南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (5): 894-903.doi: 10.12122/j.issn.1673-4254.2024.05.11
收稿日期:2023-11-20
出版日期:2024-05-20
发布日期:2024-06-06
通讯作者:
王秀
E-mail:1003857594@qq.com;wangxiuu@sina.com
作者简介:王 妍,在读硕士研究生,E-mail: 1003857594@qq.com
基金资助:
Yan WANG1,2( ), Yuqing RUAN1, Can CUI1, Xiu WANG1(
), Yuqing RUAN1, Can CUI1, Xiu WANG1( )
)
Received:2023-11-20
Online:2024-05-20
Published:2024-06-06
Contact:
Xiu WANG
E-mail:1003857594@qq.com;wangxiuu@sina.com
摘要:
目的 探讨交泰丸对阿尔茨海默病模型小鼠大脑胰岛素PI3K/AKT通路的影响。 方法 50只3月龄雄性APP/PS1双转基因小鼠随机分为5组:模型组、交泰丸低剂量组(2.1 g/kg)、交泰丸中剂量组(4.2 g/kg)、交泰丸高剂量组(8.4 g/kg)、多奈哌齐组(3 mg/kg),C57BL/6J雄性小鼠为正常组,10只/组。给药4周后,采用水迷宫和旷场实验评估各组小鼠学习记忆能力,采用HE染色、尼氏染色观察小鼠脑组织神经元病理改变,免疫组化法观察小鼠脑组织Aβ淀粉样斑块沉积情况,ELISA试剂盒测定小鼠空腹血清胰岛素水平,采用Western blot和RT-qPCR检测小鼠脑组织中Aβ42、胰岛素PI3K/AKT通路以及下游GLUTs等相关指标蛋白和mRNA表达水平。 结果 与正常组小鼠比较,模型组小鼠学习记忆能力受损,小鼠海马神经元细胞数量减少,排列稀疏,小鼠脑组织Aβ淀粉样斑块沉积显著增多(P<0.001),Aβ42蛋白表达水平升高(P<0.05),胰岛素抵抗指数升高(P<0.001),小鼠海马PI3K蛋白以及mRNA表达均降低(P<0.01),海马与皮质AKT蛋白表达(P<0.05)、AKT mRNA表达(P<0.001)、InR蛋白表达(P<0.05),GLUT1、GLUT3、GLUT4蛋白表达(P<0.05)及mRNA表达(P<0.001)均降低,GSK3β蛋白表达(P<0.01)及GSK3β mRNA表达(P<0.001)显著升高。与模型组比较,交泰丸各组和多奈哌齐组小鼠记忆能力明显改善,尤其是交泰丸中剂量组,小鼠海马神经元细胞数量增多,小鼠海马、皮质Aβ淀粉样斑块沉积减少(P<0.01),Aβ42蛋白表达下调(P<0.001),胰岛素抵抗指数降低(P<0.001),小鼠海马、皮质PI3K、AKT、InR、GLUT1、GLUT3、GLUT4蛋白(P<0.05)及mRNA(P<0.01)表达不同程度升高,GSK3β蛋白及mRNA表达降低。 结论 交泰丸可通过激活APP/PS1双转基因小鼠大脑胰岛素PI3K/AKT通路,调控其下游GLUTs表达水平,提高大脑葡萄糖代谢能力,改善小鼠学习记忆能力,延缓阿尔茨海默病发展进程。
王妍, 阮毓卿, 崔璨, 王秀. 交泰丸通过激活PI3K/AKT信号通路改善阿尔茨海默病模型小鼠大脑的葡萄糖代谢[J]. 南方医科大学学报, 2024, 44(5): 894-903.
Yan WANG, Yuqing RUAN, Can CUI, Xiu WANG. Jiaotaiwan improves brain glucose metabolism in a mouse model of Alzheimer's disease by activating the PI3K/AKT signaling pathway[J]. Journal of Southern Medical University, 2024, 44(5): 894-903.
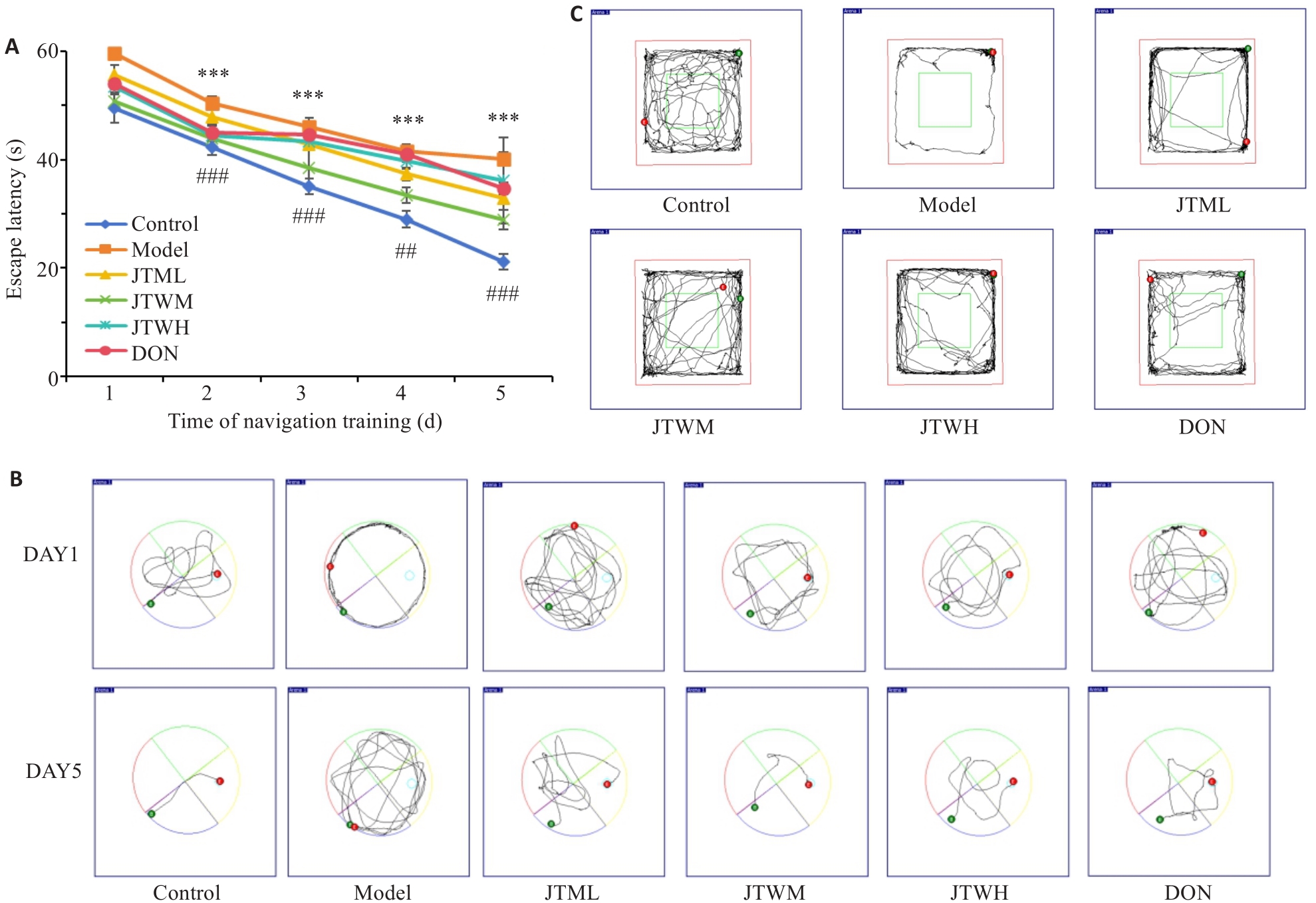
图1 各组小鼠Morris水迷宫实验以及旷场实验结果比较
Fig.1 Results of Morris water maze test and open field test. A: Comparison of escape latency of the mice among the groups (Mean±SD, n=8). B: Swimming trajectories during escape latency in each group. C: Open field behavior trajectories of the mice in each group. ***P<0.001 vs Control group; ##P<0.01, ###P<0.001 vs model group.
| Group | Times of platform crossing | Time of first platform crossing (s) | Time in platform quadrant (s) | Number of central area entry | Time in the central zone (s) |
|---|---|---|---|---|---|
| Control | 4.25±0.83 | 24.17±2.72 | 31.68±2.56 | 14.88±1.45 | 32.91±1.78 |
| Model | 1.63±0.70*** | 37.69±2.65*** | 20.11±1.99*** | 6.25±1.85*** | 16.21±1.28*** |
| JTWL | 3.13±1.05# | 32.99±2.25## | 23.21±2.81 | 9.00±1.41# | 18.10±2.56 |
| JTWM | 4.00±0.71### | 28.12±1.05### | 26.89±3.00### | 12.25±1.85### | 20.91±1.99### |
| JTWH | 3.25±0.83# | 31.27±2.02### | 25.52±2.25## | 10.63±1.58### | 19.77±2.02# |
| DON | 3.38±0.99## | 33.71±1.41# | 21.01±2.16 | 9.75±1.98## | 19.9±1.66# |
表1 各组小鼠水迷宫空间探索实验以及旷场实验比较
Tab.1 Results of water maze spatial exploration test and open field test in each group of mice (Mean±SD, n=8)
| Group | Times of platform crossing | Time of first platform crossing (s) | Time in platform quadrant (s) | Number of central area entry | Time in the central zone (s) |
|---|---|---|---|---|---|
| Control | 4.25±0.83 | 24.17±2.72 | 31.68±2.56 | 14.88±1.45 | 32.91±1.78 |
| Model | 1.63±0.70*** | 37.69±2.65*** | 20.11±1.99*** | 6.25±1.85*** | 16.21±1.28*** |
| JTWL | 3.13±1.05# | 32.99±2.25## | 23.21±2.81 | 9.00±1.41# | 18.10±2.56 |
| JTWM | 4.00±0.71### | 28.12±1.05### | 26.89±3.00### | 12.25±1.85### | 20.91±1.99### |
| JTWH | 3.25±0.83# | 31.27±2.02### | 25.52±2.25## | 10.63±1.58### | 19.77±2.02# |
| DON | 3.38±0.99## | 33.71±1.41# | 21.01±2.16 | 9.75±1.98## | 19.9±1.66# |
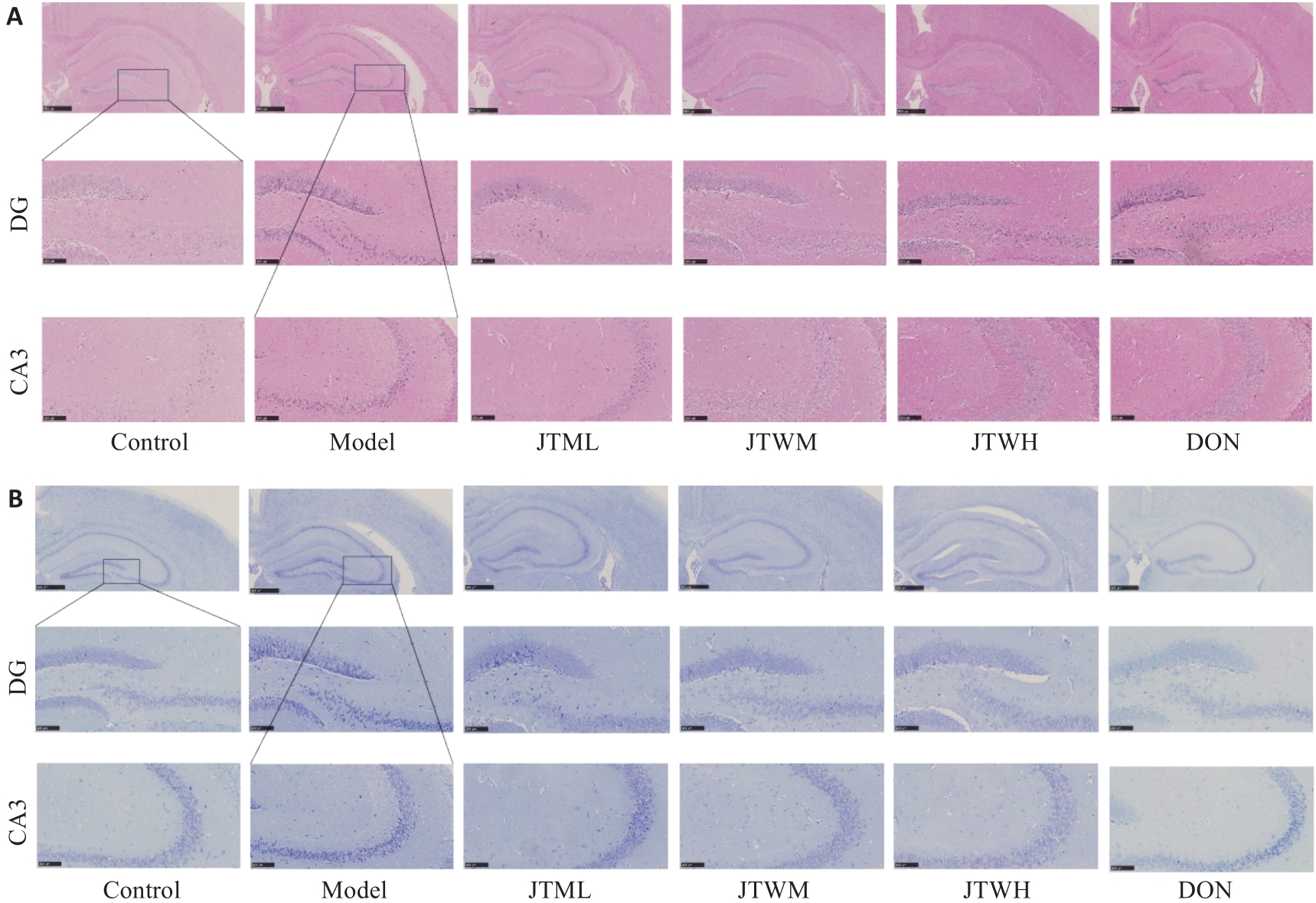
图2 交泰丸对各组小鼠海马神经元形态的影响
Fig.2 Effect of Jiaotaiwan on hippocampal neuron morphology in each group. A: Representative HE staining results of the hippocampus in each group. B: Nissl staining of the hippocampus in each group (scale bar=100 μm).
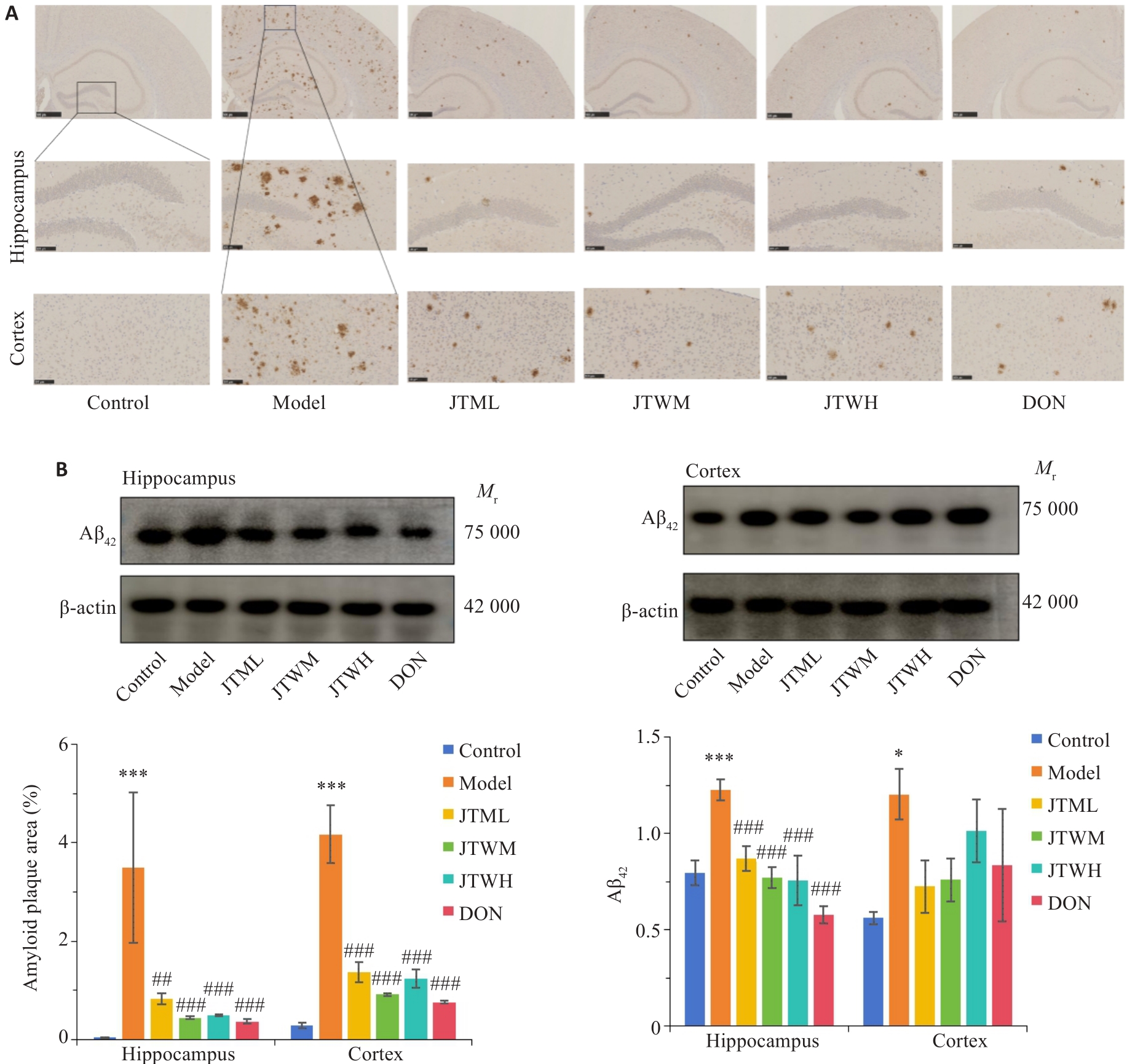
图3 交泰丸对各组小鼠海马、皮质Aβ斑块聚集和Aβ42含量的影响
Fig.3 Impact of Jiaotaiwan on aggregation of Aβ plaques and Aβ42 level in the hippocampus and cortex of the mice (Mean±SD, n=4). A: Immunohistochemistry showing Aβ plaques in the hippocampus and cortex (scale bar=100 μm. B: Quantification of Aβ42 protein expressions in the hippocampus and cortex using Western blotting. *P<0.05,***P<0.001 vs Control group; ##P<0.01,###P<0.001 vs Model group.
| Group | Fasting glucose (mmol/L) | Fasting insulin (mIU/L) | HOMA-IR |
|---|---|---|---|
| Control | 5.76±0.57 | 5.54±0.90 | 1.40±0.17 |
| Model | 11.68±1.34*** | 16.41±1.43*** | 8.52±1.24*** |
| JTWL | 8.98±2.44 | 13.98±1.16 | 5.57±1.53### |
| JTWM | 7.51±1.41## | 12.00±1.61### | 3.97±0.72### |
| JTWH | 9.60±3.71 | 13.43±3.07# | 5.29±1.12### |
| DON | 7.40±1.34## | 12.85±1.25### | 4.19±0.69### |
表2 交泰丸对各组小鼠胰岛素抵抗的影响比较
Tab.2 Effects of Jiaotaiwan on insulin resistance of the mice in each group (Mean±SD)
| Group | Fasting glucose (mmol/L) | Fasting insulin (mIU/L) | HOMA-IR |
|---|---|---|---|
| Control | 5.76±0.57 | 5.54±0.90 | 1.40±0.17 |
| Model | 11.68±1.34*** | 16.41±1.43*** | 8.52±1.24*** |
| JTWL | 8.98±2.44 | 13.98±1.16 | 5.57±1.53### |
| JTWM | 7.51±1.41## | 12.00±1.61### | 3.97±0.72### |
| JTWH | 9.60±3.71 | 13.43±3.07# | 5.29±1.12### |
| DON | 7.40±1.34## | 12.85±1.25### | 4.19±0.69### |
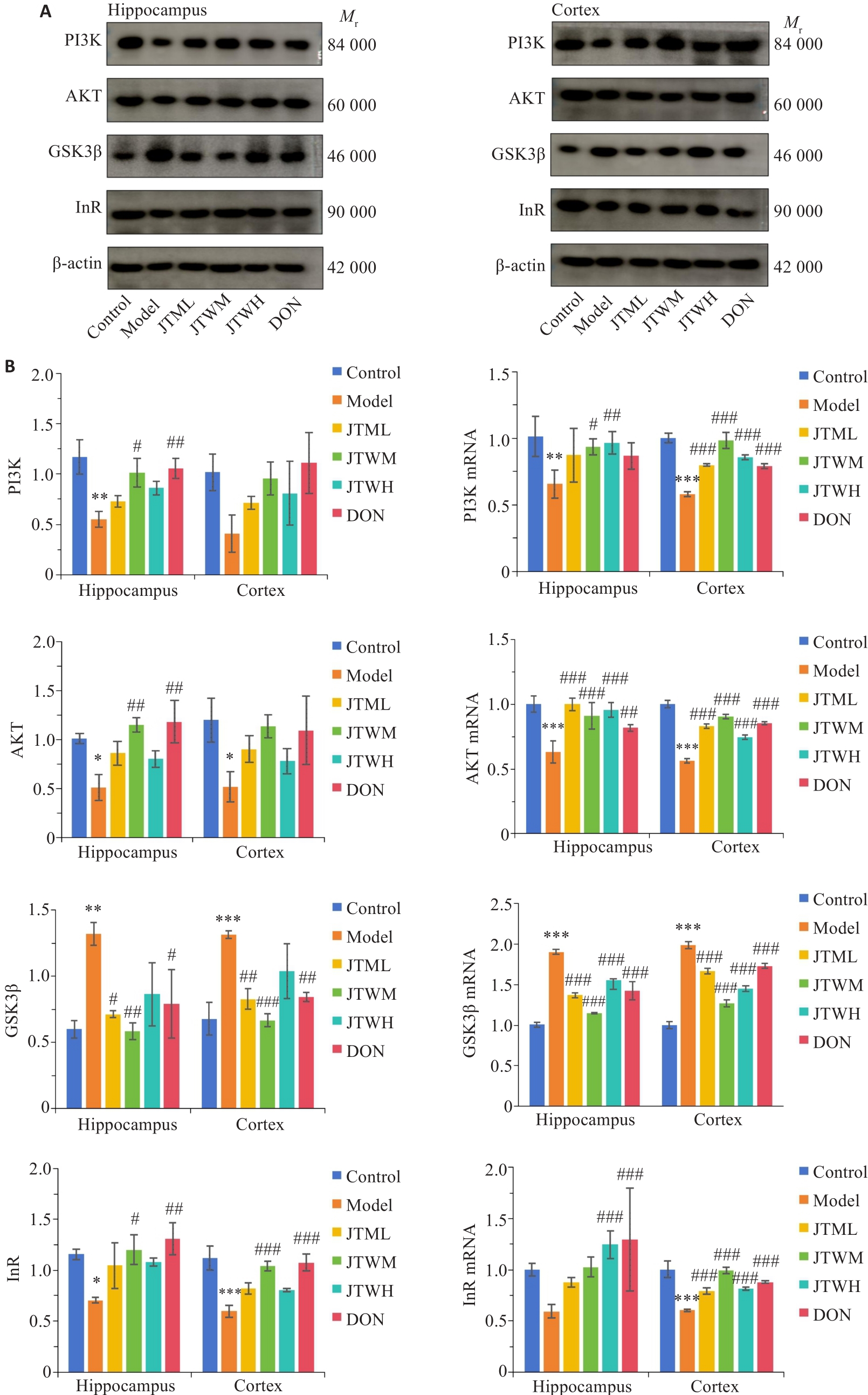
图4 交泰丸对各组小鼠海马、皮质胰岛素PI3K/AKT通路相关指标的影响
Fig.4 Effects of Jiaotaiwan on insulin-PI3K/AKT pathway in the hippocampus and cortex of the mice in each group. A: Western blots of the proteins in the insulin-PI3K/AKT pathway in the hippocampus and cortex in each group. B: Comparison of PI3K, AKT, GSK3β, and InR protein and mRNA expressions in the hippocampus and cortex among the groups (Mean±SD).*P<0.05,**P<0.01,***P<0.001 vs Control group; #P<0.05,##P<0.01,###P<0.001 vs Model group.
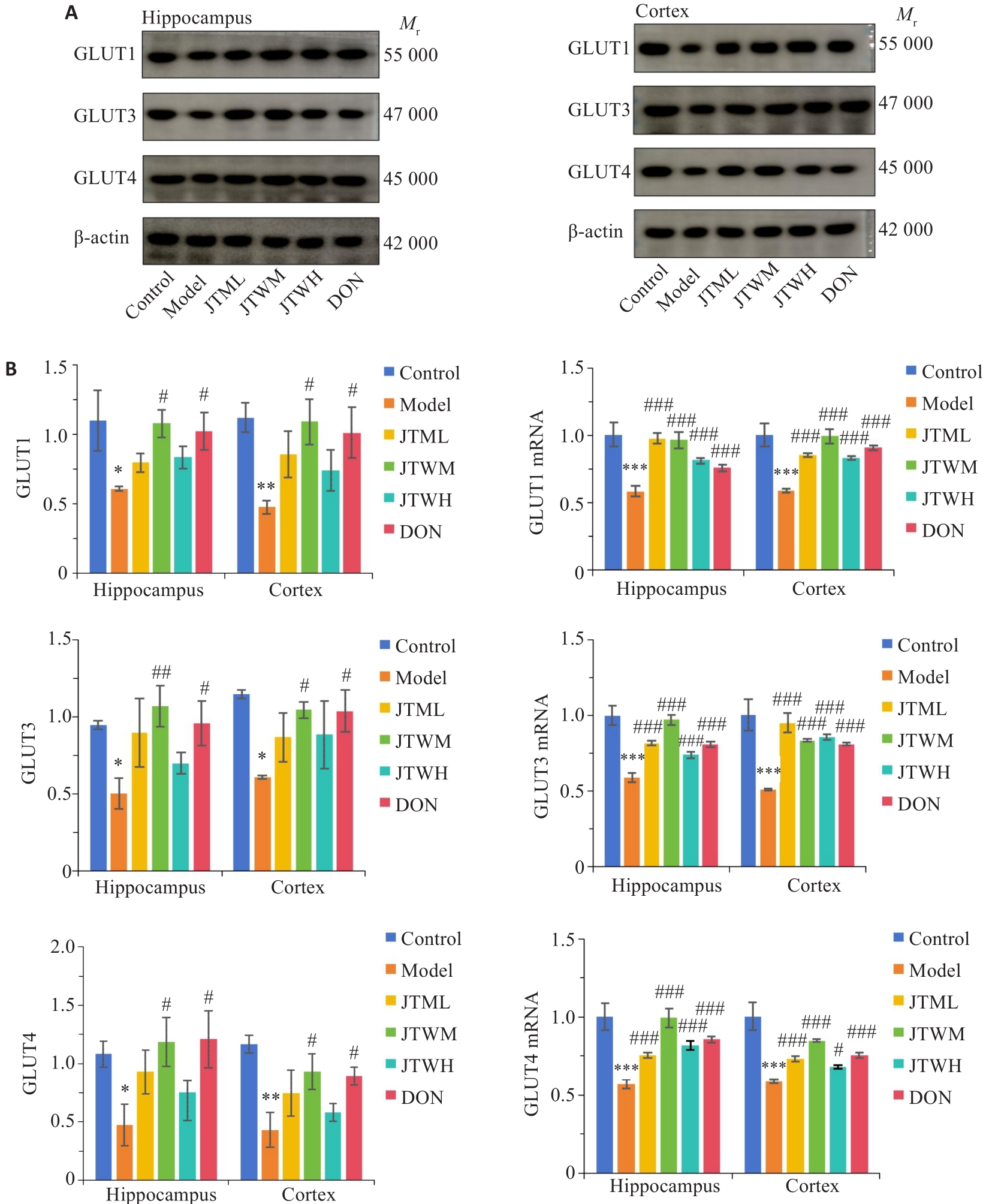
图5 交泰丸对各组小鼠海马、皮质葡萄糖转运相关指标的影响
Fig.5 Effects of Jiaotaiwan on glucose transport in the hippocampus and cortex of the mice in each group. A: Western blots of the proteins involved in glucose transport in the hippocampus and cortex in each group. B: Expression levels of GLUT1, GLUT3, and GLUT4 proteins and mRNAs in the hippocampus and cortex in each group (Mean±SD).*P<0.05, **P<0.01, ***P<0.001 vs Control group; #P<0.05, ##P<0.01, ###P<0.001 vs Model group.
| 1 | Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches[J]. Lancet Neurol, 2020, 19(9): 758-66. DOI: 10.1016/s1474-4422(20)30231-3 |
| 2 | Ou ZH, Deng LL, Lu Z, et al. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease[J]. Nutr Diabetes, 2020, 10(1): 12. DOI: 10.1038/s41387-020-0115-8 |
| 3 | Macklin L, Griffith CM, Cai Y, et al. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline[J]. Exp Gerontol, 2017, 88: 9-18. DOI: 10.1016/j.exger.2016.12.019 |
| 4 | Teipel SJ, Drzezga A, Bartenstein P, et al. Effects of donepezil on cortical metabolic response to activation during (18)FDG-PET in Alzheimer's disease: a double-blind cross-over trial[J]. Psychopharmacology, 2006, 187(1): 86-94. DOI: 10.1007/s00213-006-0408-1 |
| 5 | Chen F, He YK, Wang PW, et al. Banxia Xiexin Decoction ameliorated cognition via the regulation of insulin pathways and glucose transporters in the hippocampus of APPswe/PS1dE9 mice[J]. Int J Immunopathol Pharmacol, 2018, 32: 2058738418780066. DOI: 10.1177/2058738418780066 |
| 6 | 袁 琳, 李慧姣, 胡 娜, 等. 交泰丸不同配比组方降糖作用及相关机制探讨[J]. 中国实验方剂学杂志, 2017, 23(8): 130-7. |
| 7 | Boccardi V, Murasecco I, Mecocci P. Diabetes drugs in the fight against Alzheimer's disease[J]. Ageing Res Rev, 2019, 54: 100936. DOI: 10.1016/j.arr.2019.100936 |
| 8 | Blázquez E, Hurtado-Carneiro V, LeBaut-Ayuso Y, et al. Significance of brain glucose hypometabolism, altered insulin signal transduction, and insulin resistance in several neurological diseases[J]. Front Endocrinol, 2022, 13: 873301. DOI: 10.3389/fendo.2022.873301 |
| 9 | Yan F, Liu J, Chen MX, et al. Icariin ameliorates memory deficits through regulating brain insulin signaling and glucose transporters in 3 × Tg-AD mice[J]. Neural Regen Res, 2023, 18(1): 183-8. DOI: 10.4103/1673-5374.344840 |
| 10 | Davidy T, Yore I, Cukierman-Yaffe T, et al. A feasibility study of the combination of intranasal insulin with dulaglutide for cognition in older adults with metabolic syndrome at high dementia risk‑Study rationale and design[J]. Mech Ageing Dev, 2023, 213: 111825. DOI: 10.1016/j.mad.2024.111937 |
| 11 | 张韶君, 高江琴, 邱贵娟, 等. 黄连素通过调节磷脂酰肌醇-3-激酶/丝氨酸/苏氨酸激酶/葡萄糖转运蛋白4信号途径改善胰岛素抵抗的机制研究[J]. 中国药物与临床, 2017, 17(11): 1569-71. |
| 12 | Gomaa AA, Makboul RM, El-Mokhtar MA, et al. Evaluation of the neuroprotective effect of donepezil in type 2 diabetic rats[J]. Fundam Clin Pharmacol, 2021, 35(1): 97-112. DOI: 10.1111/fcp.12585 |
| 13 | Hugon G, Goutal S, Sarazin M, et al. Impact of donepezil on brain glucose metabolism assessed using[18F]2-fluoro-2-deoxy-D-glucose positron emission tomography imaging in a mouse model of Alzheimer’s disease induced by intracerebroventricular injection of amyloid-beta peptide[J]. Front Neurosci, 2022, 16: 835577. DOI: 10.3389/fnins.2022.835577 |
| 14 | Shimada A, Hashimoto H, Kawabe J, et al. Evaluation of therapeutic response to donepezil by positron emission tomography[J]. Osaka City Med J, 2011, 57(1): 11-9. |
| 15 | 邓晓威, 谢 宁. 黄连素治疗2型糖尿病研究进展[J]. 中国中药杂志, 2014, 39(8): 1374-8. |
| 16 | Huang WY, Dong H. Coptidis rhizoma-contained traditional formulae for insomnia: a potential to prevent diabetes?[J]. Chin J Integr Med, 2018, 24(10): 785-8. DOI: 10.1007/s11655-018-3012-4 |
| 17 | 宋宗辉, 张艺雯, 王玲洁, 等. 肉桂醛的药理活性及其研究进展[J]. 解放军药学学报, 2018, 34(6): 550-4. |
| 18 | 卫克昭, 姚平安, 刘晓宁, 等. 肉桂对糖尿病性心肌病大鼠的心脏保护作用[J]. 上海中医药杂志, 2018, 52(7): 69-74. |
| 19 | 廖作庄, 徐灵源, 王金妮, 等. 肉桂多酚对链脲佐菌素致糖尿病小鼠的保护作用[J]. 西安交通大学学报: 医学版, 2019, 40(1): 162-6. |
| 20 | 王雪萍, 张皓月, 王 婷, 等. 交泰丸中小檗碱联合cinnamtannin D1的降血糖作用[J]. 中成药, 2018, 40(12): 2613-8. |
| 21 | Nguyen TT, Ta QTH, Nguyen TKO, et al. Type 3 diabetes and its role implications in Alzheimer's disease[J]. Int J Mol Sci, 2020, 21(9): 3165. DOI: 10.3390/ijms21093165 |
| 22 | Suresh J, Khor IW, Kaur P, et al. Shared signaling pathways in Alzheimer's and metabolic disease may point to new treatment approaches[J]. FEBS J, 2021, 288(12): 3855-73. DOI: 10.1111/febs.15540 |
| 23 | Sun YN, Ma C, Sun H, et al. Metabolism: a novel shared link between diabetes mellitus and Alzheimer's disease[J]. J Diabetes Res, 2020, 2020: 4981814. DOI: 10.1155/2020/4981814 |
| 24 | Alves SS, Servilha-Menezes G, Rossi L, et al. Evidence of disturbed insulin signaling in animal models of Alzheimer's disease[J]. Neurosci Biobehav Rev, 2023, 152: 105326. DOI: 10.1016/j.neubiorev.2023.105326 |
| 25 | Kim B, Elzinga SE, Henn RE, et al. The effects of insulin and insulin-like growth factor I on amyloid precursor protein phosphorylation in in vitro and in vivo models of Alzheimer's disease[J]. Neurobiol Dis, 2019, 132: 104541. DOI: 10.1016/j.nbd.2019.104541 |
| 26 | Zhang N, Liu XY, Zhuang LL, et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: dual regulation of the PI3K/AKT and MAPK pathways[J]. Regul Toxicol Pharmacol, 2020, 110: 104544. DOI: 10.1016/j.yrtph.2019.104544 |
| 27 | Akhtar A, Sah SP. Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer's disease[J]. Neurochem Int, 2020, 135: 104707. DOI: 10.1016/j.neuint.2020.104707 |
| 28 | Wang L, Li JJ, Di LJ. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases[J]. Med Res Rev, 2022, 42(2): 946-82. DOI: 10.1002/med.21867 |
| 29 | Zhang F, Zhang ZL, Kong DS, et al. Tetramethylpyrazine reduces glucose and insulin-induced activation of hepatic stellate cells by inhibiting insulin receptor-mediated PI3K/AKT and ERK pathways[J]. Mol Cell Endocrinol, 2014, 382(1): 197-204. DOI: 10.1016/j.mce.2013.09.020 |
| 30 | Wadhwa P, Jain P, Jadhav HR. Glycogen synthase kinase 3 (GSK3): its role and inhibitors[J]. Curr Top Med Chem, 2020, 20(17): 1522-34. DOI: 10.2174/1568026620666200516153136 |
| 31 | Xu AP, Zeng QT, Tang YS, et al. Electroacupuncture protects cognition by regulating tau phosphorylation and glucose metabolism via the AKT/GSK3β signaling pathway in Alzheimer's disease model mice[J]. Front Neurosci, 2020, 14: 585476. DOI: 10.3389/fnins.2020.585476 |
| 32 | Yang W, Liu Y, Xu QQ, et al. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/akt/GSK-3β pathway in experimental models of Alzheimer's disease[J]. Oxid Med Cell Longev, 2020, 2020: 4754195. DOI: 10.1155/2020/4754195 |
| 33 | Biasibetti R, Almeida Dos Santos JP, Rodrigues L, et al. Hippocampal changes in STZ-model of Alzheimer's disease are dependent on sex[J]. Behav Brain Res, 2017, 316: 205-14. DOI: 10.1016/j.bbr.2016.08.057 |
| 34 | 李娟娥, 姜小帆. 交泰丸对糖尿病小鼠认知功能障碍的影响及机制[J]. 中国实验方剂学杂志, 2019, 25(17): 23-7. |
| 35 | 王 帅, 金 磊, 海春旭, 等. PI3K/Akt信号通路在胰岛素抵抗中作用的研究进展[J]. 毒理学杂志, 2015, 29(4): 313-6. |
| 36 | Agrawal R, Vieira-de-Abreu A, Durupt G, et al. Insulin regulates GLUT4 in the ventromedial hypothalamus to restore the sympathoadrenal response to hypoglycemia in diabetic rats[J]. Am J Physiol Endocrinol Metab, 2018, 315(6): E1286-95. DOI: 10.1152/ajpendo.00324.2018 |
| [1] | 王思飞, 齐永帅, 江 英, 池晓华, 黄 凯, 阮楚茵, 杨晓镪, 李贵平. Z-Score成像系统辅助脑血流灌注SPECT对早期阿尔茨海默病患者的诊断有较高价值[J]. 南方医科大学学报, 2021, 41(7): 1093-1100. |
| [2] | 薛继国, 刘 静, 耿 淼, 岳敬伟, 贺浩宸, 范 皎. 基于加权基因共表达网络分析阿尔茨海默病相关的核心基因[J]. 南方医科大学学报, 2021, 41(12): 1752-1762. |
| [3] | 方迎艳, 苏振宏, 司文霞, 刘圆呈, 李 洁, 曾 鹏. 白藜芦醇治疗阿尔茨海默病的作用机制:基于网络药理学方法[J]. 南方医科大学学报, 2021, 41(1): 10-19. |
| [4] | 李青峰,邢潇丹,冯前进. 基于耦合的卷积-图卷积神经网络的阿尔茨海默病的磁共振诊断方法[J]. 南方医科大学学报, 2020, 40(04): 531-537. |
| [5] | 张海英,刘亦恒,付媛,陈鹏程,陆睿,李剑星,陈明会,杨浩池,张雨生. 海马内注射PrPC抗体对APPswe/PSEN1dE9转基因小鼠学习和记忆的影响[J]. 南方医科大学学报, 2018, 38(04): 443-. |
| [6] | 罗静,赵艳,谢婧雯,刘鑫,林芳波,侯德仁. 敲低SORL1表达构建模拟阿尔茨海默病的细胞模型[J]. 南方医科大学学报, 2018, 38(01): 8-. |
| [7] | 李维,田怡,邓炎尧,奉夏露,王艳,冯辉,侯德仁. 阿尔茨海默病患者血清脂联素水平与认知功能的相关性[J]. 南方医科大学学报, 2017, 37(04): 542-. |
| [8] | 柴继侠,李徽徽,王元元,柴强,贺文欣,周艳梅,胡小冬,王震寰. 二烯丙基二硫对AD模型小鼠学习记忆能力和海马突触的影响[J]. 南方医科大学学报, 2016, 36(10): 1417-. |
| [9] | 何颜结,卫佩如,伍巧燕,张馨宇,张兴梅,刘晓加,王方. ApoE4在U87细胞中可增加GSK-3β的表达及Tau的磷酸化[J]. 南方医科大学学报, 2016, 36(07): 904-. |
| [10] | 邓锦凤,邓炎尧,李维,奉夏露,俞珠玲,赵艳,侯德仁. 血管紧张素转化酶基因位点多态性与阿尔茨海默病的相关性[J]. 南方医科大学学报, 2015, 35(09): 1325-. |
| [11] | 谭健,莫海兰,李洁,吴应玲,何晓丽,李兵. 慢性间歇性缺氧对大鼠骨骼肌葡萄糖转运蛋白4表达的影响[J]. 南方医科大学学报, 2014, 34(07): 1061-. |
| [12] | 邓炎尧,侯德仁,田密,李维,奉夏露. AD模型小鼠海马和小脑Aβ的沉积与相关miRNAs表达的变化[J]. 南方医科大学学报, 2014, 34(03): 323-. |
| [13] | 田密,侯德仁,邓炎尧,李维,奉夏露. STAT3与P-STAT3在转基因AD小鼠脑组织中的表达及意义[J]. 南方医科大学学报, 2013, 33(12): 1778-. |
| [14] | 孔卫娜,张佳,高维娟,刘清涛,周利明,柴锡庆. β淀粉样蛋白通过升高ROS水平上调晚期糖基化终末产物受体的表达[J]. 南方医科大学学报, 2013, 33(08): 1132-. |
| [15] | 田密,丁宇,侯德仁,邓炎尧,李维,奉夏露. 实时荧光定量PCR检测转基因阿尔茨海默病小鼠脑组织中miRNAs的差异表达[J]. 南方医科大学学报, 2013, 33(02): 262-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||