Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (3): 531-541.doi: 10.12122/j.issn.1673-4254.2025.03.11
Hongyan SUN1,2( ), Guoqing LU1,2, Chengwen FU1,2, Mengwen XU3, Xiaoyi ZHU3, Guoquan XING3, Leqiang LIU3, Yufei KE3, Lemei CUI3, Ruiyang CHEN3, Lei WANG1,2, Pinfang KANG1,2(
), Guoqing LU1,2, Chengwen FU1,2, Mengwen XU3, Xiaoyi ZHU3, Guoquan XING3, Leqiang LIU3, Yufei KE3, Lemei CUI3, Ruiyang CHEN3, Lei WANG1,2, Pinfang KANG1,2( ), Bi TANG1,2
), Bi TANG1,2
Received:2024-09-29
Online:2025-03-20
Published:2025-03-28
Contact:
Pinfang KANG, Bi TANG
E-mail:1210499483@qq.com;kangpinfang.1016@163.com
Supported by:Hongyan SUN, Guoqing LU, Chengwen FU, Mengwen XU, Xiaoyi ZHU, Guoquan XING, Leqiang LIU, Yufei KE, Lemei CUI, Ruiyang CHEN, Lei WANG, Pinfang KANG, Bi TANG. Quercetin ameliorates myocardial injury in diabetic rats by regulating L-type calcium channels[J]. Journal of Southern Medical University, 2025, 45(3): 531-541.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.03.11
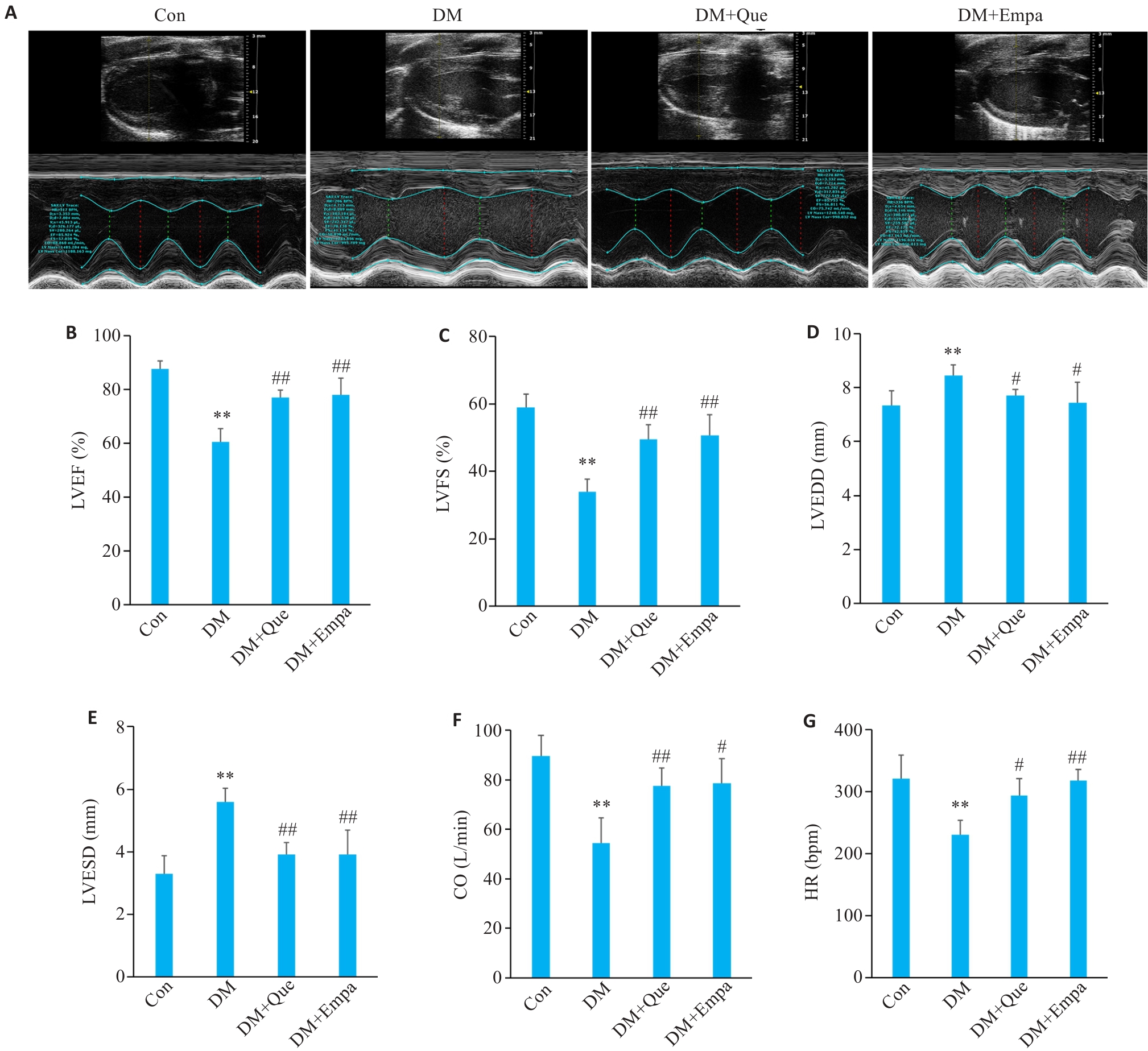
Fig.2 Effect of quercetin on cardiac function in diabetic rats. A: Comparison of echocardiographic findings in each group. B-G: LVEF%, LVFS%, LVEDD, LVESD, CO and HR of SD rats in each group. Data are presented as Mean±SD (n=6), **P<0.01 vs Con; #P<0.05, ##P<0.01 vs DM.
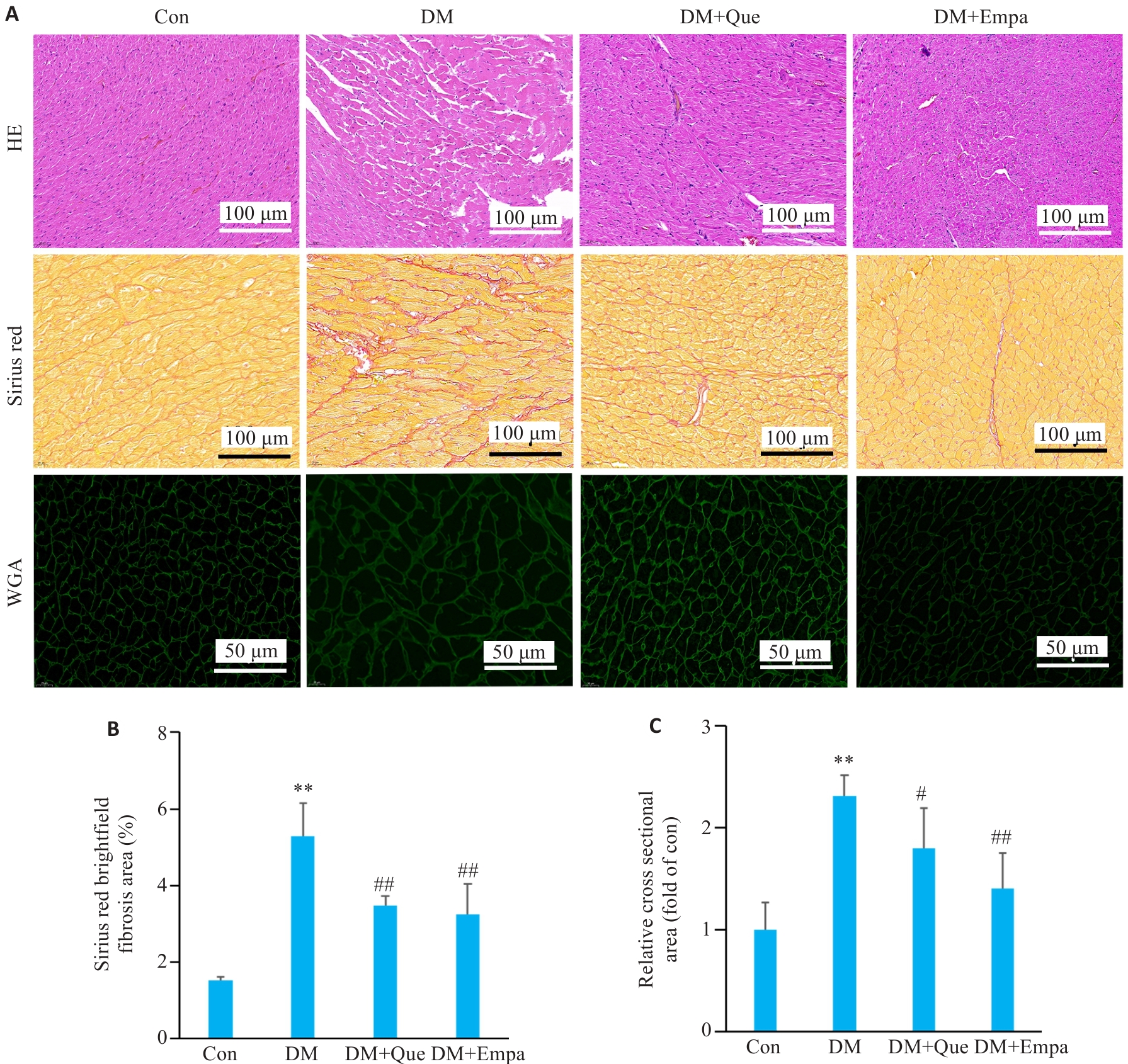
Fig.3 Effect of quercetin on myocardial structure in diabetic rats. A: HE, Sirius Red, and WGA staining results of SD rats in each group. B: Analysis of the results of Sirius Red staining in each group. C: Analysis of WGA staining in each group. Data are presented as Mean±SD (n=3). **P<0.01 vs Con, #P<0.05, ##P<0.01 vs DM.

Fig.4 Effect of quercetin on myocardial FDX1 expression and serum copper level in diabetic rats. A: Immunohistochemical staining of FDX1. B: Quantitative analysis of FDX1 expression levels; C: Serum Cu levels in each group. D, E: FDX1 protein expression levels of SD rats in different groups detected by Western blotting. Data are presented as Mean±SD (n=3). **P<0.01 vs Con; #P<0.05, ##P<0.01 vs DM; △P<0.05, △△P<0.01 vs DM+Empa.

Fig.5 Effect of quercetin on high glucose-induced injury in rat H9C2 cardiomyocytes. A, B: FDX1 protein expression levels of FDX1 protein in each group detected by Westernblotting. C, D: FDX1 expression levels in each group detected by immunofluorescence assay. E, F: CK-MB and LDH levels in each group. Data are presented as Mean±SD (n=3). **P<0.01 vs Con; #P<0.05, ##P<0.01 vs HG.
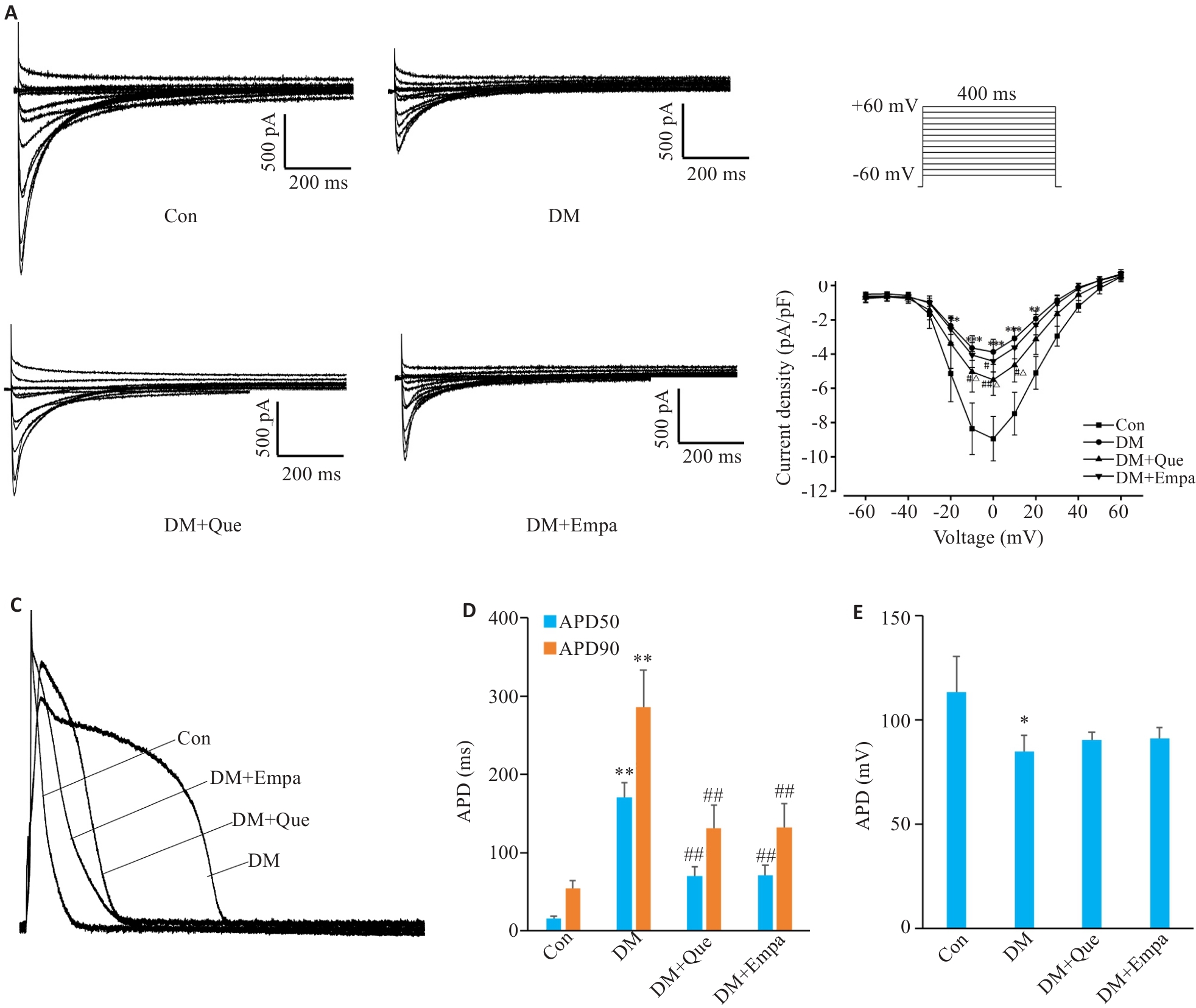
Fig.6 Effects of quercetin on myocardial ICa-L and action potentials (AP) in diabetic rats. A: Representative recording traces ICa-L currents in different groups. B: Current-voltage relationship of ICa-L currents in different groups. C: Representative action potentials (AP) in APD60 and APD90 repolarization and APA recorded in different groups. D, E: APDs and APA in different groups. Data are presented as Mean±SD (n=12), *P<0.05, **P<0.01 vs Con, #P<0.05, ##P<0.01 vs DM, △P<0.05 vs DM+Empa.
| 1 | Kyriazis ID, Hoffman M, Gaignebet L, et al. KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy[J]. Circ Res, 2021, 128(3): 335-57. |
| 2 | Dillmann WH. Diabetic cardiomyopathy[J]. Circ Res, 2019, 124(8): 1160-2. |
| 3 | Jia GH, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy[J]. Nat Rev Endocrinol, 2016, 12(3): 144-53. |
| 4 | Pan GD, Munukutla S, Kar A, et al. Type-2 diabetic aldehyde dehydrogenase 2 mutant mice (ALDH 2*2) exhibiting heart failure with preserved ejection fraction phenotype can be determined by exercise stress echocardiography[J]. PLoS One, 2018, 13(4): e0195796. |
| 5 | Paolillo S, Marsico F, Prastaro M, et al. Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications[J]. Heart Fail Clin, 2019, 15(3): 341-7. |
| 6 | Lee TI, Trang NN, Lee TW, et al. Ketogenic diet regulates cardiac remodeling and calcium homeostasis in diabetic rat cardiomyopathy[J]. Int J Mol Sci, 2023, 24(22): 16142. |
| 7 | Yuill KH, Al Kury LT, Howarth FC. Characterization of L-type calcium channel activity in atrioventricular nodal myocytes from rats with streptozotocin-induced Diabetes mellitus[J]. Physiol Rep, 2015, 3(11): e12632. |
| 8 | Korf-Klingebiel M, Reboll MR, Polten F, et al. Myeloid-derived growth factor protects against pressure overload-induced heart failure by preserving sarco/endoplasmic reticulum Ca2+-ATPase expression in cardiomyocytes[J]. Circulation, 2021, 144(15): 1227-40. |
| 9 | Arow M, Waldman M, Yadin D, et al. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy[J]. Cardiovasc Diabetol, 2020, 19(1): 7. |
| 10 | Ozturk N, Uslu S, Ozdemir S. Diabetes-induced changes in cardiac voltage-gated ion channels[J]. World J Diabetes, 2021, 12(1): 1-18. |
| 11 | Singh RM, Waqar T, Howarth FC, et al. Hyperglycemia-induced cardiac contractile dysfunction in the diabetic heart[J]. Heart Fail Rev, 2018, 23(1): 37-54. |
| 12 | Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins[J]. Science, 2022, 375(6586): 1254-61. |
| 13 | Farrant J, Dodd S, Vaughan C, et al. Rationale and design of a randomised trial of trientine in patients with hypertrophic cardiomyopathy[J]. Heart, 2023, 109(15): 1175-82. |
| 14 | Gong D, Lu J, Chen X, et al. A copper(II)-selective Chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic TGF-beta activation in the kidneys of rats used as a model of diabetes[J]. Diabetologia, 2008, 51(9): 1741-51. |
| 15 | Cui XN, Wang Y, Liu H, et al. The molecular mechanisms of defective copper metabolism in diabetic cardiomyopathy[J]. Oxid Med Cell Longev, 2022, 2022: 5418376. |
| 16 | Jia DK, Liu LL, Liu W, et al. Copper metabolism and its role in diabetic complications: a review[J]. Pharmacol Res, 2024, 206: 107264. |
| 17 | Xiao Y, Wang T, Song X, et al. Copper promotion of myocardial regeneration[J]. Exp Biol Med, 2020, 245(10): 911-21. |
| 18 | Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease[J]. Toxicology, 2011, 283(2/3): 65-87. |
| 19 | Liu ZY, Liu ZY, Lin LC, et al. Redox homeostasis in cardiac fibrosis: focus on metal ion metabolism[J]. Redox Biol, 2024, 71: 103109. |
| 20 | Aggarwal NT, Makielski JC. Redox control of cardiac excitability[J]. Antioxid Redox Signal, 2013, 18(4): 432-68. |
| 21 | Toscano CM, Filetti FM, Almenara CCP, et al. Copper exposure for 30 days at a daily dose twice the recommended increases blood pressure and cardiac contractility[J]. Life Sci, 2022, 300: 120579. |
| 22 | Zhao HJ, Wang Y, Fei DX, et al. Destruction of redox and mitochondrial dynamics co-contributes to programmed cell death in chicken kidney under arsenite or/and copper (II) exposure[J]. Ecotoxicol Environ Saf, 2019, 179: 167-74. |
| 23 | Hung Y, Chen YC, Huang SY, et al. Klotho modulates electrical activity and calcium homeostasis in pulmonary vein cardiomyocytes via PI3K/Akt signalling[J]. Europace, 2020, 22(7): 1132-41. |
| 24 | Chu SJ, Wang WJ, Zhang N, et al. Protective effects of 18β-Glycyrrhetinic acid against myocardial infarction: Involvement of PI3K/Akt pathway activation and inhibiting Ca2+ influx via L-type Ca2+ channels[J]. Food Sci Nutr, 2021, 9(12): 6831-43. |
| 25 | Clark JL, Zahradka P, Taylor CG. Efficacy of flavonoids in the management of high blood pressure[J]. Nutr Rev, 2015, 73(12): 799-822. |
| 26 | Islam MT, Tuday E, Allen S, et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age[J]. Aging Cell, 2023, 22(2): e13767. |
| 27 | Oyedemi SO, Nwaogu G, Chukwuma CI, et al. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: in silico studies of molecular interaction of quercetin with hexokinase and catalase[J]. J Food Biochem, 2020, 44(2): e13127. |
| 28 | Lazuardi M, Anjani QK, Budiatin AS, et al. Efficacy of quercetin-like compounds from the mistletoe plant of Dendrophthoe pentandra L. Miq, as oral random blood sugar lowering treatment in diabetic rats[J]. Vet Q, 2024, 44(1): 1-14. |
| 29 | Song XL, Wang YL, Gao LG. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex[J]. J Mol Model, 2020, 26(6): 133. |
| 30 | Pham TND, Stempel S, Shields MA, et al. Quercetin enhances the anti-tumor effects of BET inhibitors by suppressing hnRNPA1[J]. Int J Mol Sci, 2019, 20(17): 4293. |
| 31 | Xu HY, Li SF, Liu JY, et al. Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19[J]. Proc Natl Acad Sci USA, 2023, 120(18): e2301775120. |
| 32 | Hosseini A, Razavi BM, Banach M, et al. Quercetin and metabolic syndrome: a review[J]. Phytother Res, 2021, 35(10): 5352-64. |
| 33 | Hayamizu K, Morimoto S, Nonaka M, et al. Cardiotonic actions of quercetin and its metabolite tamarixetin through a Digitalis-like enhancement of Ca2+ transients[J]. Arch Biochem Biophys, 2018, 637: 40-7. |
| 34 | Chen YF, Qiu Q, Wang L, et al. Quercetin ameliorates myocardial injury in diabetic rats by regulating autophagy and apoptosis through AMPK/mTOR signaling pathway[J]. Am J Chin Med, 2024, 52(3): 841-64. |
| 35 | Trang NN, Chung CC, Lee TW, et al. Empagliflozin and liraglutide differentially modulate cardiac metabolism in diabetic cardiom-yopathy in rats[J]. Int J Mol Sci, 2021, 22(3): 1177. |
| 36 | Kang PF, Wang JH, Fang D, et al. Activation of ALDH2 attenuates high glucose induced rat cardiomyocyte fibrosis and necroptosis[J]. Free Radic Biol Med, 2020, 146: 198-210. |
| 37 | Huang XP, Shi ZH, Ming GF, et al. S-Allyl-L-cysteine (SAC) inhibits copper-induced apoptosis and cuproptosis to alleviate cardiomyocyte injury[J]. Biochem Biophys Res Commun, 2024, 730: 150341. |
| 38 | Nakamura K, Miyoshi T, Yoshida M, et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus[J]. Int J Mol Sci, 2022, 23(7): 3587. |
| 39 | Huo SQ, Wang Q, Shi W, et al. ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury[J]. Int J Mol Sci, 2023, 24(2): 1667. |
| 40 | Zhang L, Ward ML, Phillips ARJ, et al. Protection of the heart by treatment with a divalent-copper-selective Chelator reveals a novel mechanism underlying cardiomyopathy in diabetic rats[J]. Cardiovasc Diabetol, 2013, 12: 123. |
| 41 | Coskun O, Kanter M, Korkmaz A, et al. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas[J]. Pharmacol Res, 2005, 51(2): 117-23. |
| 42 | Chang X, Zhang T, Wang JY, et al. SIRT5-related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance[J]. Oxid Med Cell Longev, 2021, 2021: 5876841. |
| 43 | 王盼盼, 杨 赞, 刘冬兰, 等. 槲皮素对心肌缺血后线粒体能量代谢功能的影响[J]. 中国药房, 2024, 35(4): 401-6. |
| 44 | Liang YR, Zhang YY, Liu MM, et al. Protective effect of quercetin against myocardial ischemia as a Ca2+ channel inhibitor: involvement of inhibiting contractility and Ca2+ influx via L-type Ca2+ channels[J]. Arch Pharm Res, 2020, 43(8): 808-20. |
| 45 | Zhang YM, Zhang ZY, Wang RX. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury[J]. Front Physiol, 2020, 11: 956. |
| 46 | Zhong DY, Li L, Cheng H, et al. Study on computer screening and drug properties of herbs intervening in copper death[J]. Comput Math Methods Med, 2023, 2023: 3311834. |
| 47 | Schulz V, Basu S, Freibert SA, et al. Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2[J]. Nat Chem Biol, 2023, 19(2): 206-17. |
| 48 | Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes[J]. N Engl J Med, 2015, 373(22): 2117-28. |
| 49 | Preda A, Montecucco F, Carbone F, et al. SGLT2 inhibitors: from glucose-lowering to cardiovascular benefits[J]. Cardiovasc Res, 2024, 120(5): 443-60. |
| 50 | Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias[J]. Circ Res, 2017, 120(12): 1969-93. |
| 51 | Liu CH, Chang HK, Lee SP, et al. Activation of the Ca2+-sensing receptors increases currents through inward rectifier K+ channels via activation of phosphatidylinositol 4-kinase[J]. Pflugers Arch, 2016, 468(11/12): 1931-43. |
| 52 | Liu ZL, Shan ZM, Yang HY, et al. Quercetin, main active ingredient of moutan cortex, alleviates chronic orofacial pain via block of voltage-gated sodium channel[J]. Anesth Analg, 2024, 138(6): 1324-36. |
| 53 | Saponara S, Fusi F, Iovinelli D, et al. Flavonoids and hERG channels: friends or foes[J]? Eur J Pharmacol, 2021, 899: 174030. |
| [1] | Pengwei HUANG, Jie CHEN, Jinhu ZOU, Xuefeng GAO, Hong CAO. Quercetin mitigates HIV-1 gp120-induced rat astrocyte neurotoxicity via promoting G3BP1 disassembly in stress granules [J]. Journal of Southern Medical University, 2025, 45(2): 304-312. |
| [2] | Junjie GAO, Kai YE, Jing WU. Quercetin inhibits proliferation and migration of clear cell renal cell carcinoma cells by regulating TP53 gene [J]. Journal of Southern Medical University, 2025, 45(2): 313-321. |
| [3] | Xiupeng LONG, Shun TAO, Shen YANG, Suyun LI, Libing RAO, Li LI, Zhe ZHANG. Quercetin improves heart failure by inhibiting cardiomyocyte apoptosis via suppressing the MAPK signaling pathway [J]. Journal of Southern Medical University, 2025, 45(1): 187-196. |
| [4] | Qing LIU, Jing LIU, Yihang ZHENG, Jin LEI, Jianhua HUANG, Siyu LIU, Fang LIU, Qunlong PENG, Yuanfang ZHANG, Junjie WANG, Yujuan LI. Quercetin mediates the therapeutic effect of Centella asiatica on psoriasis by regulating STAT3 phosphorylation to inhibit the IL-23/IL-17A axis [J]. Journal of Southern Medical University, 2025, 45(1): 90-99. |
| [5] | Yifan JIANG, Xiaorong LI, Jiayi GENG, Yongfeng CHEN, Bi TANG, Pinfang KANG. Quercetin ameliorates diabetic kidney injury in rats by inhibiting the HMGB1/RAGE/ NF-κB signaling pathway [J]. Journal of Southern Medical University, 2024, 44(9): 1769-1775. |
| [6] | Wenxiang ZHANG, Huixian GU, Pengde CHEN, Siyu WU, Hongyan MA, Lan YAO. Compound Yuye Decoction protects diabetic rats against cardiomyopathy by inhibiting myocardial apoptosis and inflammation via regulating the PI3K/Akt signaling pathway [J]. Journal of Southern Medical University, 2024, 44(7): 1306-1314. |
| [7] | Ping SHU, Mengke YUAN, Ke YANG, Weizhi HE, Li LIU. Quercetin suppresses pyroptosis in mouse fibroblasts by inhibiting the NLRP3/caspase-1/GSDMD pathway [J]. Journal of Southern Medical University, 2024, 44(10): 1874-1880. |
| [8] | YE Hongwei, ZHANG Yuming, YUN Qi, DU Ruoli, LI Lu, LI Yuping, GAO Qin. Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression [J]. Journal of Southern Medical University, 2024, 44(1): 45-51. |
| [9] | ZHANG Ningning, QIU Qi, CHEN Yongfeng, SUN Zhengyu, LU Guoqing, WANG Lei, KANG Pingfang, WANG Hongju. Quercetin improves pulmonary arterial hypertension in rats by regulating the HMGB1/RAGE/NF-κB pathway [J]. Journal of Southern Medical University, 2023, 43(9): 1606-1612. |
| [10] | LIU Lilan, DENG Ruya, ZHOU Wenjin, LIN Min, XIA Lingzi, GAO Haitao. Mechanisms mediating the inhibitory effects of quercetin against phthalates-induced testicular oxidative damage in rats [J]. Journal of Southern Medical University, 2023, 43(4): 577-584. |
| [11] | GAO Xiaoyang, ZHAO Xiaolu, ZHANG Chunyan, YAN Yuxin, JIN Rong, MA Yuehong. Quercetin induces hepatic stellate cell apoptosis by inhibiting the PI3K/Akt signaling pathway via upregulating miR-146 [J]. Journal of Southern Medical University, 2023, 43(10): 1725-1733. |
| [12] | ZHENG Dongxiao, CHEN Linlin, WEI Qihui, ZHU Ziran, LIU Zilue, JIN Lin, YANG Guanyu, XIE Xi. Fucoxanthin regulates Nrf2/Keap1 signaling to alleviate myocardial hypertrophy in diabetic rats [J]. Journal of Southern Medical University, 2022, 42(5): 752-759. |
| [13] | TAN Xin, XIAN Wei, CHEN Yongfeng, LI Xiaorong, WANG Qiyi, KANG Pinfang, WANG Hongju. Exploring the therapeutic mechanism of quercetin for heart failure based on network pharmacology and molecular docking [J]. Journal of Southern Medical University, 2021, 41(8): 1198-1206. |
| [14] | . Quercetin alleviates lipopolysaccharide-induced acute kidney injury in mice by suppressing TLR4/NF-κB pathway [J]. Journal of Southern Medical University, 2019, 39(05): 598-. |
| [15] | . Quercetin attenuates Ox-LDL-induced calcification in vascular smooth muscle cells by regulating ROS-TLR4 signaling pathway [J]. Journal of Southern Medical University, 2018, 38(08): 980-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||