Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (9): 1840-1849.doi: 10.12122/j.issn.1673-4254.2025.09.04
Biyun LUO1,2( ), Xin YI1, Yijing CAI1, Shiqing ZHANG3, Peng WANG2, Tong LI2, Ken Kin Lam YUNG4,5, Pingzheng ZHOU1(
), Xin YI1, Yijing CAI1, Shiqing ZHANG3, Peng WANG2, Tong LI2, Ken Kin Lam YUNG4,5, Pingzheng ZHOU1( )
)
Received:2025-02-27
Online:2025-09-20
Published:2025-09-28
Contact:
Pingzheng ZHOU
E-mail:wfyyjk@163.com;pzzhou@smu.edu.cn
Biyun LUO, Xin YI, Yijing CAI, Shiqing ZHANG, Peng WANG, Tong LI, Ken Kin Lam YUNG, Pingzheng ZHOU. Ching Shum Pills alleviates non-alcoholic fatty liver disease in mice by ameliorating lipid metabolism disorders[J]. Journal of Southern Medical University, 2025, 45(9): 1840-1849.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.09.04
| Gene | Forward/Reverse primer | Primer sequences (5'-3') |
|---|---|---|
| β-actin | Forward | AAGTCCCTCACCCTCCCAAAAG |
| Reverse | AAGCAATGCTGTCACCTTCCC | |
| Tp53 | Forward | GTCACAGCACATGACGGAGG |
| Reverse | TCTTCCAGATACTCGGGATAC | |
| Cpt1 | Forward | TCCACCCTGAGGCATCTATT |
| Reverse | ATGACCTCCTGGCATTCTCC | |
| Ppara | Forward | CGGGAAAGACCAGCAACAAC |
| Reverse | ATAGCAGCCACAAACAGGGA |
Tab.1 Primer sequence for RT-qPCR in this study
| Gene | Forward/Reverse primer | Primer sequences (5'-3') |
|---|---|---|
| β-actin | Forward | AAGTCCCTCACCCTCCCAAAAG |
| Reverse | AAGCAATGCTGTCACCTTCCC | |
| Tp53 | Forward | GTCACAGCACATGACGGAGG |
| Reverse | TCTTCCAGATACTCGGGATAC | |
| Cpt1 | Forward | TCCACCCTGAGGCATCTATT |
| Reverse | ATGACCTCCTGGCATTCTCC | |
| Ppara | Forward | CGGGAAAGACCAGCAACAAC |
| Reverse | ATAGCAGCCACAAACAGGGA |
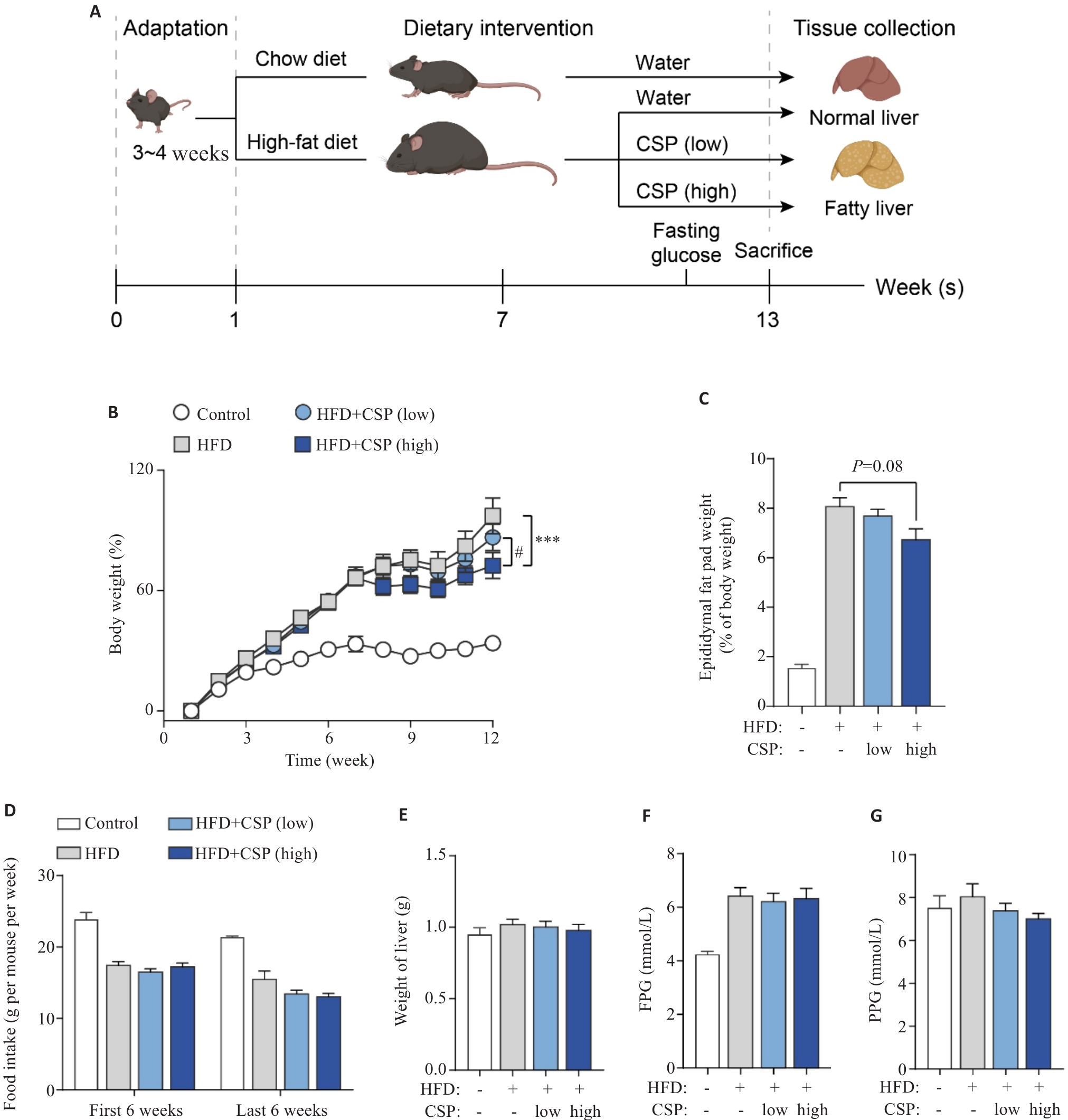
Fig.1 CSP mitigates high-fat diet-induced non-alcoholic fatty liver disease (NAFLD) in mice. A: Schematic diagram of NAFLD modeling and CSP treatment in mice. B: Body weight changes in mice during the experiment. C: Percentage of epididymal fat pad weight in body weight. D: Average food intake during the experiment. E: Weight of liver after indicated treatment. F: Fasting plasma glucose. G: Postprandial plasma glucose. ***P<0.001; #P <0.05.
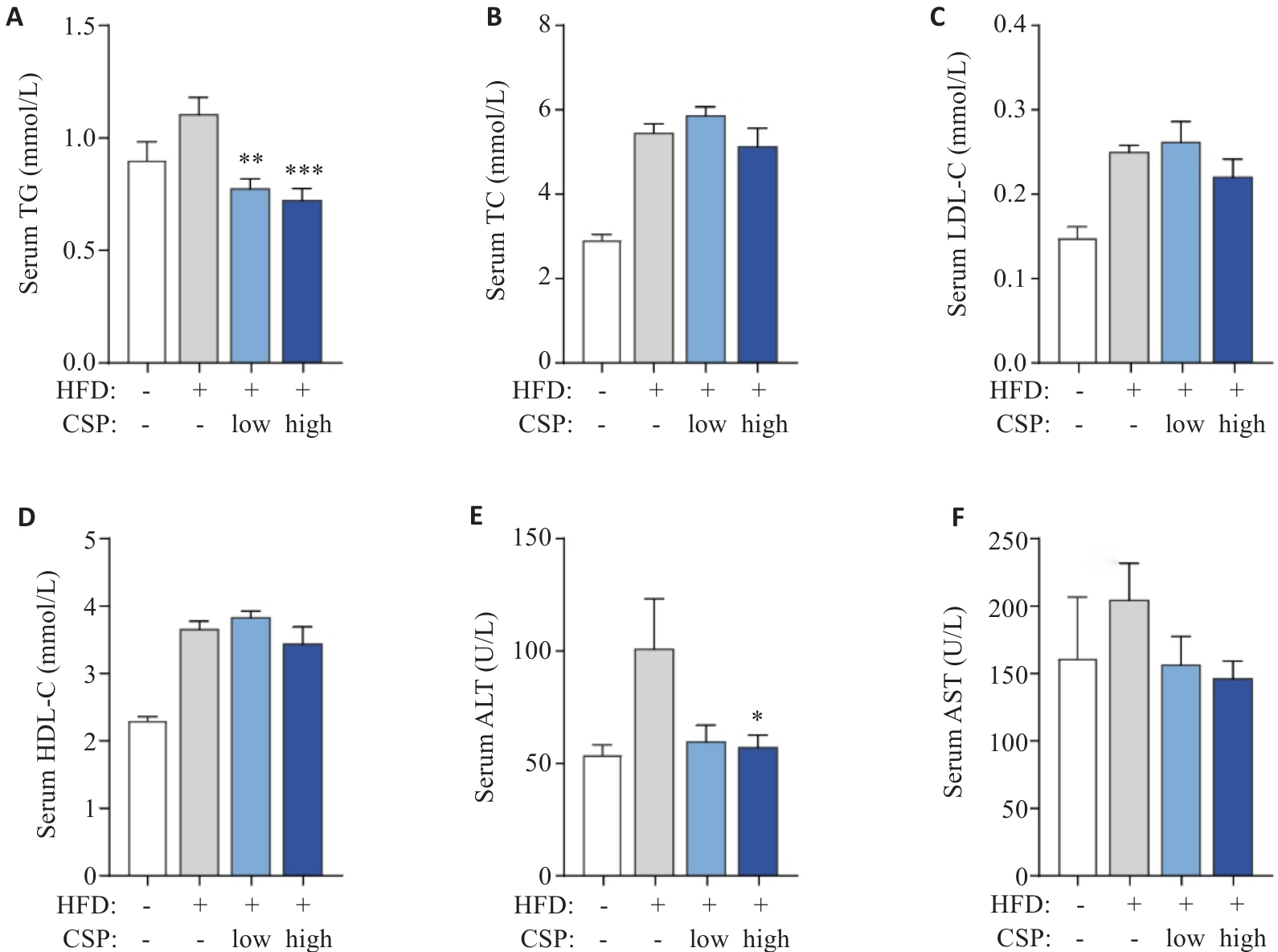
Fig.2 Serum biochemical parameters of the mice in each group. A: Triglycerides (TG) in serum. B: Total cholesterol (TC) in serum. C: Low-density lipoprotein cholesterol (LDL-C) in serum. D: High-density lipoprotein cholesterol (LDL-C) in serum. E: Alanine aminotransferase (ALT) in serum. F: Aspartate aminotransferase (AST) in serum. *P<0.05, **P <0.01, ***P<0.001 vs HFD group.
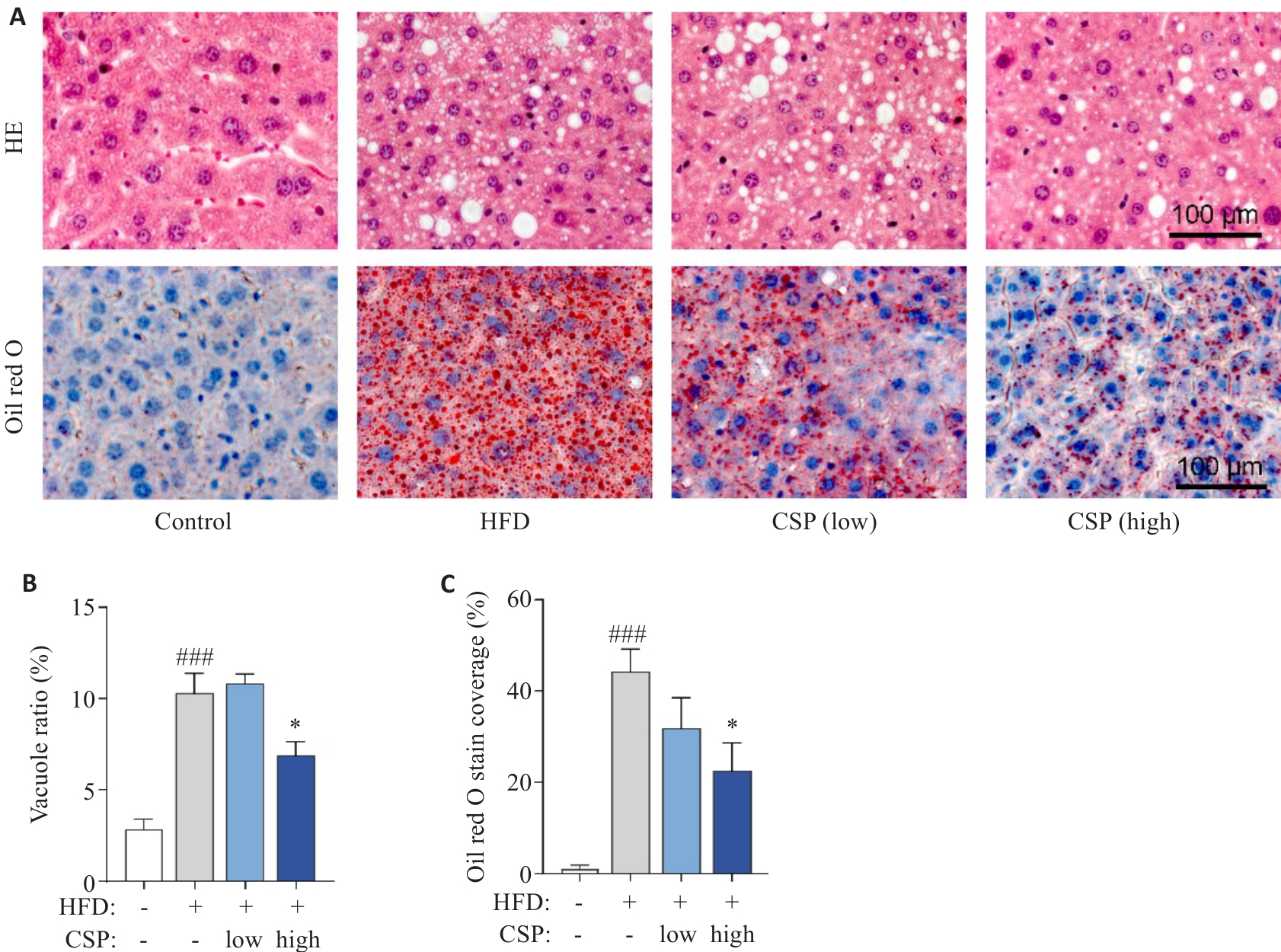
Fig.3 Histopathological staining of the liver tissues of the mice in each group. A: HE staining and Oil Red O staining of the liver tissues. B: HE bubble vacuole ratio in each group. C: Quantification of Oil Red O stain coverage in each group. *P<0.001 vs HFD group; ###P<0.001 vs Control group.
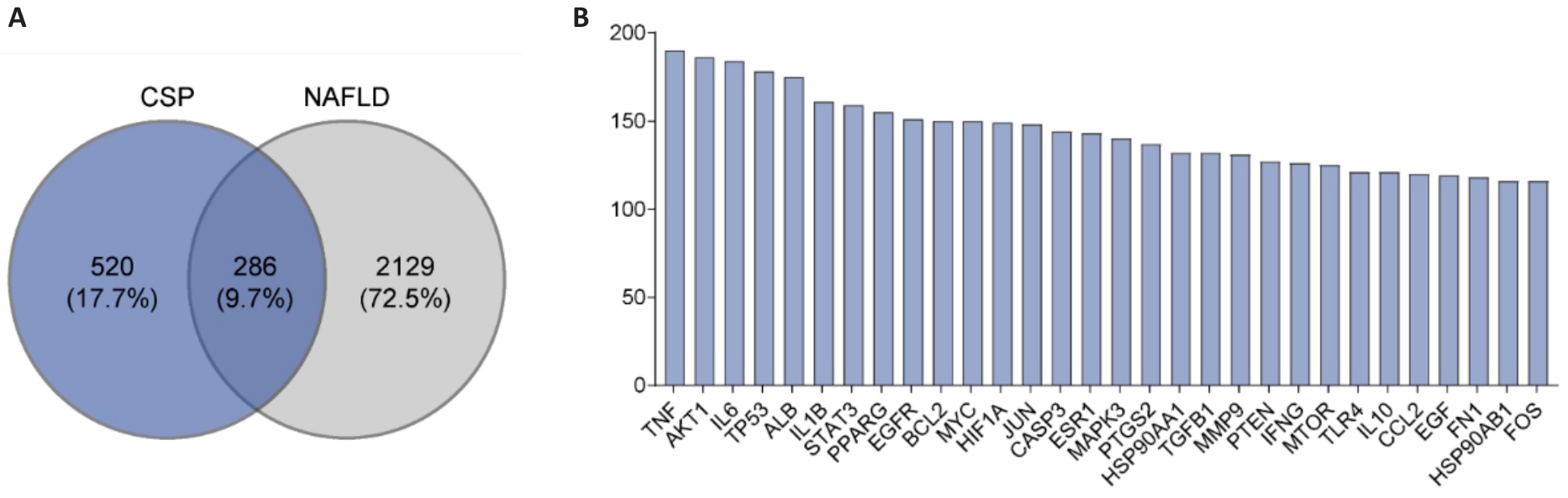
Fig.4 Common targets of Ching Shum Pill (CSP) and non-alcoholic fatty liver disease (NAFLD). A: Venn diagram of CSP- and NAFLD-related targets. B: Top 30 key targets of CSP active ingredients in NAFLD treatment.
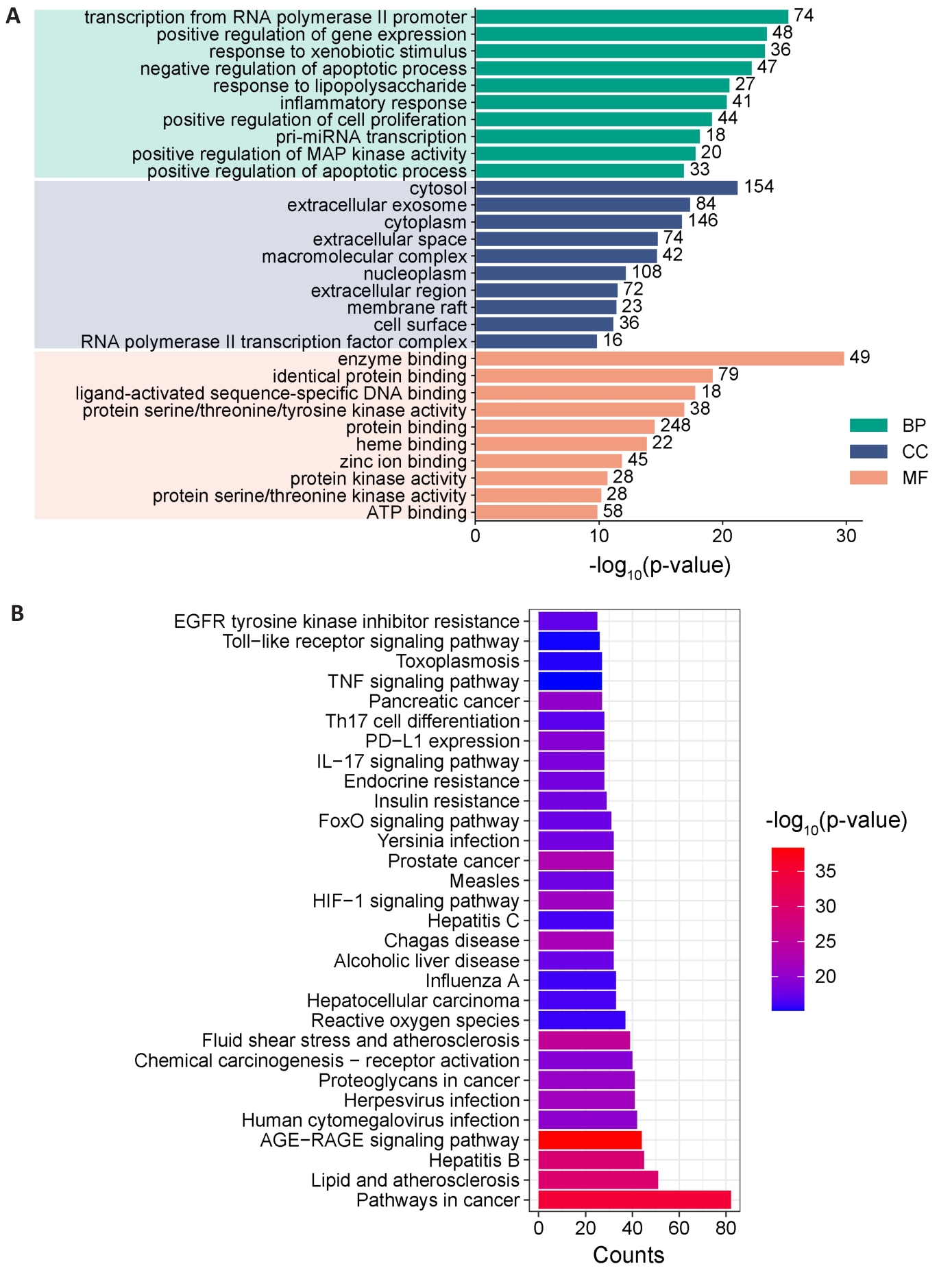
Fig.6 Key target enrichment analysis of CSP for NAFLD treatment. A: GO enrichment analysis, including biological process (BP), cellular components (CC) and molecular function (MF). B: KEGG enrichment analysis.
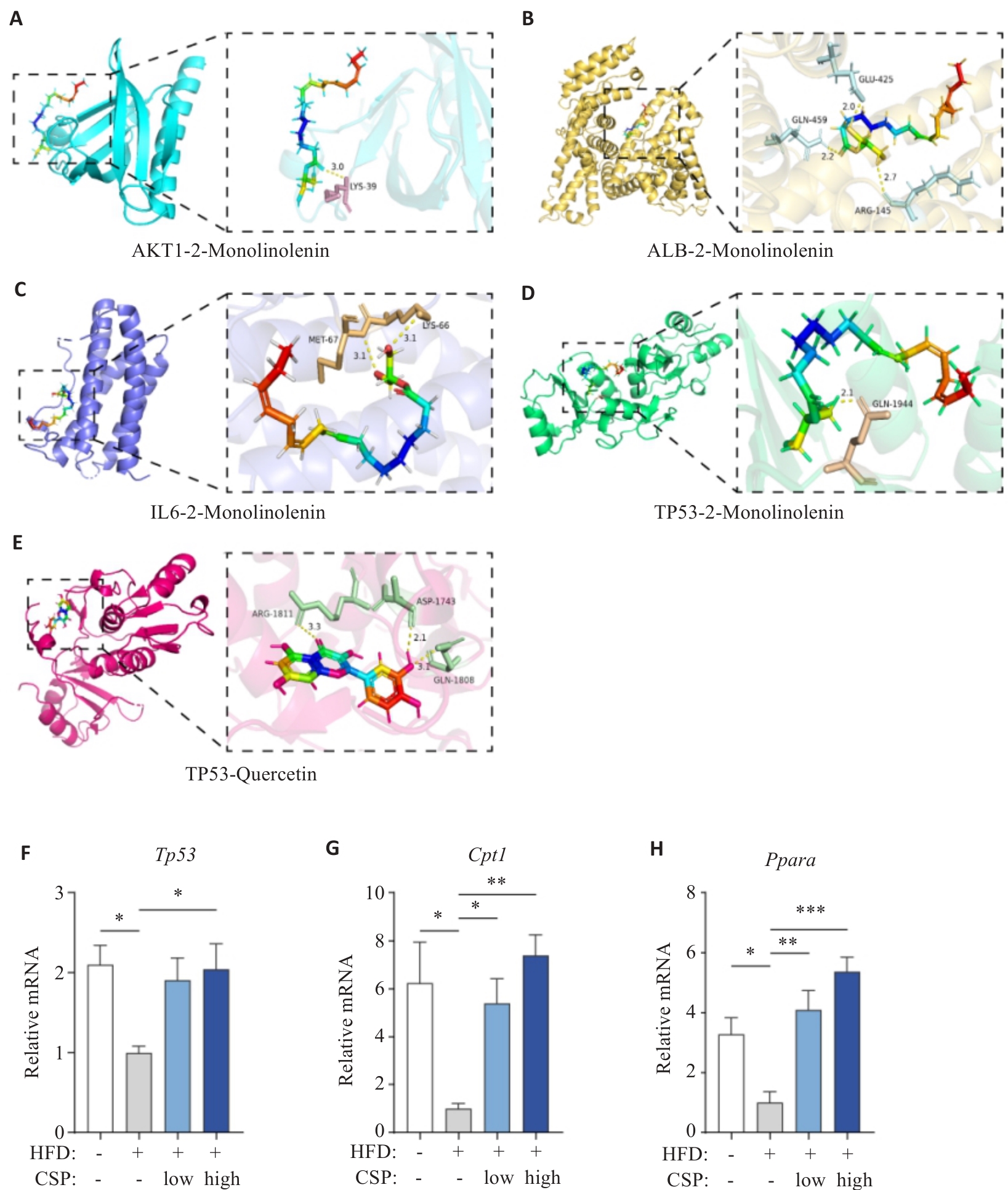
Fig.7 Molecular docking and RT-qPCR analysis of the expression levels of Tp53, Cpt1, and Ppara in mouse liver tissues. A: Docking between AKT1 and monolinolenin. B: Docking between ALB-2 and monolinolenin. C: Docking between IL-6 and monolinolenin. D: Docking between TP53 and monolinolenin. E: Docking between TP53 and quercetin. F: Tp53 mRNA expression. G: Cpt1 mRNA expression. H: Ppara mRNA expression. Data are normalized to β-actin and presented as relative values to the mean of the HFD group. *P<0.05, **P<0.01, ***P<0.001.
| [1] | 范建高, 徐小元, 南月敏, 等. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 实用肝脏病杂志, 2024, 27(4): 494-510. |
| [2] | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease[J]. Hepatology, 2023, 77(5): 1797-835. doi:10.1097/hep.0000000000000323 |
| [3] | Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease[J]. Gastroenterology, 2020, 158(7): 1851-64. doi:10.1053/j.gastro.2020.01.052 |
| [4] | Maurice J, Manousou P. Non-alcoholic fatty liver disease[J]. Clin Med, 2018, 18(3): 245-50. doi:10.7861/clinmedicine.18-3-245 |
| [5] | Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review[J]. Hepatology, 2023, 77(4): 1335-47. doi:10.1097/hep.0000000000000004 |
| [6] | Zhou J, Zhou F, Wang W, et al. Epidemiological features of NAFLD from 1999 to 2018 in China[J]. Hepatology, 2020, 71(5): 1851-64. doi:10.1002/hep.31150 |
| [7] | Stefan N, Schick F, Birkenfeld AL, et al. The role of hepatokines in NAFLD[J]. Cell Metab, 2023, 35(2): 236-52. doi:10.1016/j.cmet.2023.01.006 |
| [8] | 赵文霞, 许二平, 王宪波, 等. 非酒精性脂肪性肝炎中医诊疗指南[J]. 临床肝胆病杂志, 2023, 39(5): 1041-8. doi:10.3969/j.issn.1001-5256.2023.05.007 |
| [9] | 彭琳瑞, 王 柳, 杨晓玲, 等. 非酒精性脂肪性肝病的管理[J]. 中国实用内科杂志, 2023, 43(12): 1028-30. |
| [10] | 隆晓荣, 袁 宁, 陶然, 等. 中医药治疗非酒精性脂肪性肝病的研究进展[J]. 中西医结合肝病杂志, 2023, 33(9): 860-4. doi:10.3969/j.issn.1005-0264.2023.009.023 |
| [11] | Yan ZB, Miao XK, Zhang BZ, et al. p53 as a double-edged sword in the progression of non-alcoholic fatty liver disease[J]. Life Sci, 2018, 215: 64-72. doi:10.1016/j.lfs.2018.10.051 |
| [12] | Yao P, Zhang Z, Liu H, et al. p53 protects against alcoholic fatty liver disease via ALDH2 inhibition[J]. EMBO J, 2023, 42(8): e112304. doi:10.15252/embj.2022112304 |
| [13] | Zheng S, Xue C, Li S, et al. Chinese medicine in the treatment of non-alcoholic fatty liver disease based on network pharmacology: a review[J]. Front Pharmacol, 2024, 15: 1381712. doi:10.3389/fphar.2024.1381712 |
| [14] | Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030[J]. J Hepatol, 2018, 69(4): 896-904. doi:10.1016/j.jhep.2018.05.036 |
| [15] | 王晓杰, 张 恒, 刘素彤, 等. 小檗碱治疗非酒精性脂肪性肝病的作用机制及联合用药研究进展[J]. 中国实验方剂学杂志, 2025, 31(5): 269-81. |
| [16] | 周亚丽, 杨 萍, 李喜香, 等. 半夏化学成分与药理作用研究进展及其质量标志物(Q-Marker)预测[J]. 中草药, 2024, 55(14): 4939-52. doi:10.7501/j.issn.0253-2670.2024.14.029 |
| [17] | 白庆云, 陶思敏, 田锦鸿, 等. 黄芩对肝病的防治作用及机制研究进展[J]. 中国中药杂志, 2020, 45(12): 2808-16. doi:10.19540/j.cnki.cjcmm.20200224.403 |
| [18] | 贺雯茜, 张程亮, 向 东, 等. 基于血清药理学技术研究体外培育牛黄抑制肝细胞脂质沉积的作用机制[J]. 中国中药杂志, 2019, 44(17): 3780-5. doi:10.19540/j.cnki.cjcmm.20190416.402 |
| [19] | Jiang L, Yi R, Chen H, et al. Quercetin alleviates metabolic-associated fatty liver disease by tuning hepatic lipid metabolism, oxidative stress and inflammation[J]. Anim Biotechnol, 2025, 36(1): 2442351. doi:10.1080/10495398.2024.2442351 |
| [20] | Katsaros I, Sotiropoulou M, Vailas M, et al. The effect of quercetin on non-alcoholic fatty liver disease (NAFLD) and the role of Beclin1, P62, and LC3: an experimental study[J]. Nutrients, 2024, 16(24): 4282. doi:10.3390/nu16244282 |
| [21] | Gao XR, Chen Z, Fang K, et al. Protective effect of quercetin against the metabolic dysfunction of glucose and lipids and its associated learning and memory impairments in NAFLD rats[J]. Lipids Health Dis, 2021, 20(1): 164. doi:10.1186/s12944-021-01590-x |
| [22] | Liu L, Gao C, Yao P, et al. Quercetin alleviates high-fat diet-induced oxidized low-density lipoprotein accumulation in the liver: implication for autophagy regulation[J]. Biomed Res Int, 2015, 2015: 607531. doi:10.1155/2015/607531 |
| [23] | Saleh Al-Maamari JN, Rahmadi M, Panggono SM, et al. The effects of quercetin on the expression of SREBP-1c mRNA in high-fat diet-induced NAFLD in mice[J]. J Basic Clin Physiol Pharmacol, 2021, 32(4): 637-44. doi:10.1515/jbcpp-2020-0423 |
| [24] | Dong J, Zhang X, Zhang L, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1[J]. J Lipid Res, 2014, 55(3): 363-74. doi:10.1194/jlr.m038786 |
| [25] | Fan YY, Ren YJ, Deng LQ, et al. Testosterone deficiency aggravates diet-induced non-alcoholic fatty liver disease by inducing hepatocyte ferroptosis via targeting BMAL1 in mice[J]. Int Immunopharmacol, 2025, 144: 113641. doi:10.1016/j.intimp.2024.113641 |
| [26] | Kasarinaite A, Sinton M, Saunders PTK, et al. The influence of sex hormones in liver function and disease[J]. Cells, 2023, 12(12): 1604. doi:10.3390/cells12121604 |
| [27] | 郭 艳, 郑明明, 李晓钰, 等. α-亚麻酸植物甾醇酯改善非酒精性脂肪性肝病作用[J]. 中国公共卫生, 2021, 37(3): 512-5. |
| [28] | Kruse M, Kemper M, Gancheva S, et al. Dietary rapeseed oil supplementation reduces hepatic steatosis in obese men-a randomized controlled trial[J]. Mol Nutr Food Res, 2020, 64(21): e2000419. doi:10.1002/mnfr.202000419 |
| [29] | 李 钰. α-亚麻酸植物甾醇酯基于AMPK调节线粒体功能及氧化应激改善非酒精性脂肪性肝病的作用和分子机制[D]. 山西医科大学, 2021. |
| [30] | Shan Z, Zhang H, He C, et al. High-protein mulberry leaves improve glucose and lipid metabolism via activation of the PI3K/Akt/PPARα/CPT-1 pathway[J]. Int J Mol Sci, 2024, 25(16): 8726. doi:10.3390/ijms25168726 |
| [31] | Lacroix M, Riscal R, Arena G, et al. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer[J]. Mol Metab, 2020, 33: 2-22. doi:10.1016/j.molmet.2019.10.002 |
| [32] | Chu XR, Zhou Y, Zhang SM, et al. Chaetomorpha linum polysaccharides alleviate NAFLD in mice by enhancing the PPARα/CPT-1/MCAD signaling[J]. Lipds Health Dis, 2022, 21(1): 140. doi:10.1186/s12944-022-01730-x |
| [1] | Ruimin HAN, Manke ZHAO, Junfang YUAN, Zhenhong SHI, Zhen WANG, Defeng WANG. Live combined Bacillus subtilis and Enterococcus faecium improves glucose and lipid metabolism in type 2 diabetic mice with circadian rhythm disruption via the SCFAs/GPR43/GLP-1 pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1490-1497. |
| [2] | Dandan LI, Jiaxin CHU, Yan YAN, Wenjun XU, Xingchun ZHU, Yun SUN, Haofeng DING, Li REN, Bo ZHU. Curcumin inhibits lipid metabolism in non-small cell lung cancer by downregulating the HIF-1α pathway [J]. Journal of Southern Medical University, 2025, 45(5): 1039-1046. |
| [3] | Hongyan SUN, Guoqing LU, Chengwen FU, Mengwen XU, Xiaoyi ZHU, Guoquan XING, Leqiang LIU, Yufei KE, Lemei CUI, Ruiyang CHEN, Lei WANG, Pinfang KANG, Bi TANG. Quercetin ameliorates myocardial injury in diabetic rats by regulating L-type calcium channels [J]. Journal of Southern Medical University, 2025, 45(3): 531-541. |
| [4] | Pengwei HUANG, Jie CHEN, Jinhu ZOU, Xuefeng GAO, Hong CAO. Quercetin mitigates HIV-1 gp120-induced rat astrocyte neurotoxicity via promoting G3BP1 disassembly in stress granules [J]. Journal of Southern Medical University, 2025, 45(2): 304-312. |
| [5] | Junjie GAO, Kai YE, Jing WU. Quercetin inhibits proliferation and migration of clear cell renal cell carcinoma cells by regulating TP53 gene [J]. Journal of Southern Medical University, 2025, 45(2): 313-321. |
| [6] | Qing SHI, Suye RAN, Lingyu SONG, Hong YANG, Wenjuan WANG, Hanlin LIU, Qi LIU. NLRP6 overexpression improves nonalcoholic fatty liver disease by promoting lipid oxidation and decomposition in hepatocytes through the AMPK/CPT1A/PGC1A pathway [J]. Journal of Southern Medical University, 2025, 45(1): 118-125. |
| [7] | Xiupeng LONG, Shun TAO, Shen YANG, Suyun LI, Libing RAO, Li LI, Zhe ZHANG. Quercetin improves heart failure by inhibiting cardiomyocyte apoptosis via suppressing the MAPK signaling pathway [J]. Journal of Southern Medical University, 2025, 45(1): 187-196. |
| [8] | Qing LIU, Jing LIU, Yihang ZHENG, Jin LEI, Jianhua HUANG, Siyu LIU, Fang LIU, Qunlong PENG, Yuanfang ZHANG, Junjie WANG, Yujuan LI. Quercetin mediates the therapeutic effect of Centella asiatica on psoriasis by regulating STAT3 phosphorylation to inhibit the IL-23/IL-17A axis [J]. Journal of Southern Medical University, 2025, 45(1): 90-99. |
| [9] | Yifan JIANG, Xiaorong LI, Jiayi GENG, Yongfeng CHEN, Bi TANG, Pinfang KANG. Quercetin ameliorates diabetic kidney injury in rats by inhibiting the HMGB1/RAGE/ NF-κB signaling pathway [J]. Journal of Southern Medical University, 2024, 44(9): 1769-1775. |
| [10] | Xianheng ZHANG, Jian LIU, Qi HAN, Yiming CHEN, Xiang DING, Xiaolu CHEN. Huangqin Qingrechubi Capsule alleviates inflammation and uric acid and lipid metabolism imbalance in rats with gouty arthritis by inhibiting the PTEN/PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(8): 1450-1458. |
| [11] | Jing XIAO, Ying LI, Min FANG, Hong GONG, Wen LI, Chunyan ZHANG, Fangyao CHEN, Yan ZHANG, Tuo HAN. Triglyceride-glucose index in non-obese individuals: its association with and predictive value for non-alcoholic fatty liver disease [J]. Journal of Southern Medical University, 2024, 44(7): 1266-1271. |
| [12] | Ping SHU, Mengke YUAN, Ke YANG, Weizhi HE, Li LIU. Quercetin suppresses pyroptosis in mouse fibroblasts by inhibiting the NLRP3/caspase-1/GSDMD pathway [J]. Journal of Southern Medical University, 2024, 44(10): 1874-1880. |
| [13] | Chengling CUI, Yuzhen XU, Chaoqun TANG, Jiaying JIANG, Ying HU, Jie SHUANG. Molecular mechanism of high-altitude hypoxia-induced lipid metabolism disorder in mouse spleen tissue [J]. Journal of Southern Medical University, 2024, 44(10): 2024-2032. |
| [14] | KONG Xiang, ZHANG Teng, ZHANG Yan, GAO Linxi, WANG Wen, WANG Mengyan, WANG Guodong, LÜ Kun. Overexpression of lncRNA HEM2M alleviates liver injury in mice with non-alcoholic fatty liver disease [J]. Journal of Southern Medical University, 2024, 44(1): 1-8. |
| [15] | ZHANG Ningning, QIU Qi, CHEN Yongfeng, SUN Zhengyu, LU Guoqing, WANG Lei, KANG Pingfang, WANG Hongju. Quercetin improves pulmonary arterial hypertension in rats by regulating the HMGB1/RAGE/NF-κB pathway [J]. Journal of Southern Medical University, 2023, 43(9): 1606-1612. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||