Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (9): 1830-1839.doi: 10.12122/j.issn.1673-4254.2025.09.03
Qinjun YANG1( ), Hongyu ZHU1, Yuan GAO1, Cheng YANG2, Tong LIU1, Lu ZHANG1, Jiabing TONG1,2,3, Zegeng LI2,3,4(
), Hongyu ZHU1, Yuan GAO1, Cheng YANG2, Tong LIU1, Lu ZHANG1, Jiabing TONG1,2,3, Zegeng LI2,3,4( )
)
Received:2025-04-08
Online:2025-09-20
Published:2025-09-28
Contact:
Zegeng LI
E-mail:yangqjahtcm@163.com;li6609@126.com
Supported by:Qinjun YANG, Hongyu ZHU, Yuan GAO, Cheng YANG, Tong LIU, Lu ZHANG, Jiabing TONG, Zegeng LI. Sangma Zhike Formula alleviates airway inflammation and hyperresponsiveness in rats with postinfectious cough by inhibiting the TRPV1-SP/CGRP and pyroptosis pathways[J]. Journal of Southern Medical University, 2025, 45(9): 1830-1839.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.09.03
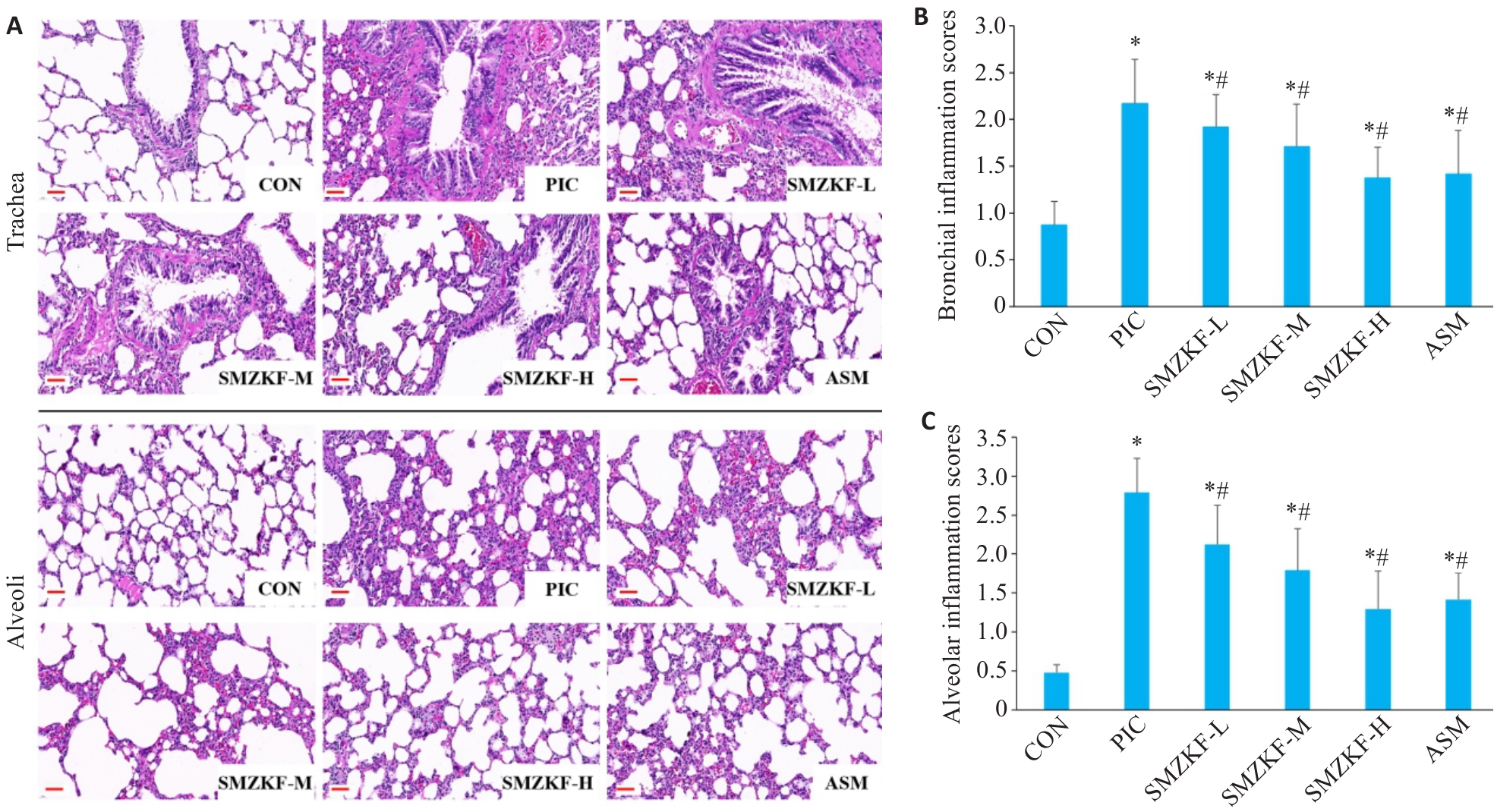
Fig.3 Lung tissue pathology and bronchial and alveolar inflammation scores in each group of rats. A: HE staining of rat lung tissue (Original magnification: ×400). B: Bronchial inflammation scores. C: Alveolar inflammation scores. *P<0.05 vs CON group, #P<0.05 vs PIC group.
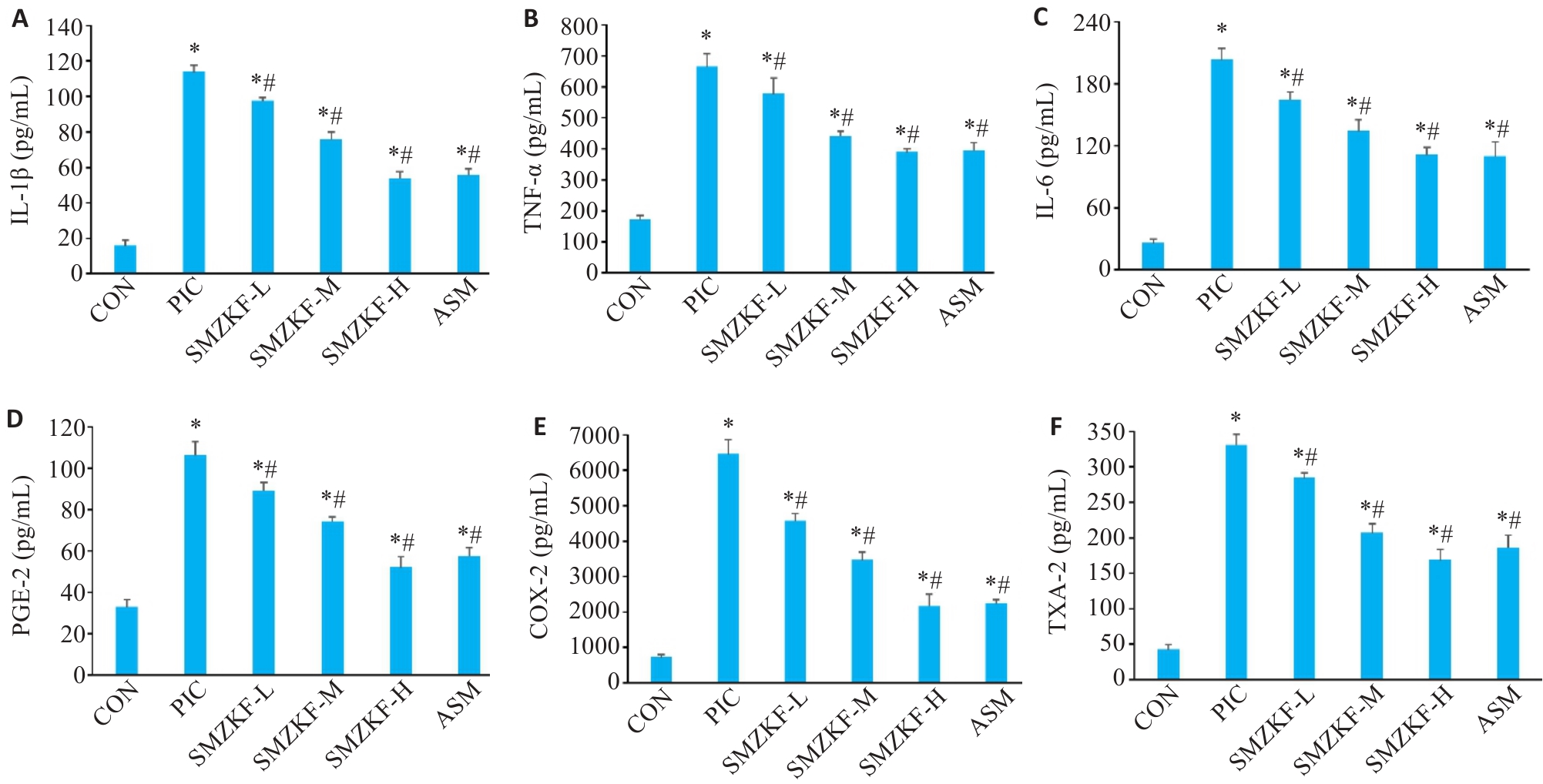
Fig.5 Levels of inflammatory factors and mediators in the BALF of the rats in each group. A: IL-1β. B: TNF-α. C: IL-6. D: PGE-2. E: COX-2. F: TXA-2. *P<0.05 vs CON group, #P<0.05 vs PIC group.
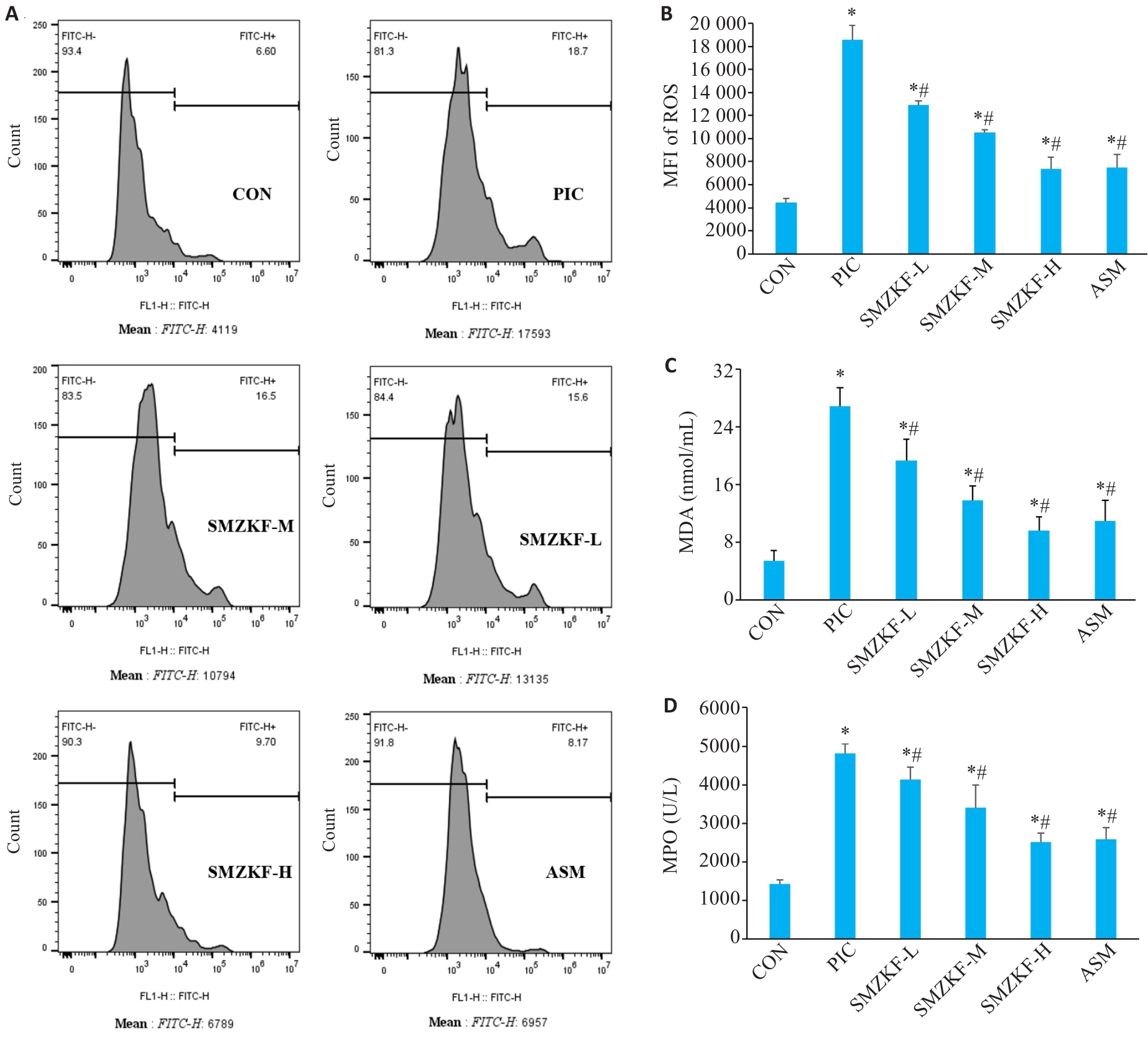
Fig.6 Levels of ROS and MDA in the lung tissues of the rats in each group. A: Flow cytometry for detecting ROS in the lung tissue. B: ROS. C: MDA. D: MPO. *P<0.05 vs CON group, #P<0.05 vs PIC group.
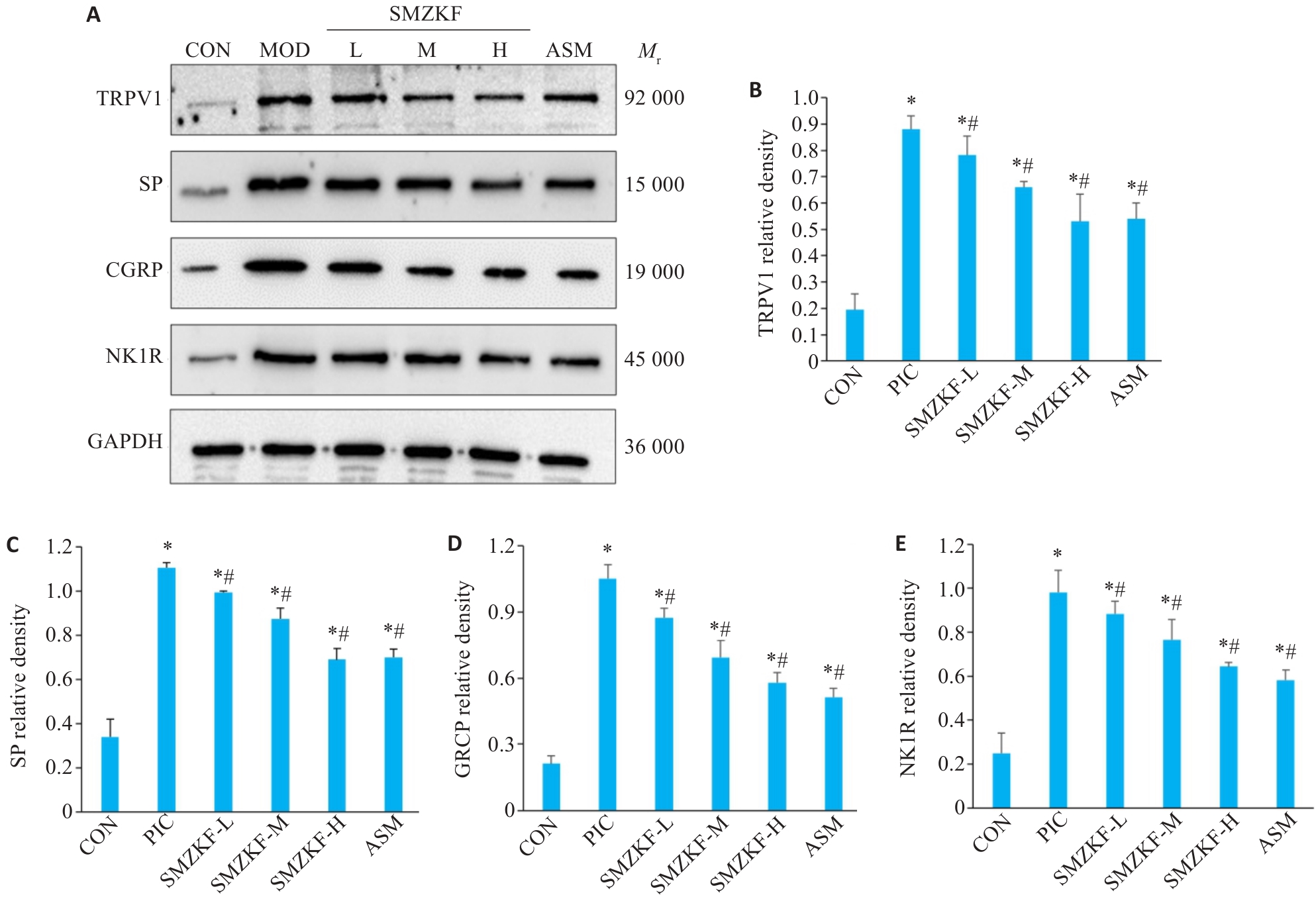
Fig.7 Expressions of airway sensitivity-related proteins in the lung tissues of the rats in each group. A: Western blotting for detecting expressions of TRPV1, SP, CGRP and NK1R in the lung tissue. B: TRPV1. C: SP. D: CGRP. E: NK1R. *P<0.05 vs CON group, #P<0.05 vs PIC group.
| [1] | 赖克方, 聂怡初. 感染后咳嗽发病机制、诊断与治疗研究进展[J]. 中华肺部疾病杂志, 2014, 7(5): 481-5. |
| [2] | 吴 蕾, 黄敏玲, 许银姬, 等. 感染后咳嗽中医诊疗方案建立与评价的思路探讨[J]. 中华中医药杂志, 2010, 25(6): 888-90. |
| [3] | Ishida T, Yokoyama T, Iwasaku M, et al. Clinical investigation of postinfectious cough among adult patients with prolonged cough[J]. Nihon Kokyuki Gakkai Zasshi, 2010, 48(3): 179-85. |
| [4] | 那飞扬, 杨 译, 王 雍, 等. 2014-2023年兰州地区儿童慢性咳嗽的病因研究[J].中国全科医学, 2025, 1-6[2025-03-20]. |
| [5] | 花盛浩, 邵雪君, 徐 俊. 新型冠状病毒肺炎常态化防控下儿童常见呼吸道病毒感染谱的变化[J]. 中华传染病杂志, 2021, 39(10): 621-5. |
| [6] | Ryan NM, Vertigan AE, Ferguson J, et al. Clinical and physiological features of postinfectious chronic cough associated with H1N1 infection[J]. Respir Med, 2012, 106(1): 138-44. doi:10.1016/j.rmed.2011.10.007 |
| [7] | 胡诣璋, 赵 俊, 崔 瑷. 感染后咳嗽发病机制及治疗进展[J]. 临床药物治疗杂志, 2016, 14(1): 11-6. |
| [8] | Yang Y, Huang L, Tian C, et al. Magnesium isoglycyrrhizinate inhibits airway inflammation in rats with chronic obstructive pulmonary disease[J]. BMC Pulm Med, 2021, 21(1): 371. doi:10.1186/s12890-021-01745-7 |
| [9] | Li Y, Zhao RH, Zhang MY, et al. Xingbei antitussive granules ameliorate cough hypersensitivity in post-infectious cough guinea pigs by regulating tryptase/PAR2/TRPV1 pathway[J]. J Ethnopharmacol, 2024, 319: 117243. doi:10.1016/j.jep.2023.117243 |
| [10] | 张恩光, 赵兰才. 赵兰才感染后咳嗽证治经验[J]. 中华中医药杂志, 2019, 34(7): 3069-71. |
| [11] | Luo J, Deng Y, Ding Y, et al. Xiebai Zengye decoction improves respiratory function and attenuates inflammation in juvenile rats with postinfection cough via regulating ERK signaling pathway[J]. Cell Biochem Funct, 2023, 41(7): 857-67. doi:10.1002/cbf.3835 |
| [12] | Ridzuan N, Zakaria N, Widera D, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD)[J]. Stem Cell Res Ther, 2021, 12(1): 54. doi:10.1186/s13287-020-02088-6 |
| [13] | 杨勤军, 王 卉, 徐淑钰, 等. 参芪调肾方减轻慢阻肺肺肾气虚证大鼠气道炎症的机制: 基于铁死亡途径[J]. 南方医科大学学报, 2024, 44(10): 1937-46. doi:10.12122/j.issn.1673-4254.2024.10.12 |
| [14] | Tamasauskiene L, Sitkauskiene B. Immune system in the pathogenesis of chronic cough[J]. Immunol Lett, 2020, 218: 40-3. doi:10.1016/j.imlet.2019.12.013 |
| [15] | 任红芳, 罗 燕, 杨玉凤, 等. 经方射干麻黄汤配合氨溴特罗治疗感染后咳嗽(风寒恋肺证)疗效及对中医证候积分和神经源性气道炎症介质的影响[J]. 中国中医急症, 2019, 28(2): 269-72. |
| [16] | Regulska M, Szuster-Głuszczak M, Trojan E, et al. The emerging role of the double-edged impact of arachidonic acid- derived eicosanoids in the neuroinflammatory background of depression[J]. Curr Neuropharmacol, 2021, 19(2): 278-93. doi:10.2174/1570159x18666200807144530 |
| [17] | 甘 雨, 马跃海, 包玉龙, 等. 射干止咳胶囊缓解感染后咳嗽及对花生四烯酸代谢通路的影响[J]. 时珍国医国药, 2023, 34(4): 828-31. |
| [18] | 卢 朋, 韩 梁, 白 雷, 等.中药治疗感染后咳嗽的作用机制研究进展[J].药物评价研究, 2025, 48(3): 757-69. |
| [19] | Gao X, Zhuang J, Zhao L, et al. Cross-effect of TRPV1 and EP3 receptor on coughs and bronchopulmonary C-neural activities[J]. PLoS One, 2021, 16(2): e0246375. doi:10.1371/journal.pone.0246375 |
| [20] | 周 勇, 赵心安, 崔 勇. 脂类炎症介质拮抗剂治疗哮喘的研究进展[J]. 中国医院药学杂志, 1999, 19(3): 43-5. |
| [21] | Chlopicki S, Olszanecki R, Janiszewski M, et al. Functional role of NADPH oxidase in activation of platelets[J]. Antioxid Redox Signal, 2004, 6(4): 691-8. doi:10.1089/1523086041361640 |
| [22] | 魏鑫淼, 杨启帆, 田家豪, 等. TRPV1通道的功能、门控机制及其调节剂在药物研发中的应用[J]. 生物化学与生物物理进展, 2023, 50(3):421-36 |
| [23] | Zhang L, Sun TY, Liu LT, et al. The research of the possible mechanism and the treatment for capsaicin-induced cough[J]. Pulm Pharmacol Ther, 2018, 49: 1-9. doi:10.1016/j.pupt.2017.12.008 |
| [24] | Liang M, Meng Y, Wang X, et al. The effectiveness of wogonin on treating cough mice with Mycoplasma pneumoniae infection[J]. Front Mol Biosci, 2022, 9: 803842. doi:10.3389/fmolb.2022.803842 |
| [25] | Canning BJ. Anatomy and neurophysiology of the cough reflex ACCP evidence-based clinical practice guidelines[J]. Chest, 2006, 129(1): 33S-47S. doi:10.1378/chest.129.1_suppl.33s |
| [26] | 王 胜, 张曦煜, 郑莉莉. 基于TRPV1探讨调气止咳方改善感染后咳嗽大鼠气道神经源性炎症的机制[J]. 时珍国医国药, 2023, 34(11):2615-9. |
| [27] | Raffaelli B, Reuter U. The biology of monoclonal antibodies: focus on calcitonin gene-related peptide for prophylactic migraine therapy[J]. Neurotherapeutics, 2018, 15(2): 324-35. doi:10.1007/s13311-018-0622-7 |
| [28] | Zhong X, Zhang Z, Li J, et al. Effects of electroacupuncture on gastrointestinal motility function, pain, and inflammation via transient receptor potential vanilloid 1 in a rat model after colonic anastomoses[J]. Dis Markers, 2022, 2022: 5113473. doi:10.1155/2022/5113473 |
| [29] | Piao JJ, Kim S, Shin D, et al. Cannabidiol alleviates chronic prostatitis and chronic pelvic pain syndrome via CB2 receptor activation and TRPV1 desensitization[J]. World J Mens Health, 2025, 43(1): 228-38. doi:10.5534/wjmh.230352 |
| [30] | 中华医学会呼吸病学分会哮喘学组.咳嗽的诊断与治疗指南(2015)[J].中华结核和呼吸杂志, 2016, 39(5): 323-54. |
| [31] | Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases[J]. Signal Transduct Target Ther, 2021, 6(1): 128. doi:10.1038/s41392-021-00507-5 |
| [32] | Hirota JA, Gold MJ, Hiebert PR, et al. The nucleotide-binding domain, leucine-rich repeat protein 3 inflammasome/IL-1 receptor I axis mediates innate, but not adaptive, immune responses after exposure to particulate matter under 10 μm[J]. Am J Respir Cell Mol Biol, 2015, 52(1): 96-105. doi:10.1165/rcmb.2014-0158oc |
| [33] | Rao Z, Zhu Y, Yang P, et al. Pyroptosis in inflammatory diseases and cancer[J]. Theranostics, 2022, 12(9): 4310-29. doi:10.7150/thno.71086 |
| [34] | Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation[J]. Int J Mol Sci, 2019, 20(13): E3328. doi:10.3390/ijms20133328 |
| [35] | Li Y, Wang J, Huang D, et al. Baicalin alleviates contrast-induced acute kidney injury through ROS/NLRP3/caspase-1/GSDMD pathway-mediated proptosis in vitro [J]. Drug Des Devel Ther, 2022, 16: 3353-64. doi:10.2147/dddt.s379629 |
| [36] | Qadri M, Jay GD, Zhang LX, et al. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages[J]. Arthritis Res Ther, 2018, 20(1): 192. doi:10.1186/s13075-018-1693-x |
| [37] | Guo W, Liu W, Chen Z, et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis[J]. Nat Commun, 2017, 8(1): 2168. doi:10.1038/s41467-017-02351-0 |
| [38] | Li Q, Hua X, Li L, et al. AIP1 suppresses neovascularization by inhibiting the NOX4-induced NLRP3/NLRP6 imbalance in a murine corneal alkali burn model[J]. Cell Commun Signal, 2022, 20(1): 59. doi:10.1186/s12964-022-00877-5 |
| [39] | Li M, Wang C, Yu Z, et al. MgIG exerts therapeutic effects on crizotinib-induced hepatotoxicity by limiting ROS-mediated autophagy and pyroptosis[J]. J Cell Mol Med, 2022, 26(16): 4492-505. doi:10.1111/jcmm.17474 |
| [40] | Jing GQ, Zuo J, Fang Q, et al. Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1α/Xbp1s-Ca2+ axis[J]. J Neuroinflammation, 2022, 19(1): 237. doi:10.1186/s12974-022-02598-5 |
| [41] | Zhuang J, Cui H, Zhuang L, et al. Bronchial epithelial pyroptosis promotes airway inflammation in a murine model of toluene diisocyanate-induced asthma[J]. Biomed Pharmacother, 2020, 125: 109925. doi:10.1016/j.biopha.2020.109925 |
| [1] | Ziyu ZHENG, Xiaying YANG, Shengjie WU, Shijie ZHANG, Guorong LYU, Peizhong LIU, Jun WANG, Shaozheng HE. A multi-feature fusion-based model for fetal orientation classification from intrapartum ultrasound videos [J]. Journal of Southern Medical University, 2025, 45(7): 1563-1570. |
| [2] | Xinheng WANG, Xiaohan SHAO, Tongtong LI, Lu ZHANG, Qinjun YANG, Weidong YE, Jiabing TONG, Zegeng LI, Xiangming FANG. Pingchuanning Formula suppresses airway inflammation in a rat model of asthmatic cold syndrome by regulating the HMGB1/Beclin-1 axis-mediated autophagy [J]. Journal of Southern Medical University, 2025, 45(6): 1153-1162. |
| [3] | Huanyu JI, Rui WANG, Shengxiang GAO, Wengang CHE. SG-UNet: a melanoma segmentation model enhanced with global attention and self-calibrated convolution [J]. Journal of Southern Medical University, 2025, 45(6): 1317-1326. |
| [4] | Suqiang LI, Zhouyang WANG, Sixian CHAN, Xiaolong ZHOU. AConvLSTM U-Net: a multi-scale jaw cyst segmentation model based on bidirectional dense connection and attention mechanism [J]. Journal of Southern Medical University, 2025, 45(5): 1082-1092. |
| [5] | Yancai CHEN, Gaofeng ZHANG, Shubo LI, Nianxiang LUO, Yi ZHANG. Biomechanical analysis of a novel bridging plate for treating Rockwood III acromioclavicular joint dislocation [J]. Journal of Southern Medical University, 2025, 45(5): 1103-1112. |
| [6] | Tianli SONG, Yimin WANG, Tong SUN, Xu LIU, Sheng HUANG, Yun RAN. Zheng Gan Decoction inhibits diethylnitrosamine-induced hepatocellular carcinoma in rats by activating the Hippo/YAP signaling pathway [J]. Journal of Southern Medical University, 2025, 45(4): 799-809. |
| [7] | Yan HUANG, Xi CHEN, Mengchen QIN, Lei GAO. Core targets and immune regulatory mechanisms of Huoluo Xiaoling Pellet for promoting zebrafish fin regeneration [J]. Journal of Southern Medical University, 2025, 45(3): 494-505. |
| [8] | Yuying REN, Lingxiao HUANG, Fang DU, Xinbo YAO. An efficient and lightweight skin pathology detection method based on multi-scale feature fusion using an improved RT-DETR model [J]. Journal of Southern Medical University, 2025, 45(2): 409-421. |
| [9] | Hui ZHANG, Yangyang LIU, Xiapeng LI, Mengyao WANG, Li LI, Haitao WEI. High KHSRP expression promotes gastric adenocarcinoma metastasis: the mediating role of the JAK1/STAT3 signaling axis [J]. Journal of Southern Medical University, 2024, 44(9): 1760-1768. |
| [10] | Qinjun YANG, Hui WANG, Shuyu XU, Cheng YANG, Huanzhang DING, Di WU, Jie ZHU, Jiabing TONG, Zegeng LI. Shenqi Tiaoshen Formula alleviates airway inflammation in rats with chronic obstructive pulmonary disease and kidney qi deficiency syndrome by inhibiting ferroptosis via regulating the Nrf2/SLC7A11/GPX4 signaling pathway [J]. Journal of Southern Medical University, 2024, 44(10): 1937-1946. |
| [11] | DA Rong, ZHOU Yi, CHENG Yue, LV Jia, HAN Bei. UhpTE350Q mutation along with the presence of fosA6/5 genes in the genome probably contributes to inherent fosfomycin resistance of Klebsiella pneumoniae [J]. Journal of Southern Medical University, 2023, 43(7): 1110-1115. |
| [12] | CHEN Rujuan, WU Wei, QIU Yinping. Circular RNA hsa_circ_0087893 participates in intraventricular hemorrhage occurrence and progression possibly as a competitive endogenous RNA in preterm infants [J]. Journal of Southern Medical University, 2023, 43(5): 749-754. |
| [13] | GONG Gao, CAO Shi, XIAO Hui, FANG Weiyang, QUE Yuqing, LIU Ziwei, CHEN Chaomin. Prediction of microvascular invasion in hepatocellular carcinoma with magnetic resonance imaging using models combining deep attention mechanism with clinical features [J]. Journal of Southern Medical University, 2023, 43(5): 839-851. |
| [14] | LIU Lihe, ZHU Mingrui, WANG Yifan, WAN Bo, JIANG Zhi. Molecular pathological mechanism of liver metabolic disorder in mice with severe spinal muscular atrophy [J]. Journal of Southern Medical University, 2023, 43(5): 852-858. |
| [15] | ZHAO Yuxi, ZHAO Xu, ZHU Qingnan, ZHU Bingrui, ZHANG Zhenbin, CHEN Jing. Therapeutic mechanism of Guizhi Gancao Decoction for heart failure: a network pharmacology-based analysis [J]. Journal of Southern Medical University, 2023, 43(5): 772-782. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||