
| 13 | Kim HJ, Moon KD, Oh SY, et al. Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus[J]. Neurosci Lett, 2001, 314(1/2): 65-8. |
| 14 | Zeng XH, Zhang SM, Zhang L, et al. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro [J]. Planta Med, 2006, 72(15): 1359-65. |
| 15 | Deng CJ, Chen HZ, Meng ZY, et al. Gastrodin and vascular dementia: advances and current perspectives[J]. Evid Based Complement Alternat Med, 2022: 2563934. |
| 16 | Liu Y, Gao JL, Peng M, et al. A review on central nervous system effects of gastrodin[J]. Front Pharmacol, 2018, 9: 24. |
| 17 | Zhang JQ, Li LY, Liu Q, et al. Gastrodin programs an Arg-1+ microglial phenotype in hippocampus to ameliorate depression- and anxiety-like behaviors via the Nrf2 pathway in mice[J]. Phytomedicine, 2023, 113: 154725. |
| 18 | Guo J, Zhang XL, Bao ZR, et al. Gastrodin regulates the Notch signaling pathway and Sirt3 in activated microglia in cerebral hypoxic-ischemia neonatal rats and in activated BV-2 microglia[J]. Neuromolecular Med, 2021, 23(3): 348-62. |
| 19 | Zhu M, Li D, Wu Y, et al. TREM-2 promotes macrophage-mediated eradication of Pseudomonas aeruginosa via a PI3K/Akt pathway[J]. Scand J Immunol, 2014, 79(3): 187-96. |
| 20 | Lv MR, Li B, Wang MG, et al. RETRACTED: activation of the PI3K-Akt pathway promotes neuroprotection of the δ‑opioid receptor agonist against cerebral ischemia-reperfusion injury in rat models[J]. Biomedecine Pharmacother, 2017, 93: 230-7. |
| 21 | Tu XK, Zhang HB, Shi SS, et al. 5-LOX inhibitor zileuton reduces inflammatory reaction and ischemic brain damage through the activation of PI3K/akt signaling pathway[J]. Neurochem Res, 2016, 41(10): 2779-87. |
| 22 | Liu SJ, Liu XY, Li JH, et al. Gastrodin attenuates microglia activation through renin-angiotensin system and Sirtuin3 pathway[J]. Neurochem Int, 2018, 120: 49-63. |
| 23 | Chen Z, Hu Y, Lu RF, et al. MicroRNA-374a-5p inhibits neuroinflammation in neonatal hypoxic-ischemic encephalopathy via regulating NLRP3 inflammasome targeted Smad6[J]. Life Sci, 2020, 252: 117664. |
| 24 | Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury[J]. Br J Pharmacol, 2016, 173(4): 692-702. |
| 25 | Shrivastava SK, Dalko E, Delcroix-Genete D, et al. Uptake of parasite-derived vesicles by astrocytes and microglial phagocytosis of infected erythrocytes may drive neuroinflammation in cerebral malaria[J]. Glia, 2017, 65(1): 75-92. |
| 26 | Nayak D, Roth TL, McGavern DB. Microglia development and function[J]. Annu Rev Immunol, 2014, 32: 367-402. |
| 27 | Lalancette-Hébert M, Gowing G, Simard A, et al. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain[J]. J Neurosci, 2007, 27(10): 2596-605. |
| 28 | Tsuji S, di Martino E, Mukai T, et al. Aggravated brain injury after neonatal hypoxic ischemia in microglia-depleted mice[J]. J Neuroinflammation, 2020, 17(1): 111. |
| 29 | Serdar M, Kempe K, Rizazad M, et al. Early pro-inflammatory microglia activation after inflammation-sensitized hypoxic-ischemic brain injury in neonatal rats[J]. Front Cell Neurosci, 2019, 13: 237. |
| 30 | Dai JN, Zong Y, Zhong LM, et al. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways[J]. PLoS One, 2011, 6(7): e21891. |
| 31 | Peng Z, Wang H, Zhang R, et al. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder[J]. Physiol Res, 2013, 62(5): 537-45. |
| 32 | Jafari M, Ghadami E, Dadkhah T, et al. PI3k/AKT signaling pathway: erythropoiesis and beyond[J]. J Cell Physiol, 2019, 234(3): 2373-85. |
| 33 | Wang JT, Ma WM, Liu YD. Long non-coding RNA HULC promotes bladder cancer cells proliferation but inhibits apoptosis via regulation of ZIC2 and PI3K/AKT signaling pathway[J]. Cancer Biomark, 2017, 20(4): 425-34. |
| 34 | Choudhury R, Bonacci T, Wang XX, et al. The E3 ubiquitin ligase SCF(cyclin F) transmits AKT signaling to the cell-cycle machinery[J]. Cell Rep, 2017, 20(13): 3212-22. |
| 35 | Xian MH, Cai JL, Zheng KN, et al. Aloe-emodin prevents nerve injury and neuroinflammation caused by ischemic stroke via the PI3K/AKT/mTOR and NF-κB pathway[J]. Food Funct, 2021, 12(17): 8056-67. |
| 36 | Wang XS, Tian Z, Zhang N, et al. Protective effects of gastrodin against autophagy-mediated astrocyte death[J]. Phytother Res, 2016, 30(3): 386-96. |
| [1] | 周雪利, 李华, 陈青宇, 靳美娜, 李海波, 白炜, 贾楚璇, 魏翠英. 慢性间歇低氧和复氧对大鼠胰岛素抵抗及骨骼肌miR-27a-3p/PPARγ/IRS1/PI3K/AKT表达的影响[J]. 南方医科大学学报, 2024, 44(9): 1729-1737. |
| [2] | 张先恒, 刘健, 韩琦, 陈一鸣, 丁香, 陈晓露. 黄芩清热除痹胶囊通过PTEN/PI3K/AKT信号通路改善痛风性关节炎大鼠的炎症反应及尿酸、脂质代谢失衡[J]. 南方医科大学学报, 2024, 44(8): 1450-1458. |
| [3] | 耿志军, 杨晶晶, 牛民主, 刘馨悦, 施金冉, 刘亦珂, 姚新宇, 张雨路, 张小凤, 胡建国. 桑黄酮G通过调控PI3K/AKT/mTOR通路抑制胃癌细胞的生长、迁移和侵袭[J]. 南方医科大学学报, 2024, 44(8): 1476-1484. |
| [4] | 刘硕, 李静, 吴兴旺. Swertiamarin通过抑制肠上皮细胞细胞凋亡改善TNBS诱导的实验性结肠炎[J]. 南方医科大学学报, 2024, 44(8): 1545-1552. |
| [5] | 向珊, 张宗星, 江露, 刘道忠, 李玮怡, 包卓玛, 田瑞, 陈丹, 袁林. 三百棒通过调控PI3K/Akt信号通路改善胶原诱导性类风湿性关节炎大鼠的血管翳[J]. 南方医科大学学报, 2024, 44(8): 1582-1588. |
| [6] | 程瑶, 王远迎, 姚飞扬, 胡盼, 陈铭勰, 吴宁. 黄芩苷通过调控PI3K/AKT信号通路抑制登革病毒感染诱导的人静脉内皮细胞的自噬[J]. 南方医科大学学报, 2024, 44(7): 1272-1283. |
| [7] | 房锦存, 刘立威, 林俊豪, 陈逢生. CDHR2过表达通过抑制PI3K/Akt通路抑制乳腺癌细胞增殖[J]. 南方医科大学学报, 2024, 44(6): 1117-1125. |
| [8] | 王妍, 阮毓卿, 崔璨, 王秀. 交泰丸通过激活PI3K/AKT信号通路改善阿尔茨海默病模型小鼠大脑的葡萄糖代谢[J]. 南方医科大学学报, 2024, 44(5): 894-903. |
| [9] | 王媛媛, 陈腾, 从小凡, 李依然, 陈蕊, 张配, 孙小锦, 赵素容. 扁蒴藤素通过活性氧调控PI3K/AKT通路增强顺铂诱导鼻咽癌细胞凋亡[J]. 南方医科大学学报, 2024, 44(5): 904-912. |
| [10] | 夏勇生, 王炼, 陈孝华, 张雨路, 孙奥飞, 陈德利. 过表达TSR2通过下调PI3K/AKT信号通路抑制胃癌细胞的增殖和侵袭[J]. 南方医科大学学报, 2024, 44(5): 913-919. |
| [11] | 何 程, 陈 炜, 张念志, 栾 军, 王三凤, 张 尤. 参七虫草方通过ASS1/src/STAT3信号通路改善肺纤维化大鼠的炎症反应[J]. 南方医科大学学报, 2024, 44(4): 644-651. |
| [12] | 包汉生, 王苏童, 吕穆杰, 王永成, 姜 萍, 李 晓. 激活α7nAchR促进肥胖小鼠的脂肪稳态和米色脂肪生成及产热作用[J]. 南方医科大学学报, 2024, 44(3): 499-506. |
| [13] | 吴广阳, 宋添力, 唐 浪, 王一民, 刘 绪, 黄 胜. 竹节参总皂苷缓解CCl4诱导的大鼠急性肝损伤:基于调控PI3K/Akt/NF-κB信号通路[J]. 南方医科大学学报, 2024, 44(2): 244-251. |
| [14] | 段 婷, 张 震, 施金冉, 肖林雨, 杨晶晶, 殷丽霞, 张小凤, 耿志军, 陆国玉. CPNE3在胃癌中高表达并与患者的预后不良相关[J]. 南方医科大学学报, 2024, 44(1): 129-137. |
| [15] | 孙晓鹏, 史 航, 张 磊, 刘 中, 李克威, 钱玲玲, 朱星宇, 杨康佳, 付 强, 丁 华. 外胚层间充质干细胞来源的外泌体通过控制炎症和氧化损伤减少M1型小胶质细胞并促进H2O2处理后PC12细胞的存活[J]. 南方医科大学学报, 2024, 44(1): 119-128. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||