南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (12): 2265-2275.doi: 10.12122/j.issn.1673-4254.2024.12.01
• •
张榆雪1( ), 蓝洁莹1, 马昕怡2, 周琼1, 秦梦晨1, 高磊1,3,4(
), 蓝洁莹1, 马昕怡2, 周琼1, 秦梦晨1, 高磊1,3,4( )
)
收稿日期:2024-08-21
出版日期:2024-12-20
发布日期:2024-12-26
通讯作者:
高磊
E-mail:1610045163@qq.com;rayg@foxmail.com
作者简介:张榆雪,在读硕士研究生,E-mail: 1610045163@qq.com
基金资助:
Yuxue ZAHNG1( ), Jieying LAN1, Xinyi MA2, Qiong ZHOU1, Mengchen QIN1, Lei GAO1,3,4(
), Jieying LAN1, Xinyi MA2, Qiong ZHOU1, Mengchen QIN1, Lei GAO1,3,4( )
)
Received:2024-08-21
Online:2024-12-20
Published:2024-12-26
Contact:
Lei GAO
E-mail:1610045163@qq.com;rayg@foxmail.com
Supported by:摘要:
目的 探讨化橘红配方颗粒治疗脂肪性肝病(FLD)的药效作用和具体机制。 方法 将受精后3 d的斑马鱼幼鱼随机分为空白组、模型对照组、化橘红配方颗粒处理组(16、32、64 μg/mL),分别构建斑马鱼幼鱼非酒精性脂肪肝(NAFLD)和酒精性脂肪肝(ALD)模型,采用生存分析、病理组织切片、全鱼油红O染色和实时定量荧光PCR实验评价化橘红配方颗粒治疗FLD的药效,并从CAV1和铁代谢及铁死亡角度探讨其治疗FLD的具体机制,通过普鲁士蓝染色、DCFH-DA活体探针、丙二醛含量检测、实时定量荧光PCR实验和CAV1免疫组织化学染色分析化橘红配方颗粒治疗FLD的药效验证和具体机制。 结果 化橘红配方颗粒能减轻斑马鱼幼鱼NAFLD和ALD模型中的脂质累积,减少脂肪酸合成酶、固醇调节元件结合蛋白1、3-羟基-3-甲基戊二酰辅酶A还原酶、肿瘤坏死因子-α和白介素6的表达水平,升高载脂蛋白A1和PPARα表达水平。化橘红配方颗粒可以减少斑马鱼幼鱼NAFLD和ALD模型中肝脏的铁沉积,减少幼鱼体内的丙二醛和活性氧含量(P<0.05)。NAFLD状态下,不同浓度的化橘红配方颗粒可升高Tf、TfR、FPN和SLC7A11表达(P<0.05),中、高浓度的化橘红配方颗粒可升高谷胱甘肽过氧化物酶4(GPX4)的表达(P<0.05);ALD状态下,斑马鱼幼鱼的Tf、TfR和FPN表达升高,不同浓度的化橘红配方颗粒可降低这些基因的表达(P<0.05)。化橘红配方颗粒对ALD状态下SLC7A11的表达无明显影响,但高浓度的化橘红配方颗粒可升高GPX4的表达(P<0.05),免疫组织化学染色和实时定量荧光PCR实验结果显示,在NAFLD和ALD状态下,斑马鱼幼鱼肝脏的CAV1升高,而中、高浓度的化橘红配方颗粒可明显改善评价两种脂肪肝状态下斑马鱼幼鱼肝脏的CAV1表达。 结论 化橘红配方颗粒可减轻NAFLD和ALD的脂质累积和炎症反应,并通过减少CAV1的表达纠正NAFLD和ALD的铁稳态失衡、脂质过氧化和铁死亡。
张榆雪, 蓝洁莹, 马昕怡, 周琼, 秦梦晨, 高磊. 化橘红配方颗粒通过维持铁稳态并抑制脂质过氧化和铁死亡缓解斑马鱼脂肪性肝病[J]. 南方医科大学学报, 2024, 44(12): 2265-2275.
Yuxue ZAHNG, Jieying LAN, Xinyi MA, Qiong ZHOU, Mengchen QIN, Lei GAO. Exocarpium Citri Grandis formula granules alleviate fatty liver disease in Zebrafish by maintaining iron homeostasis and suppressing lipid peroxidation and ferroptosis[J]. Journal of Southern Medical University, 2024, 44(12): 2265-2275.
| Primer | Primer sequence (5'-3') | Primer sequence (5'-3') |
|---|---|---|
| β-actin | ATGGATGAGGAAATCGCTGCC | CTCCCTGATGTCTGGGTCGTC |
| FASN | GAGAAAGCTTGCCAAACAGG | GAGGGTCTTGCAGGAGACAG |
| SREBP1 | CATCCACATGGCTCTGAGTG | CTCATCCACAAAGAAGCGGT |
| HMGCRA | CCTGTTAGCCGTCAGTGGA | TCTTTGACCACTCGTGCCG |
| PPARα | TGCTGGACTACCAGAACTGTGACA | TGCTGGCTGAGAACACTTCTGAG |
| Apoa1 | GCACTAAGCTGACCGAGCGT | GGAGGTCCTGGGTGTGTGGA |
| TNF-α | GCTTATGAGCCATGCAGTGA | TGCCCAGTCTGTCTCCTTCT |
| IL6 | CCTCAAACCTTCAGACCGCT | GAACAGGATCGAGTGGACCG |
| Tf | GTTGATGGTGGCCAGGTGTA | AGCGTAGTAACTTGCGGTCC |
| TfR | CCGCTCATACTCGCGGTTTA | TGGTTCAGAACGACCTGTGG |
| FPN | GCCAACGTCACCGTTTTTGA | CGGTCAAGTCGAAGGACCAA |
| GPX4 | GAGGTTTACGCATCCTGGCT | GGCTGATCCTTCAGCCACTT |
| SLC7A11 | TGTGGGAATGTCACTGGTGG | ACGCCTCCAGAATGTACGTG |
表1 引物序列
Tab.1 Primer sequences for RT-qPCR
| Primer | Primer sequence (5'-3') | Primer sequence (5'-3') |
|---|---|---|
| β-actin | ATGGATGAGGAAATCGCTGCC | CTCCCTGATGTCTGGGTCGTC |
| FASN | GAGAAAGCTTGCCAAACAGG | GAGGGTCTTGCAGGAGACAG |
| SREBP1 | CATCCACATGGCTCTGAGTG | CTCATCCACAAAGAAGCGGT |
| HMGCRA | CCTGTTAGCCGTCAGTGGA | TCTTTGACCACTCGTGCCG |
| PPARα | TGCTGGACTACCAGAACTGTGACA | TGCTGGCTGAGAACACTTCTGAG |
| Apoa1 | GCACTAAGCTGACCGAGCGT | GGAGGTCCTGGGTGTGTGGA |
| TNF-α | GCTTATGAGCCATGCAGTGA | TGCCCAGTCTGTCTCCTTCT |
| IL6 | CCTCAAACCTTCAGACCGCT | GAACAGGATCGAGTGGACCG |
| Tf | GTTGATGGTGGCCAGGTGTA | AGCGTAGTAACTTGCGGTCC |
| TfR | CCGCTCATACTCGCGGTTTA | TGGTTCAGAACGACCTGTGG |
| FPN | GCCAACGTCACCGTTTTTGA | CGGTCAAGTCGAAGGACCAA |
| GPX4 | GAGGTTTACGCATCCTGGCT | GGCTGATCCTTCAGCCACTT |
| SLC7A11 | TGTGGGAATGTCACTGGTGG | ACGCCTCCAGAATGTACGTG |
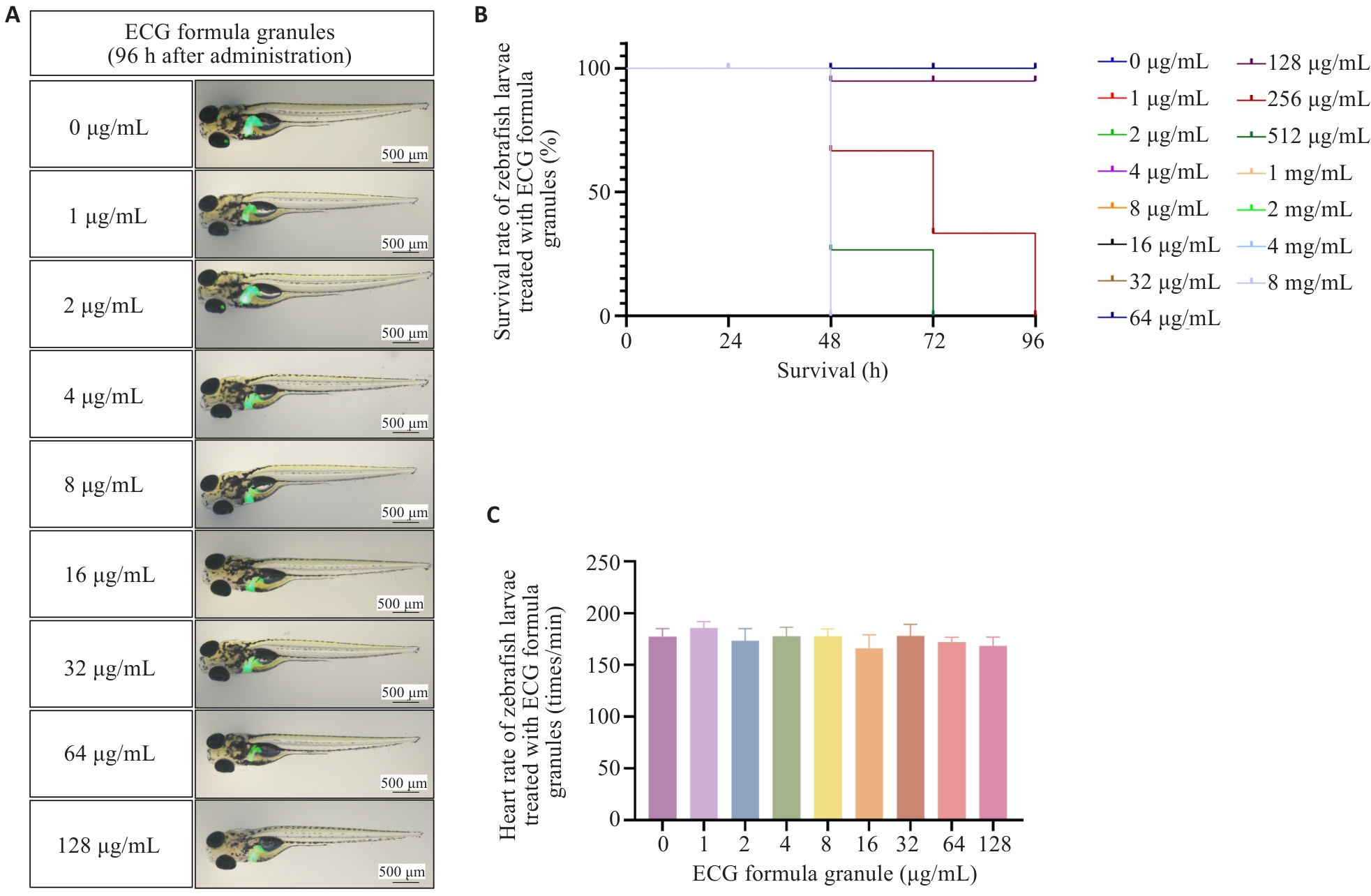
图1 化橘红配方颗粒的斑马鱼急性毒性实验结果
Fig.1 Results of acute toxicity test of Exocarpium Citri Grandis (ECG) in zebrafish larvae. A: Development of zebrafish larvae 96 h after ECG administration. B: Effects of ECG granules on survival of zebrafish. C: Effects of ECG granules on heart rate of zebrafish at 96 h after administration.

图2 化橘红配方颗粒治疗非酒精性脂肪肝的药效评价
Fig.2 Efficacy evaluation of ECG decoction tablets and ECG granules for treatment of NAFLD in zebrafish larvae. A: HE staining of the liver of zebrafish larvae in each group. B: Whole fish oil red O staining in different groups. C: Effects of ECG decoction tablets and ECG granules on lipid metabolism-related genes and inflammatory factors. ECGFG:Exocarpium Citri Grandis formula granules.*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
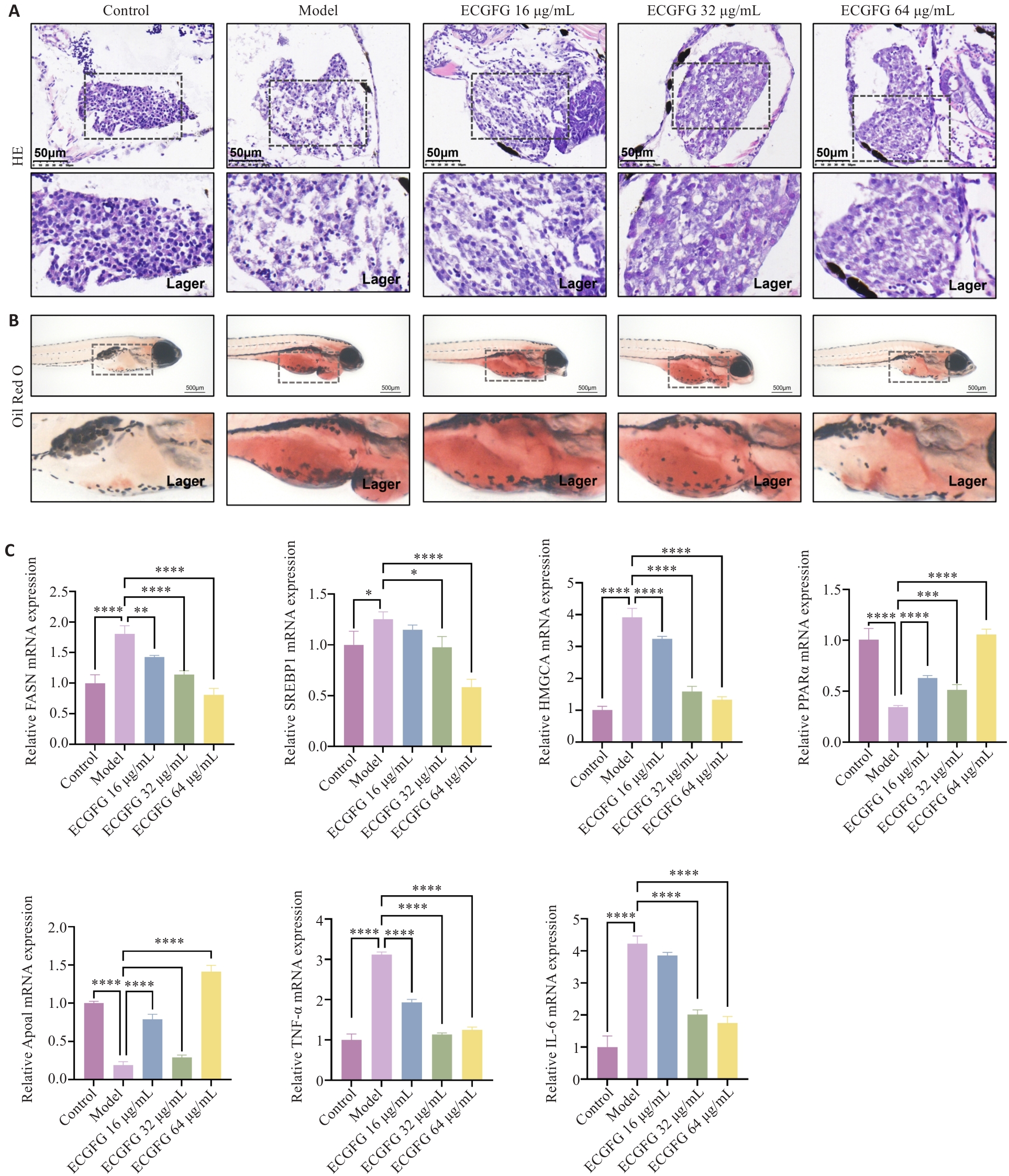
图3 化橘红配方颗粒治疗酒精性脂肪肝的药效评价
Fig.3 Efficacy evaluation of ECG decoction tablets and ECG granules for treatment of ALD in zebrafish larvae. A: HE staining of the liver of zebrafish larvae in each group. B: Whole fish oil red O staining in different groups. C: Effects of ECG decoction tablets and ECG granules on lipid metabolism-related genes and inflammatory factors. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
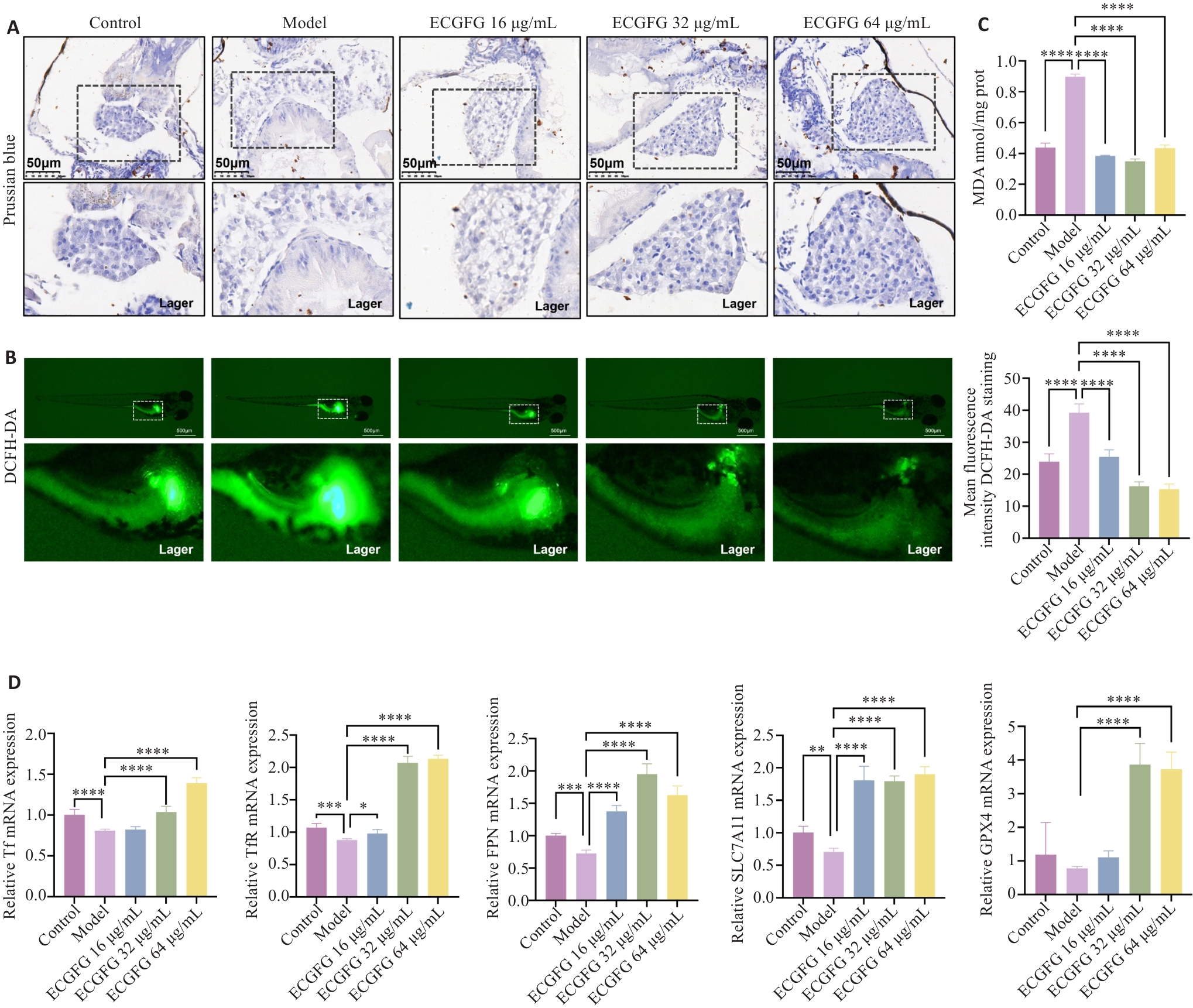
图4 化橘红配方颗粒对非酒精性脂肪肝铁代谢和铁死亡的作用评估
Fig.4 Effect of ECG granules on iron metabolism and ferroptosis in zebrafish larvae models of NAFLD. A: Prussian blue staining of the liver of zebrafish larvae in each group. B: DCFH-DA probe detection of zebrafish larvae in each group. C: Detection of MDA content in zebrafish larvae in each group. D: Effects of ECG granules on iron metabolism- and ferroptosis-related genes. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
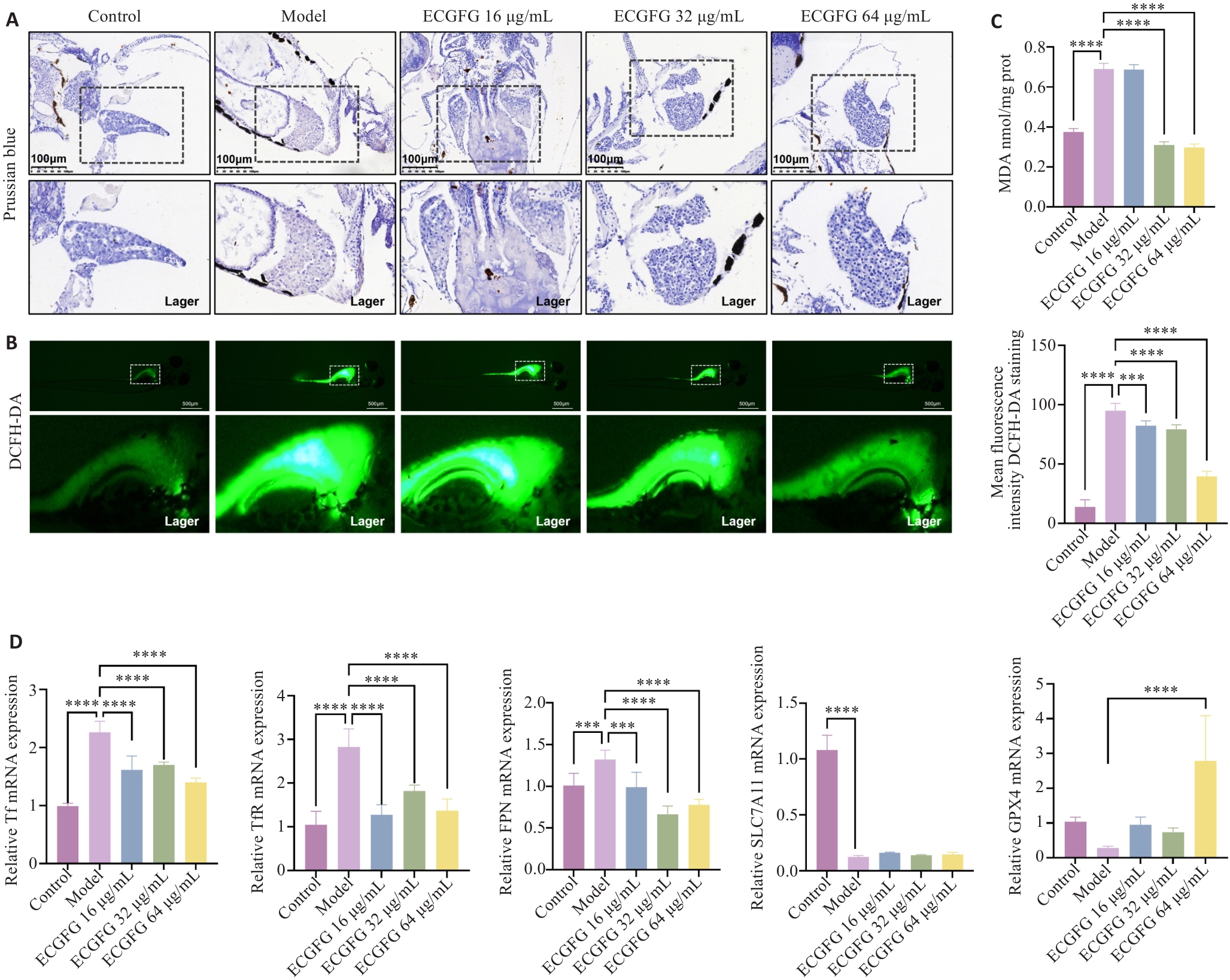
图5 化橘红配方颗粒对酒精性脂肪肝铁代谢和铁死亡的作用评估
Fig.5 Effect of ECG granules on iron metabolism and ferroptosis in zebrafish larvae models of ALD. A: Prussian blue staining of the liver of zebrafish larvae in each group. B: DCFH-DA probe detection of zebrafish larvae in each group. C: Detection of MDA content in zebrafish larvae in each group. D: Effects of ECG granules on expressions of iron metabolism- and ferroptosis-related genes. ***P<0.001, ****P<0.0001.
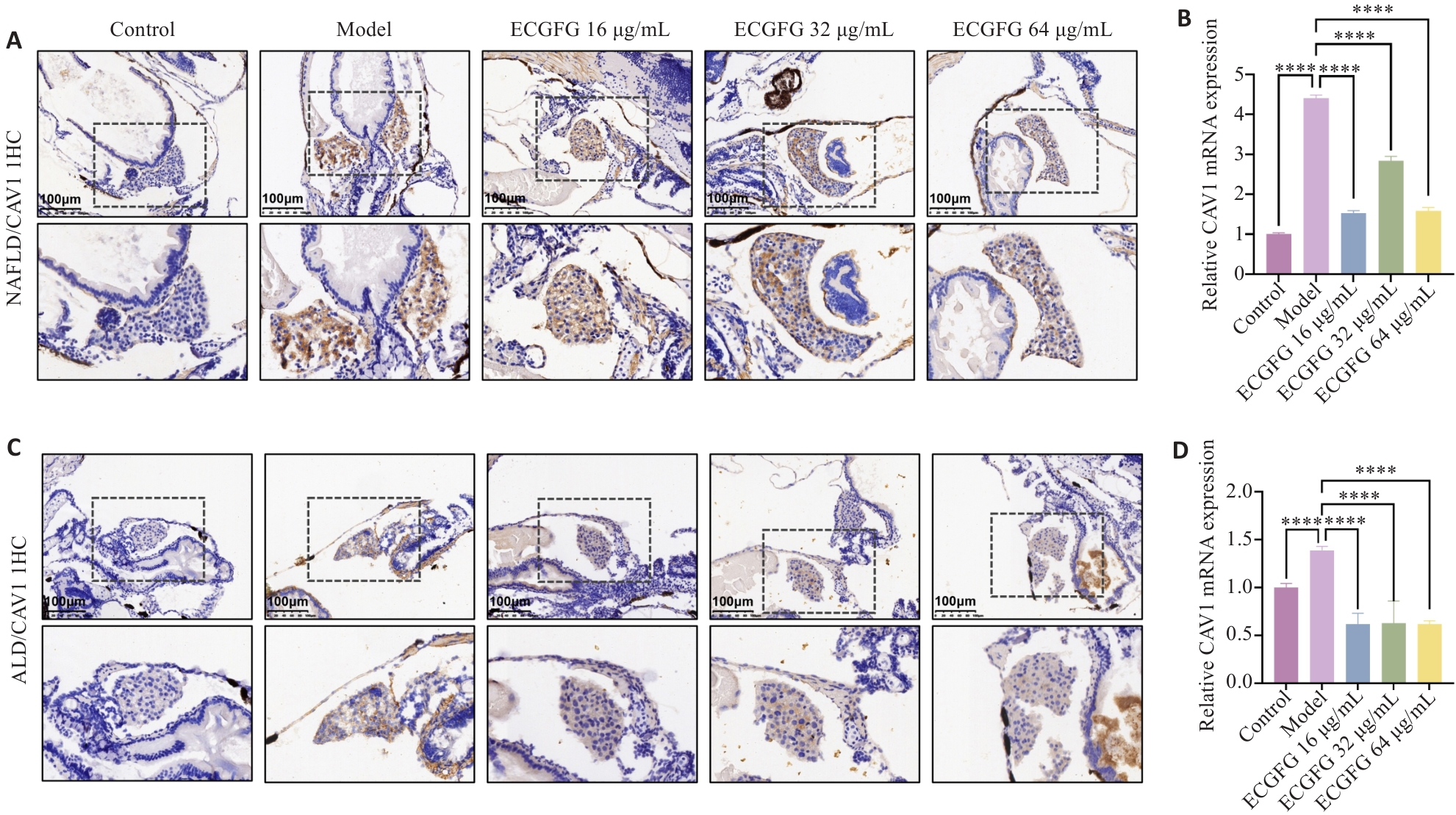
图6 化橘红配方颗粒可能通过CAV1调控肝铁代谢和铁死亡
Fig.6 ECG granules regulate hepatic iron metabolism and ferroptosis possibly through CAV1 in zebrafish larvae. A: Liver CAV1 immunohistochemical staining in zebrafish larvae models of NAFLD. B: Effect of ECG granules on CAV1 expression in zebrafish larvae models of NAFLD. C: Liver CAV1 immunohistochemical staining in zebrafish larvae models of ALD. D: Effect of ECG granules on CAV1 expression in zebrafish larvae models of ALD. ****P<0.0001.
| 1 | 国家药典委员会. 中华人民共和国药典-一部: 2020年版[M]. 北京: 中国 医药科技出版社, 2020. |
| 2 | 范会云, 邓乔华, 宋松泉. “一片值千金”: 化橘红[J]. 生命世界, 2020(9): 40-1. |
| 3 | Musso G, Bo S, Cassader M, et al. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism[J]. Am J Clin Nutr, 2013, 98(4): 895-906. |
| 4 | 中华中医药学会脾胃病分会, 张 莉, 季 光, 等. 非酒精性脂肪性肝病中医诊疗专家共识(2023)[J]. 中国中西医结合消化杂志, 2024, 10(1): 1-7. |
| 5 | 毕占阳, 杨 柱, 郭 斌, 等. 基于酒伤理论探讨酒精性肝病的中医治疗[J]. 云南中医中药杂志, 2022, 43(5): 16-9. |
| 6 | 李锦坤, 金刚亮, 王艳慧, 等. 化橘红提取物的制备工艺优化及其降脂作用[J/OL]. 现代食品 科技, 2024,06. 10.13982/j.mfst.1673-9078.2024.10.0892 |
| 7 | 吴道顺, 王梦晨, 张雪涟, 等. 化橘红水提物对酒精诱导的急性肝损伤保护作用[J]. 中国实验方剂学杂志, 2022, 28(19): 42-8. |
| 8 | Deng GH, Liu C, Zhao JM, et al. Exocarpium Citri Grandis alleviates the aggravation of NAFLD by mitigating lipid accumulation and iron metabolism disorders[J]. J Ethnopharmacol, 2023, 313: 116559. |
| 9 | 吴雄志, 卞兆祥, 陈新宇, 等. 中药配方颗粒临床应用国际专家共识[J]. TMR经典中医研究, 2021, 7(1): 19-26. |
| 10 | 谭婉清, 王术玲, 刘潇潇, 等. 化橘红配方颗粒与饮片汤剂的化学等量性和药效等效性研究[J]. 中药新药与临床药理, 2023, 34(4): 541-9. |
| 11 | 米永佳, 王茂泓, 李龙华, 等. 全国名中医张小萍教授治疗非酒精性脂肪肝经验[J]. 中国民族民间医药, 2024, 33(12): 80-3. |
| 12 | 方俐晖, 郭志玲, 张轶斐, 等. 田德禄教授三期三脏辨治酒精性肝病经验[J]. 现代中医临床, 2021, 28(5): 38-42. |
| 13 | 王艳慧. 化橘红的研究进展[J]. 世界科学技术-中医药现代化, 2017, 19(6): 1076-82. |
| 14 | 苏志鹏. 化橘红活性成分的综合利用及对降血糖血脂活性的研究[D]. 广州: 广东药科大学, 2019. |
| 15 | 吴 祎. 化橘红解酒功效与保健食品的研究[D]. 广州: 广州中医药大学, 2014. |
| 16 | 黄凯伟, 任应宗, 张 辉, 等. 化橘红(柚)饮片、标准汤剂和配方颗粒相关性研究[J]. 中国药业, 2023, 32(17): 57-62. |
| 17 | Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies[J]. Biochim Biophys Acta, 2015, 1852(7): 1347-59. |
| 18 | Vogt AC S, Arsiwala T, Mohsen M, et al. On iron metabolism and its regulation[J]. Int J Mol Sci, 2021, 22(9): 4591. |
| 19 | Graham RM, Chua AC, Herbison CE, et al. Liver iron transport[J]. World J Gastroenterol, 2007, 13(35): 4725-36. |
| 20 | Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver[J]. World J Hepatol, 2015, 7(2): 177-88. |
| 21 | Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression[J]. Cell Mol Life Sci, 2019, 76(1): 99-128. |
| 22 | Sun KX, Zhao JV, Nelson EAS, et al. Iron status and non-alcoholic fatty liver disease: a Mendelian randomization study[J]. Nutrition, 2024, 118: 112295. |
| 23 | Yu GF, Liu L, Qin T, et al. Associations of serum iron status with MAFLD and liver fibrosis in the USA: a nationwide cross-section study[J]. Biol Trace Elem Res, 2024, 202(1): 87-98. |
| 24 | Deng GH, Liu C, Zhao JM, et al. Exocarpium Citri Grandis alleviates |
| the aggravation of NAFLD by mitigating lipid accumulation and iron | |
| metabolism disorders[J]. J Ethnopharmacol, 2023, 313: 116559. | |
| 25 | Ali N, Ferrao K, Mehta KJ. Liver iron loading in alcohol-associated liver disease[J]. Am J Pathol, 2023, 193(10): 1427-39. |
| 26 | Li LX, Guo FF, Liu H, et al. Iron overload in alcoholic liver disease: |
| mechanismsunderlying, effectsdetrimental, and therapeuticpotential | |
| targets[J]. Cell Mol Life Sci, 2022, 79(4): 201. | |
| 27 | Chen JY, Li XP, Ge CD, et al. The multifaceted role of ferroptosis in liver disease[J]. Cell Death Differ, 2022, 29(3): 467-80. |
| 28 | Chen X, Li J, Kang R, et al. Ferroptosis: machinery and regulation[J]. Autophagy, 2021, 17(9): 2054-81. |
| 29 | Koppula P, Zhuang L, Gan BY. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy[J]. Protein Cell, 2021, 12(8): 599-620. |
| 30 | Xie YC, Kang R, Klionsky DJ, et al. GPX4 in cell death, autophagy, and disease[J]. Autophagy, 2023, 19(10): 2621-38. |
| 31 | Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications[J]. Cell, 2022, 185(14): 2401-21. |
| 32 | Chen X, Li JB, Kang R, et al. Ferroptosis: machinery and regulation[J]. Autophagy, 2021, 17(9): 2054-81. |
| 33 | Sui YT, Geng X, Wang ZW, et al. Targeting the regulation of iron homeostasis as a potential therapeutic strategy for nonalcoholic fatty liver disease[J]. Metabolism, 2024, 157: 155953. |
| 34 | Luo J, Song G, Chen NN, et al. Ferroptosis contributes to ethanol-induced hepatic cell death via labile iron accumulation and GPx4 inactivation[J]. Cell Death Discov, 2023, 9(1): 311. |
| 35 | Fernández-Rojo MA, Gongora M, Fitzsimmons RL, et al. Caveolin-1 is necessary for hepatic oxidative lipid metabolism: evidence for crosstalk between caveolin-1 and bile acid signaling[J]. Cell Rep, 2013, 4(2): 238-47. |
| 36 | Haddad D, Al Madhoun A, Nizam R, et al. Role of caveolin-1 in diabetes and its complications[J]. Oxid Med Cell Longev, 2020, 2020: 9761539. |
| 37 | Jiang Y, Krantz S, Qin X, et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy[J]. Redox Biol, 2022, 52: 102304. |
| 38 | Fernández MA, Albor C, Ingelmo-Torres M, et al. Caveolin-1 is essential for liver regeneration[J]. Science, 2006, 313(5793): 1628-32. |
| 39 | Fernandez-Rojo MA, Ramm GA. Caveolin-1 function in liver physiology and disease[J]. Trends Mol Med, 2016, 22(10): 889-904. |
| 40 | Gao L, Zhou YC, Zhong WC, et al. Caveolin-1 is essential for protecting against binge drinking-induced liver damage through inhibiting reactive nitrogen species[J]. Hepatology, 2014, 60(2): 687-99. |
| 41 | Tao L, Wang JP, Wang K, et al. Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin-1[J]. Aging Cell, 2024, 23(8): e14181. |
| 42 | Zhang T, Yang F, Dai XY, et al. Role of Caveolin-1 on the molybdenum and cadmium exposure induces pulmonary ferroptosis and fibrosis in the sheep[J]. Environ Pollut, 2023, 334: 122207. |
| 43 | Liu WH, Luo GH. CAV1 inhibits Xc (-) system through IFNGR1 to promote ferroptosis to inhibit stemness and improves anti-PD-1 efficacy in breast cancer[J]. Transl Oncol, 2024, 50: 102149. |
| 44 | Deng GH, Li YJ, Ma SY, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress[J]. Free Radic Biol Med, 2020, 148: 151-61. |
| 45 | Huang S, Wang YH, Xie SW, et al. Isoliquiritigenin alleviates liver |
| fibrosis through caveolin-1-mediated hepatic stellate cells ferroptosis | |
| in zebrafish and mice[J]. Phytomedicine, 2022, 101: 154117. | |
| 46 | Amali AA, Rekha RD, Lin CJ, et al. Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis[J]. J Biomed Sci, 2006, 13(2): 225-32. |
| 47 | Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis[J]. Expert Opin Investig Drugs, 2018, 27(3): 301-11. |
| 48 | Bautista AP. Neutrophilic infiltration in alcoholic hepatitis[J]. Alcohol, 2002, 27(1): 17-21. |
| [1] | 陈凯, 孟兆菲, 闵静婷, 王佳慧, 李正红, 高琴, 胡俊锋. 姜黄素通过抑制TXNIP/TRX-1/GPX4通路介导的铁死亡减轻脓毒症小鼠肺损伤[J]. 南方医科大学学报, 2024, 44(9): 1805-1813. |
| [2] | 欧阳明子, 崔佳琦, 王慧, 梁正, 皮大锦, 陈利国, 陈前军, 吴迎朝. 开心散通过减轻前额叶皮质铁死亡缓解小鼠的阿霉素化疗性抑郁[J]. 南方医科大学学报, 2024, 44(8): 1441-1449. |
| [3] | 张银亮, 骆泽谭, 赵睿, 赵娜, 徐志东, 奥迪, 丛古一, 刘新宇, 郑海伦. 血根碱通过调控STUB1/GPX4诱导直肠癌细胞发生铁死亡[J]. 南方医科大学学报, 2024, 44(8): 1537-1544. |
| [4] | 王元国, 张鹏. 铁死亡抑制基因在食管癌中的高表达分析[J]. 南方医科大学学报, 2024, 44(7): 1389-1396. |
| [5] | 何华星, 刘璐琳, 刘颖茵, 陈纳川, 孙素霞. 丁酸钠与索拉非尼可能通过YAP诱导铁死亡协同抑制肝癌细胞增殖[J]. 南方医科大学学报, 2024, 44(7): 1425-1430. |
| [6] | 任智先, 周倍贤, 王林鑫, 李菁, 张荣平, 潘锡平. 5-羟基-6,7-二甲氧基黄酮抑制流感病毒诱导A549细胞炎症反应和铁死亡的作用及机制[J]. 南方医科大学学报, 2024, 44(6): 1070-1078. |
| [7] | 张方圆, 刘刚. 右美托咪定通过激活Nrf2/HO-1/GPX4通路抑制肾小管上皮细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(6): 1135-1140. |
| [8] | 王南, 石斌, 马小兰, 吴伟超, 曹佳. FMRP通过激活RAS/MAPK信号通路抑制结直肠肿瘤细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(5): 885-893. |
| [9] | 郭新邓, 郭卓琳, 孙冬梅, 邹丽芳, 区锦莹, 余林中, 卢子滨, 曹惠慧, 刘俊珊. 连翘配方颗粒与饮片的抗炎、抗肿瘤和抑菌效果的比较研究[J]. 南方医科大学学报, 2024, 44(3): 594-604. |
| [10] | 李淑贤, 于淑平, 穆亚铭, 王 凯, 刘 玉, 张美华. 二甲双胍通过抑制铁死亡改善PM2.5导致的胎盘滋养细胞功能损伤[J]. 南方医科大学学报, 2024, 44(3): 437-446. |
| [11] | 赵培培, 周志刚, 杨媛媛, 黄树升, 涂逸轩, 涂剑. 铁死亡诱导剂Erastin下调ACSL4抑制肝癌细胞体外增殖[J]. 南方医科大学学报, 2024, 44(11): 2131-2136. |
| [12] | 蔡华俊, 陈致岐, 胡文婷, 谭伟, 吴昊, 王超. 鼠曲草总黄酮通过激活Nrf2/SLC7A11/GPX-4信号通路抑制肝细胞铁死亡缓解对乙酰氨基酚诱导的小鼠急性肝损伤[J]. 南方医科大学学报, 2024, 44(11): 2201-2208. |
| [13] | 杨勤军, 王卉, 徐淑钰, 杨程, 丁焕章, 吴迪, 朱洁, 童佳兵, 李泽庚. 参芪调肾方减轻慢阻肺肺肾气虚证大鼠气道炎症的机制:基于铁死亡途径[J]. 南方医科大学学报, 2024, 44(10): 1937-1946. |
| [14] | 孙 硕, 黄 鑫, 李国东, 张春云, 卢泽梅, 张伟伟, 李泽彦, 杨清竹. 敲低结肠癌转移相关基因1促进RSL3诱导的结直肠癌细胞铁死亡[J]. 南方医科大学学报, 2024, 44(1): 173-178. |
| [15] | 张晓红, 赵 品, 蒯建科, 常 超, 袁 庆. 亚精胺通过抑制细胞凋亡、ROS生成及铁死亡减轻脂多糖诱导的小鼠心肌损伤[J]. 南方医科大学学报, 2024, 44(1): 166-172. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||