南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (12): 2404-2411.doi: 10.12122/j.issn.1673-4254.2024.12.17
• • 上一篇
匡红( ), 蔡文涵, 刘一鸣, 温佳新, 田硕, 薛志强(
), 蔡文涵, 刘一鸣, 温佳新, 田硕, 薛志强( )
)
收稿日期:2024-04-01
出版日期:2024-12-20
发布日期:2024-12-26
通讯作者:
薛志强
E-mail:jojomonica1@hotmail.com;xuezhiqiang301@126.com
作者简介:匡 红,硕士,主管护师,E-mail: jojomonica1@hotmail.com
基金资助:
Hong KUANG( ), Wenhan CAI, Yiming LIU, Jiaxin WEN, Shuo TIAN, Zhiqiang XUE(
), Wenhan CAI, Yiming LIU, Jiaxin WEN, Shuo TIAN, Zhiqiang XUE( )
)
Received:2024-04-01
Online:2024-12-20
Published:2024-12-26
Contact:
Zhiqiang XUE
E-mail:jojomonica1@hotmail.com;xuezhiqiang301@126.com
摘要:
目的 探讨细胞能量代谢中的葡萄糖转运蛋白SLC2A1对肺腺癌增殖和迁移能力的影响及其分子机制。 方法 分析TCGA数据库中SLC2A1基因在肺腺癌正常组织和肿瘤组织中的表达差异及其对预后的影响。收集我院临床肺腺癌患者的肿瘤组织和癌旁配对组织,进行免疫组化染色,分析SLC2A1蛋白在肿瘤中的表达水平。验证肺腺癌肿瘤组织和癌旁组织中SLC2A1蛋白的表达差异,探讨该蛋白与患者临床病理特征之间的关系。体外构建稳定过表达和稳定干预SLC2A1基因的细胞系,通过Western blotting实验和qRT-PCR实验验证干预效果。采用CCK-8和Transwell实验体外验证细胞表型,明确其对肿瘤细胞增殖、迁移能力的影响。检测干预SLC2A1后细胞的铁死亡和细胞自噬相关蛋白的表达水平,并通过ROS荧光染色、Fe2+荧光染色明确铁死亡的发生过程。 结果 与正常肺组织相比,SLC2A1在肺腺癌肿瘤组织中高表达(P<0.05),且与肺腺癌患者的病理参数及不良预后相关(P<0.05)。与对照组相比,过表达SLC2A1,细胞中该基因上调(P<0.05),细胞增殖活性增加(P<0.05),细胞侵袭和迁移能力增强(P<0.05)。干预SLC2A1基因表达后,细胞死亡比例增加(P<0.05),侵袭和增殖能力均被抑制(P<0.05)。干预SLC2A1激活了铁死亡,GPX4、xCT等铁死亡指标表达下调,细胞内ROS累积,Fe2+增多(P<0.05)。同时检测到细胞自噬的发生,LC3B增加,且这一过程可被3-MA挽救。 结论 SLC2A1在肿瘤组织中高表达且与不良预后有关。靶向抑制SLC2A1可以促进肺腺癌中的铁死亡和自噬发生,有望成为肺腺癌诊断和治疗的新潜在分子靶点。
匡红, 蔡文涵, 刘一鸣, 温佳新, 田硕, 薛志强. SLC2A1抑制肺腺癌铁死亡并促进肺腺癌细胞的增殖和侵袭[J]. 南方医科大学学报, 2024, 44(12): 2404-2411.
Hong KUANG, Wenhan CAI, Yiming LIU, Jiaxin WEN, Shuo TIAN, Zhiqiang XUE. High expression of SLC2A1 inhibits ferroptosis and promotes proliferation and invasion of lung adenocarcinoma cells[J]. Journal of Southern Medical University, 2024, 44(12): 2404-2411.
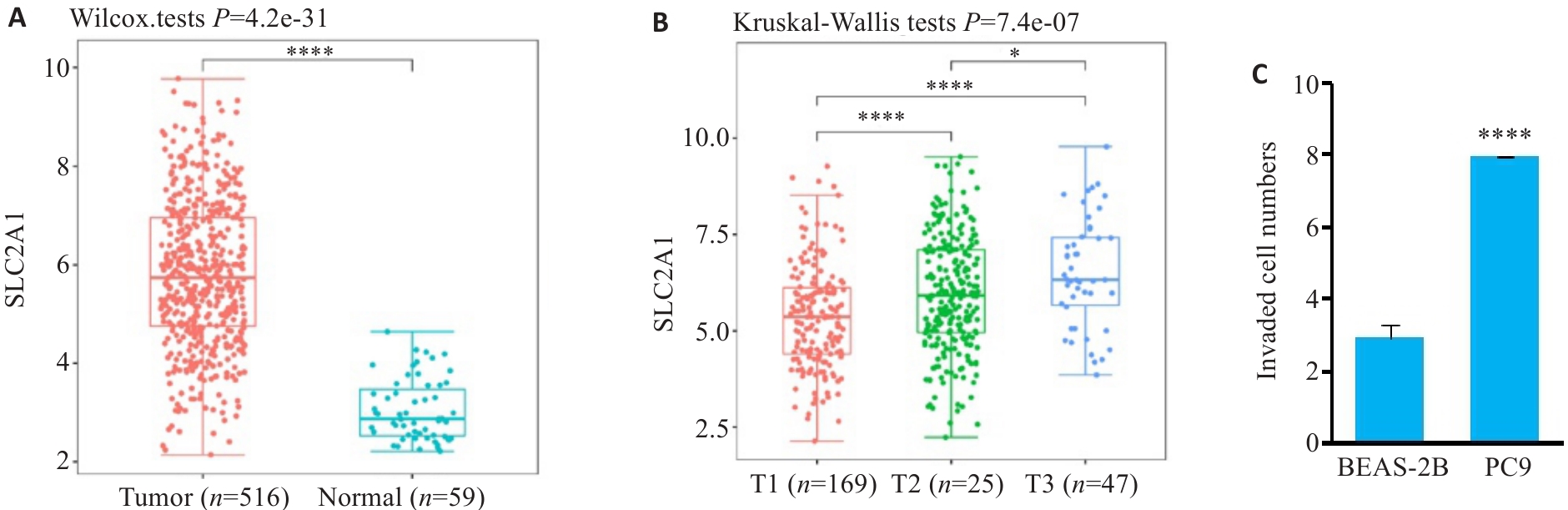
图1 SLC2A1在肺腺癌和正常组织表达情况
Fig.1 Expression of SLC2A1 in lung adenocarcinoma (LUAD) and normal lung tissues and cells. A: Expression of SLC2A1 in LUAD and normal lung tissue. B: Expression of SLC2A1 in LUAD patients with different T grades. C: Expression of SLC2A1 in PC9 and BEAS-2B cell lines. *P<0.05, ****P<0.0001.
| Clinicopathological feature | n | SLC2A1 Low | SLC2A1 High | P |
|---|---|---|---|---|
| Gender | 0.0577 | |||
| Male | 238 | 79 | 159 | |
| Female | 278 | 112 | 166 | |
| Age (year) | <0.0001 | |||
| <65 | 365 | 135 | 230 | |
| ≥65 | 151 | 67 | 84 | |
| Tumor diameter (cm ) | 0.0351 | |||
| <4 | 280 | 136 | 144 | |
| ≥4 | 236 | 62 | 174 | |
| Fuhrman | 0.2935 | |||
| 1-2 | 353 | 155 | 198 | |
| 3-4 | 163 | 58 | 105 | |
表1 肺腺癌患者临床特征与SLC2A1表达相关性
Tab.1 Relation between SLC2A1 and clinicopathological features of patients with LUAD (n)
| Clinicopathological feature | n | SLC2A1 Low | SLC2A1 High | P |
|---|---|---|---|---|
| Gender | 0.0577 | |||
| Male | 238 | 79 | 159 | |
| Female | 278 | 112 | 166 | |
| Age (year) | <0.0001 | |||
| <65 | 365 | 135 | 230 | |
| ≥65 | 151 | 67 | 84 | |
| Tumor diameter (cm ) | 0.0351 | |||
| <4 | 280 | 136 | 144 | |
| ≥4 | 236 | 62 | 174 | |
| Fuhrman | 0.2935 | |||
| 1-2 | 353 | 155 | 198 | |
| 3-4 | 163 | 58 | 105 | |
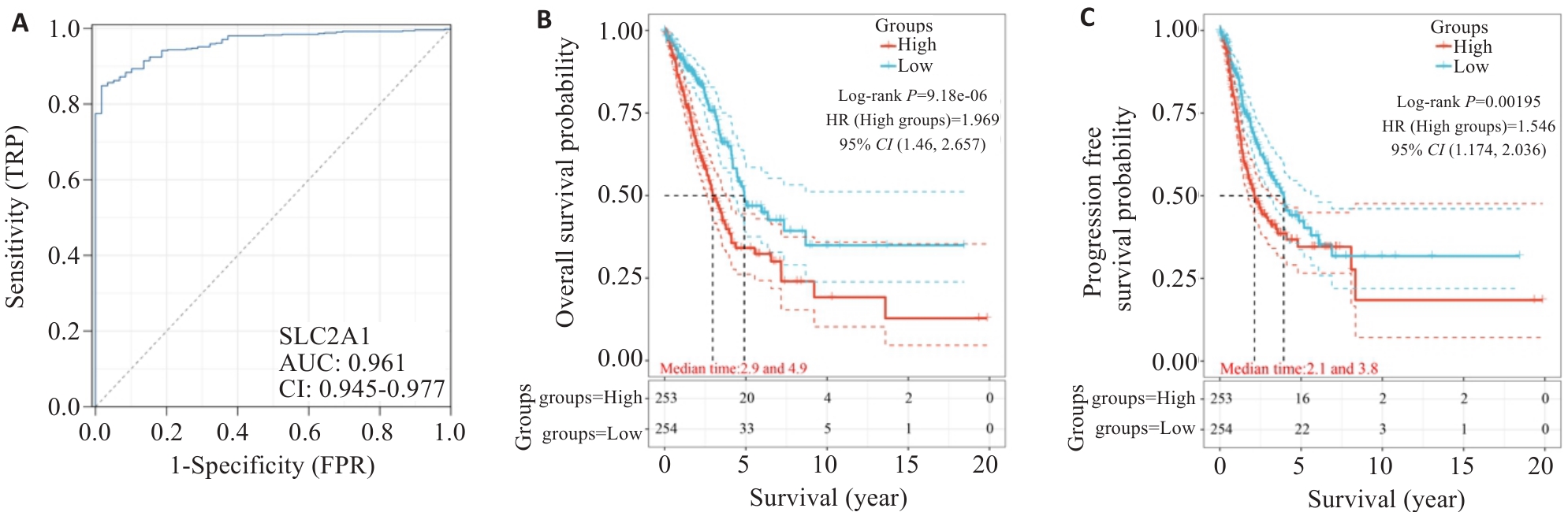
图2 SLC2A1在肺腺癌诊断和预后情况
Fig.2 Diagnostic and prognostic values of SLC2A1 in LUAD. A: AUC curve feature of SLC2A1 in LUAD. B: Overall survival of LUAD patients with different levels of SLC2A1. C: Progression-free survival of LUAD patients with different levels of SLC2A1.
| Variable | n | Mean Survival (months) | P |
|---|---|---|---|
| Gender | 0.2986 | ||
| Male | 156 | 69.2 | |
| Female | 51 | 62.3 | |
| Age (year) | 0.0620 | ||
| <65 | 165 | 70.0 | |
| ≥65 | 42 | 63.6 | |
| Tumor diameter ( cm ) | 0.0053 | ||
| <4 | 71 | 71.2 | |
| ≥4 | 136 | 67.0 | |
| Fuhrman | 0.0270 | ||
| 1-2 | 153 | 71.7 | |
| 3-4 | 54 | 60.1 | |
| SLC2A1 expression | 0.0079 | ||
| Low | 59 | 71.7 | |
| High | 148 | 69.2 | |
| T stage | 0.1082 | ||
| T1 | 52 | 71.0 | |
| T2 | 77 | 70.4 | |
| T3 | 78 | 65.5 | |
表2 单因素分析SLC2A1表达与肺腺癌患者临床特征及预后相关性
Tab.2 Univariate analysis of the correlations of SLC2A1 expression and clinicopathologic variables with overall survival of LUAD patients
| Variable | n | Mean Survival (months) | P |
|---|---|---|---|
| Gender | 0.2986 | ||
| Male | 156 | 69.2 | |
| Female | 51 | 62.3 | |
| Age (year) | 0.0620 | ||
| <65 | 165 | 70.0 | |
| ≥65 | 42 | 63.6 | |
| Tumor diameter ( cm ) | 0.0053 | ||
| <4 | 71 | 71.2 | |
| ≥4 | 136 | 67.0 | |
| Fuhrman | 0.0270 | ||
| 1-2 | 153 | 71.7 | |
| 3-4 | 54 | 60.1 | |
| SLC2A1 expression | 0.0079 | ||
| Low | 59 | 71.7 | |
| High | 148 | 69.2 | |
| T stage | 0.1082 | ||
| T1 | 52 | 71.0 | |
| T2 | 77 | 70.4 | |
| T3 | 78 | 65.5 | |
| Variables | Hazard Ratio (95% CI) | P |
|---|---|---|
| Tumor diameter | 1.239(1.136-1.352) | 0.220 |
| SLC2A1 expression | 1.912(1.841-1.988) | 0.024 |
| Fuhrman | 2.029(1.995-2.063) | 0.194 |
表3 COX多因素分析临床指标相关性
Tab.3 COX multivariate analysis of clinicopathological parameters and overall survival of the patients
| Variables | Hazard Ratio (95% CI) | P |
|---|---|---|
| Tumor diameter | 1.239(1.136-1.352) | 0.220 |
| SLC2A1 expression | 1.912(1.841-1.988) | 0.024 |
| Fuhrman | 2.029(1.995-2.063) | 0.194 |
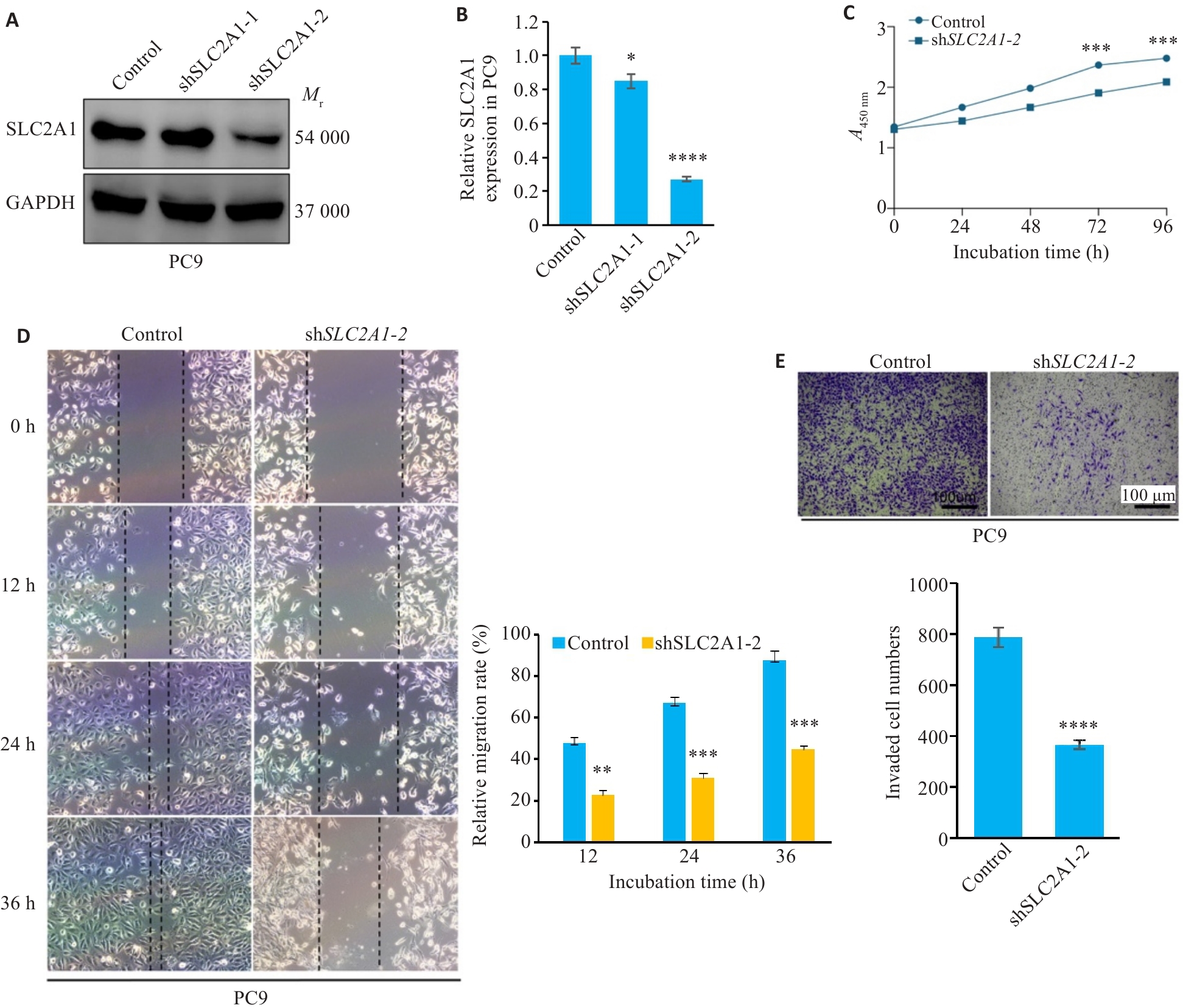
图4 干预SLC2A1抑制肺腺癌细胞的增殖迁移能力
Fig.4 Knockdown of SLC2A1 inhibits proliferation and migration of LUAD cells. A: Efficiency of SLC2A1 knockdown in PC9 cells. B: qRT-PCR data showing SLC2A1 mRNA expression in PC9 with or without shRNA. C: CCK-8 assay for assessing proliferation of PC9 cells with SLC2A1 downregulation. D: Migration ability of PC9 cells with SLC2A1 downregulation. E: Invasion ability of PC9 cells with SLC2A1 downregulation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
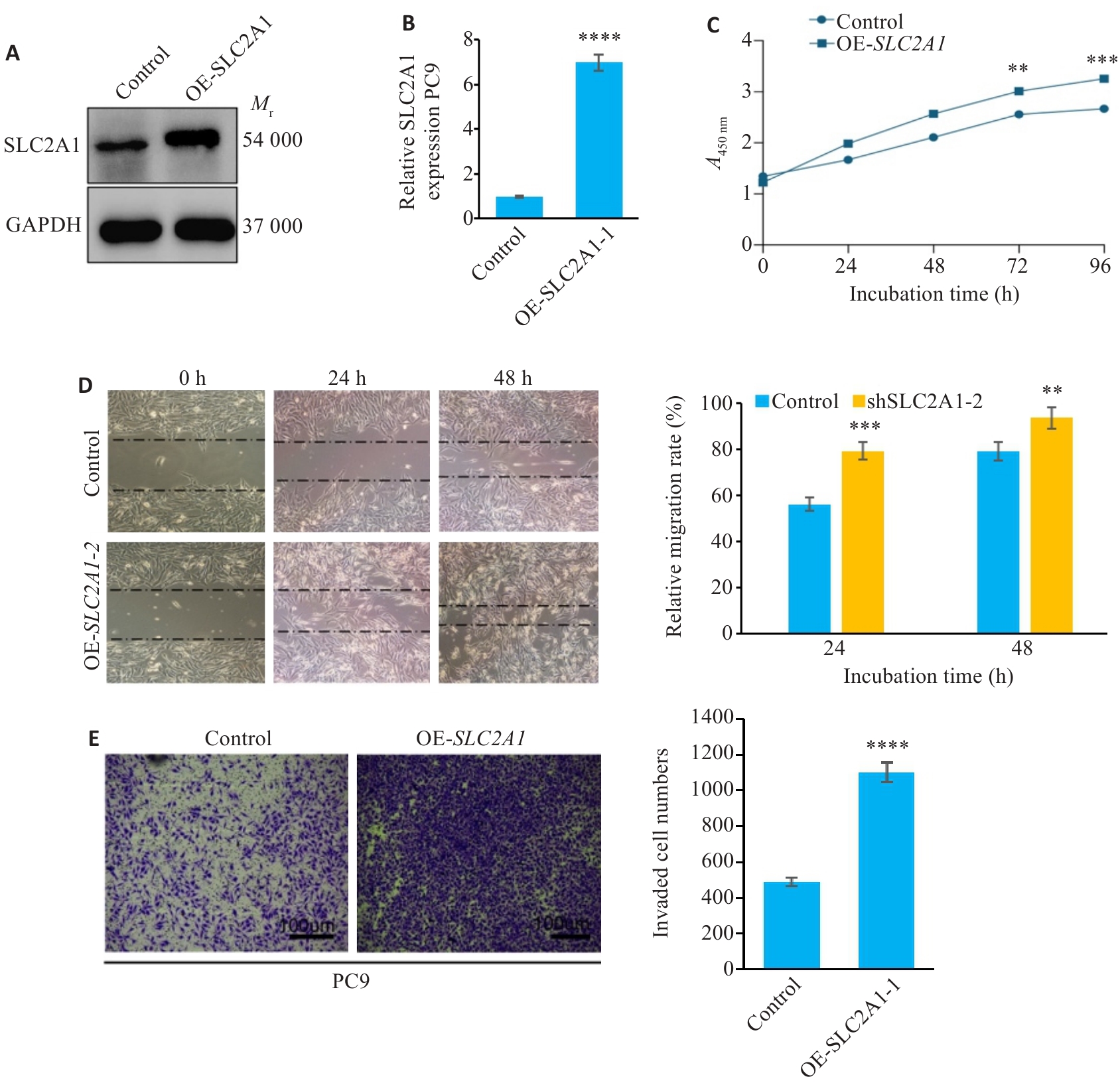
图5 过表达SLC2A1促进肺腺癌细胞的增殖迁移能力
Fig.5 Overexpression of SLC2A1 promotes proliferation and migration of LUAD cells.A: Efficiency of SLC2A1 overexpression in PC9 cells. B: qRT-PCR of SLC2A1 mRNA expression in PC9 cells with or without SLC2A1 overexpression. C: CCK-8 for assessing proliferation of PC9 cells overexpressing SLC2A1. D: Migration ability of PC9 cells overexpressing SLC2A1. E: Invasion of PC9 cells overexpressing SLC2A1. Scar bar=100 μm. **P<0.01, ***P<0.001, ****P<0.0001 vs control group.
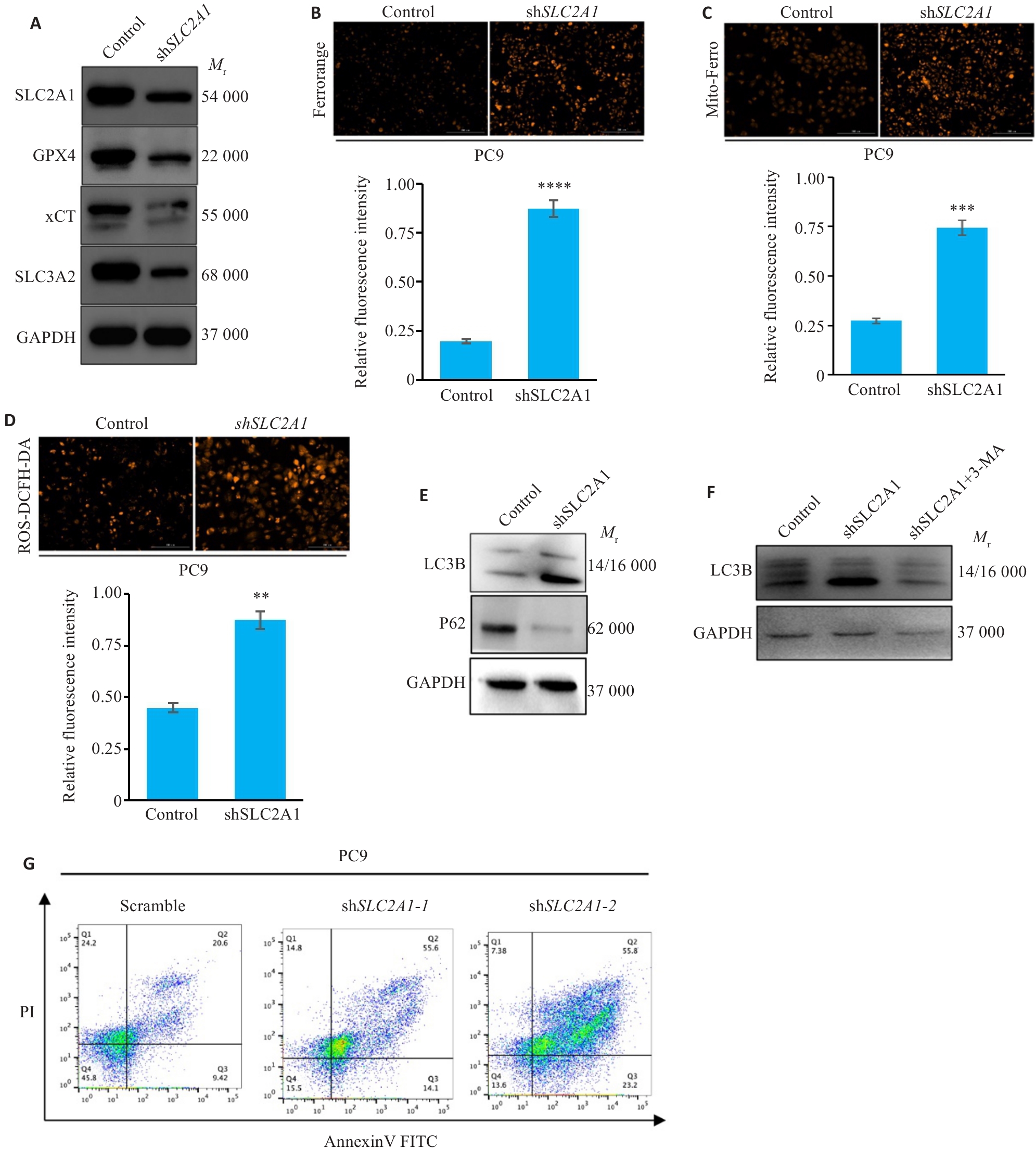
图6 干预SLC2A1促进肺腺癌发生自噬与铁死亡
Fig.6 SLC2A1 knockdown promotes autophagy and iron death in PC9 cells. A: Western blotting of ferroptosis-related proteins in PC9 cells with SLC2A1 knockdown. B: Intracellular Fe2+ level in PC9 cells with SLC2A1 knockdown. C: Mito-Fe2+ level in PC9 cells with SLC2A1 knockdown. D: Intracellular ROS level in PC9 cells with SLC2A1 knockdown. E: Knockdown of SLC2A1 induces autophagy in PC9 cells. F: 3-MA resuced SLC2A1 knockdown-induced autophagy of PC9 cells. G: Knockdown of SLC2A1 induces cell death in PC9 cells. Scar bar=100 μm. **P<0.01, ***P<0.001, ****P<0.0001.
| 1 | Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022 [J]. CA Cancer J Clin, 2022, 72(1): 7-33. |
| 2 | Lin WL, Chen Y, Wu BM, et al. Identification of the pyroptosis-related prognostic gene signature and the associated regulation axis in lung adenocarcinoma[J]. Cell Death Discov, 2021, 7(1): 161. |
| 3 | Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations[J]. J Clin Oncol, 2022, 40(6): 611-25. |
| 4 | Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family[J]. Annu Rev Nutr, 1996, 16: 235-56. |
| 5 | Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma[J]. Cancer, 2003, 97(4): 1015-24. |
| 6 | Amann T, Maegdefrau U, Hartmann A, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis[J]. Am J Pathol, 2009, 174(4): 1544-52. |
| 7 | Wachi S, Yoneda K, Wu RE. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues[J]. Bioinformatics, 2005, 21(23): 4205-8. |
| 8 | Krzeslak A, Wojcik-Krowiranda K, Forma E, et al. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers[J]. Pathol Oncol Res, 2012, 18(3): 721-8. |
| 9 | Dixon SJ. Ferroptosis: bug or feature?[J]. Immunol Rev, 2017, 277(1): 150-7. |
| 10 | Chen X, Kang R, Kroemer G, et al. Ferroptosis in infection, inflammation, and immunity [J]. J Exp Med, 2021, 218(6): 223-35. |
| 11 | Jiang XJ, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-82. |
| 12 | Liu XS, Yang JW, Zeng J, et al. SLC2A1 is a diagnostic biomarker involved in immune infiltration of colorectal cancer and associated with m6A modification and ceRNA[J]. Front Cell Dev Biol, 2022, 10: 853596. |
| 13 | Relli V, Trerotola M, Guerra E, et al. Abandoning the notion of non-small cell lung cancer[J]. Trends Mol Med, 2019, 25(7): 585-94. |
| 14 | Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives[J]. Crit Rev Oncol Hematol, 2021, 157: 103194. |
| 15 | Yao J, Tang SM, Shi CY, et al. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma[J]. Autophagy, 2023, 19(4): 1221-38. |
| 16 | Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion[J]. Nat Rev Cancer, 2019, 19(7): 405-14. |
| 17 | Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death [J]. Cell, 2012, 149(5): 1060-72. |
| 18 | Larraufie MH, Yang WS, Jiang E, et al. Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility[J]. Bioorg Med Chem Lett, 2015, 25(21): 4787-92. |
| 19 | Ou WJ, Mulik RS, Anwar A, et al. Low-density lipoprotein docosa-hexaenoic acid nanoparticles induce ferroptotic cell death in hepato-cellular carcinoma[J]. Free Radic Biol Med, 2017, 112: 597-607. |
| 20 | Yuan H, Li XM, Zhang XY, et al. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation[J]. Biochem Biophys Res Commun, 2016, 478(2): 838-44. |
| 21 | Bai T, Wang S, Zhao YP, et al. Haloperidol, a Sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells[J]. Biochem Biophys Res Commun, 2017, 491(4): 919-25. |
| 22 | Hao SH, Yu J, He WM, et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells[J]. Neoplasia, 2017, 19(12): 1022-32. |
| 23 | Galadari S, Rahman A, Pallichankandy S, et al. Reactive oxygen species and cancer paradox: To promote or to suppress [J]. Free Radic Biol Med, 2017, 104: 144-64. |
| 24 | Sun XF, Niu XH, Chen RC, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis[J]. Hepatology, 2016, 64(2): 488-500. |
| 25 | Zheng DW, Lei Q, Zhu JY, et al. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy[J]. Nano Lett, 2017, 17(1): 284-91. |
| 26 | Zhu S, Zhang QH, Sun XF, et al. HSPA5 regulates ferroptotic cell death in cancer cells[J]. Cancer Res, 2017, 77(8): 2064-77. |
| [1] | 陈凯, 孟兆菲, 闵静婷, 王佳慧, 李正红, 高琴, 胡俊锋. 姜黄素通过抑制TXNIP/TRX-1/GPX4通路介导的铁死亡减轻脓毒症小鼠肺损伤[J]. 南方医科大学学报, 2024, 44(9): 1805-1813. |
| [2] | 欧阳明子, 崔佳琦, 王慧, 梁正, 皮大锦, 陈利国, 陈前军, 吴迎朝. 开心散通过减轻前额叶皮质铁死亡缓解小鼠的阿霉素化疗性抑郁[J]. 南方医科大学学报, 2024, 44(8): 1441-1449. |
| [3] | 朱名扬, 王博康, 张秀森, 周克旭, 苗泽宇, 孙江涛. 基线水平CCL19+树突状细胞可有效预测肺腺癌患者免疫治疗的敏感性[J]. 南方医科大学学报, 2024, 44(8): 1529-1536. |
| [4] | 张银亮, 骆泽谭, 赵睿, 赵娜, 徐志东, 奥迪, 丛古一, 刘新宇, 郑海伦. 血根碱通过调控STUB1/GPX4诱导直肠癌细胞发生铁死亡[J]. 南方医科大学学报, 2024, 44(8): 1537-1544. |
| [5] | 王元国, 张鹏. 铁死亡抑制基因在食管癌中的高表达分析[J]. 南方医科大学学报, 2024, 44(7): 1389-1396. |
| [6] | 何华星, 刘璐琳, 刘颖茵, 陈纳川, 孙素霞. 丁酸钠与索拉非尼可能通过YAP诱导铁死亡协同抑制肝癌细胞增殖[J]. 南方医科大学学报, 2024, 44(7): 1425-1430. |
| [7] | 任智先, 周倍贤, 王林鑫, 李菁, 张荣平, 潘锡平. 5-羟基-6,7-二甲氧基黄酮抑制流感病毒诱导A549细胞炎症反应和铁死亡的作用及机制[J]. 南方医科大学学报, 2024, 44(6): 1070-1078. |
| [8] | 张方圆, 刘刚. 右美托咪定通过激活Nrf2/HO-1/GPX4通路抑制肾小管上皮细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(6): 1135-1140. |
| [9] | 王南, 石斌, 马小兰, 吴伟超, 曹佳. FMRP通过激活RAS/MAPK信号通路抑制结直肠肿瘤细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(5): 885-893. |
| [10] | 申磊磊, 陈莹, 云天洋, 郭俊唐, 柳曦, 张涛, 梁朝阳, 刘阳. IB期肺腺癌患者辅助治疗方案的筛选[J]. 南方医科大学学报, 2024, 44(5): 989-997. |
| [11] | 李淑贤, 于淑平, 穆亚铭, 王 凯, 刘 玉, 张美华. 二甲双胍通过抑制铁死亡改善PM2.5导致的胎盘滋养细胞功能损伤[J]. 南方医科大学学报, 2024, 44(3): 437-446. |
| [12] | 刘云泽, 李宬润, 郭俊唐, 刘 阳. 基于临床-影像组学列线图模型鉴别局灶性机化性肺炎与肺腺癌[J]. 南方医科大学学报, 2024, 44(2): 397-404. |
| [13] | 张榆雪, 蓝洁莹, 马昕怡, 周琼, 秦梦晨, 高磊. 化橘红配方颗粒通过维持铁稳态并抑制脂质过氧化和铁死亡缓解斑马鱼脂肪性肝病[J]. 南方医科大学学报, 2024, 44(12): 2265-2275. |
| [14] | 赵培培, 周志刚, 杨媛媛, 黄树升, 涂逸轩, 涂剑. 铁死亡诱导剂Erastin下调ACSL4抑制肝癌细胞体外增殖[J]. 南方医科大学学报, 2024, 44(11): 2131-2136. |
| [15] | 蔡华俊, 陈致岐, 胡文婷, 谭伟, 吴昊, 王超. 鼠曲草总黄酮通过激活Nrf2/SLC7A11/GPX-4信号通路抑制肝细胞铁死亡缓解对乙酰氨基酚诱导的小鼠急性肝损伤[J]. 南方医科大学学报, 2024, 44(11): 2201-2208. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||