Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (12): 2541-2550.doi: 10.12122/j.issn.1673-4254.2025.12.02
Changlong FU1,2( ), Ruolan CHEN1, Shiqi XU3, Jinxin YOU1, Qing LIN3, Yanfeng HUANG1,2(
), Ruolan CHEN1, Shiqi XU3, Jinxin YOU1, Qing LIN3, Yanfeng HUANG1,2( )
)
Received:2025-06-17
Online:2025-12-20
Published:2025-12-22
Contact:
Yanfeng HUANG
E-mail:993001232@qq.com;banglongnet@126.com
Supported by:Changlong FU, Ruolan CHEN, Shiqi XU, Jinxin YOU, Qing LIN, Yanfeng HUANG. Morinda officinalis polysaccharide delays osteoarthritis mouse chondrocyte degeneration by modulating the glycolysis-pyroptosis axis via targeting the lncRNA XIST[J]. Journal of Southern Medical University, 2025, 45(12): 2541-2550.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.12.02
| Gene | Primer sequences | Produet length (bp) |
|---|---|---|
| GLUT1 | F:5'-ACCATCTTGGAGCTGTTCCG-3' R:5'-GCCTTCTCGAAGATGCTCGT-3' | 131 |
| HK2 | F:5'-CCAGAGCATCCTCCTCAAGT-3' R:5'-GGTCTTCATAGCCACAAGTCATC-3' | 173 |
| PKM2 | F:5'-AGGCTGCCATCTACCACTTG-3' R:5'-CCAGACTTGGTGAGCACGAT-3' | 148 |
| LDHA | F:5'-CAGACTTGGCTGAGAGCATAATG-3' R:5'-CCTTCACAACATCCGAGATTCC-3' | 147 |
| PFKFB3 | F:5'-CAGGATCTTGTCCAACGCCT-3' R:5'-AGGCTAGTAGGCAGCGTAGA-3' | 103 |
| NLRP3 | F:5'-TCCCAGACACTCATGTTGCC-3' R:5'-GTCCAGTTCAGTGAGGCTCC-3' | 116 |
| Caspase-1 | F:5'-ACTGACTGGGACCCTCAAGT-3' R:5'-GCAAGACGTGTACGAGTGGT-3' | 111 |
| GSDMD | F:5'-TTAATTGAGGCGGCAGACTTC-3' R:5'-CGATGTTCACCAGTATCTGTTGT-3' | 109 |
| GAPDH | F:5'-TGGAAAGCTGTGGCGTGATG-3' R:5'-TACTTGGCAGGTTTCTCCAGG-3' | 189 |
Tab.1 Primer sequences for RT-qPCR
| Gene | Primer sequences | Produet length (bp) |
|---|---|---|
| GLUT1 | F:5'-ACCATCTTGGAGCTGTTCCG-3' R:5'-GCCTTCTCGAAGATGCTCGT-3' | 131 |
| HK2 | F:5'-CCAGAGCATCCTCCTCAAGT-3' R:5'-GGTCTTCATAGCCACAAGTCATC-3' | 173 |
| PKM2 | F:5'-AGGCTGCCATCTACCACTTG-3' R:5'-CCAGACTTGGTGAGCACGAT-3' | 148 |
| LDHA | F:5'-CAGACTTGGCTGAGAGCATAATG-3' R:5'-CCTTCACAACATCCGAGATTCC-3' | 147 |
| PFKFB3 | F:5'-CAGGATCTTGTCCAACGCCT-3' R:5'-AGGCTAGTAGGCAGCGTAGA-3' | 103 |
| NLRP3 | F:5'-TCCCAGACACTCATGTTGCC-3' R:5'-GTCCAGTTCAGTGAGGCTCC-3' | 116 |
| Caspase-1 | F:5'-ACTGACTGGGACCCTCAAGT-3' R:5'-GCAAGACGTGTACGAGTGGT-3' | 111 |
| GSDMD | F:5'-TTAATTGAGGCGGCAGACTTC-3' R:5'-CGATGTTCACCAGTATCTGTTGT-3' | 109 |
| GAPDH | F:5'-TGGAAAGCTGTGGCGTGATG-3' R:5'-TACTTGGCAGGTTTCTCCAGG-3' | 189 |
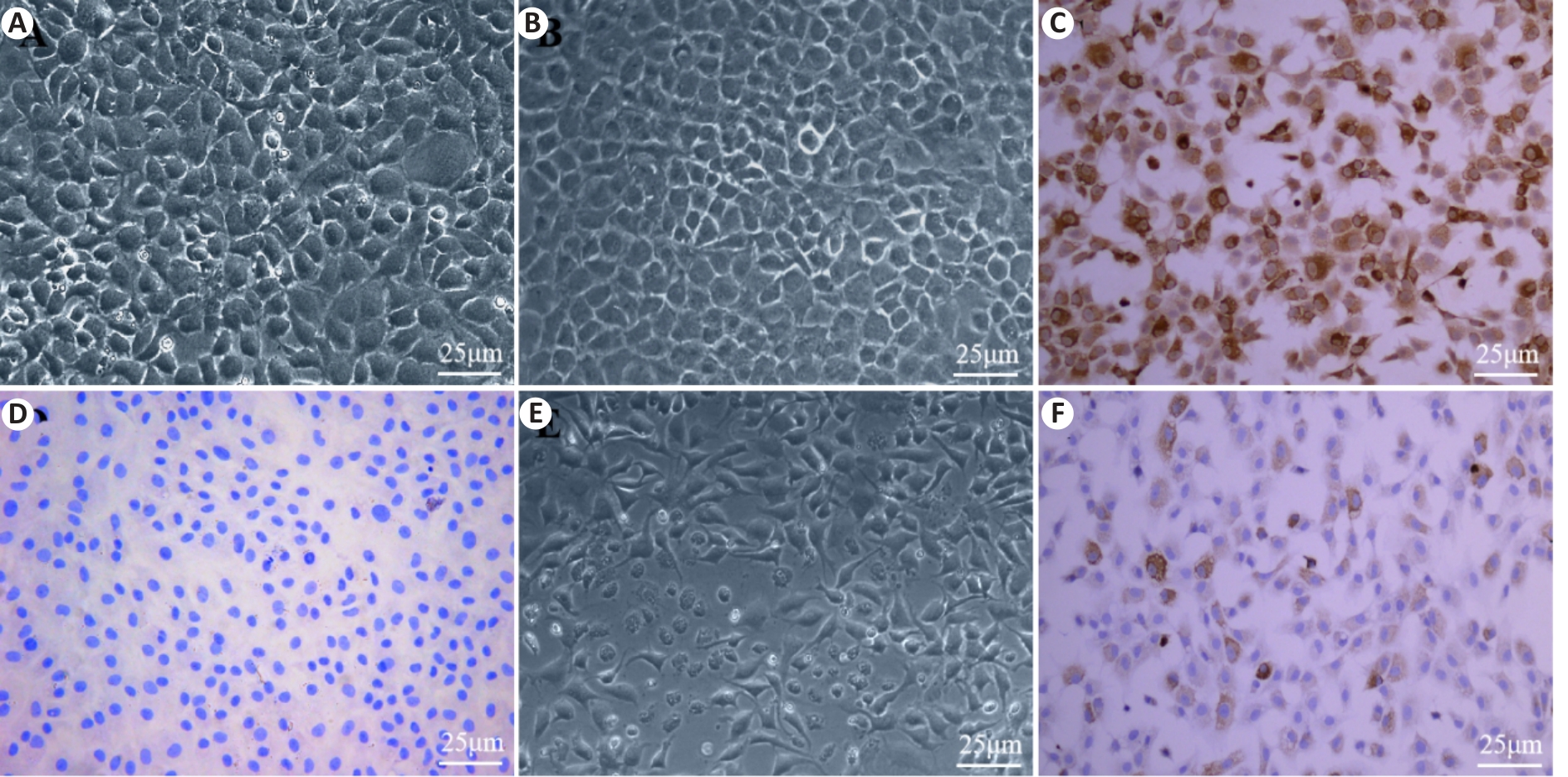
Fig.1 Primary culture, induction, and identification of mouse chondrocytes. A: Morphology of primary chondrocytes. B: Morphology of the second-passage chondrocytes. C: Positive expression of collagen II protein. D: Negative expression of collagen II protein. E: Morphology of chondrocytes induced by IL-1β. F: IL-1β induces positive expression of collagen II protein.
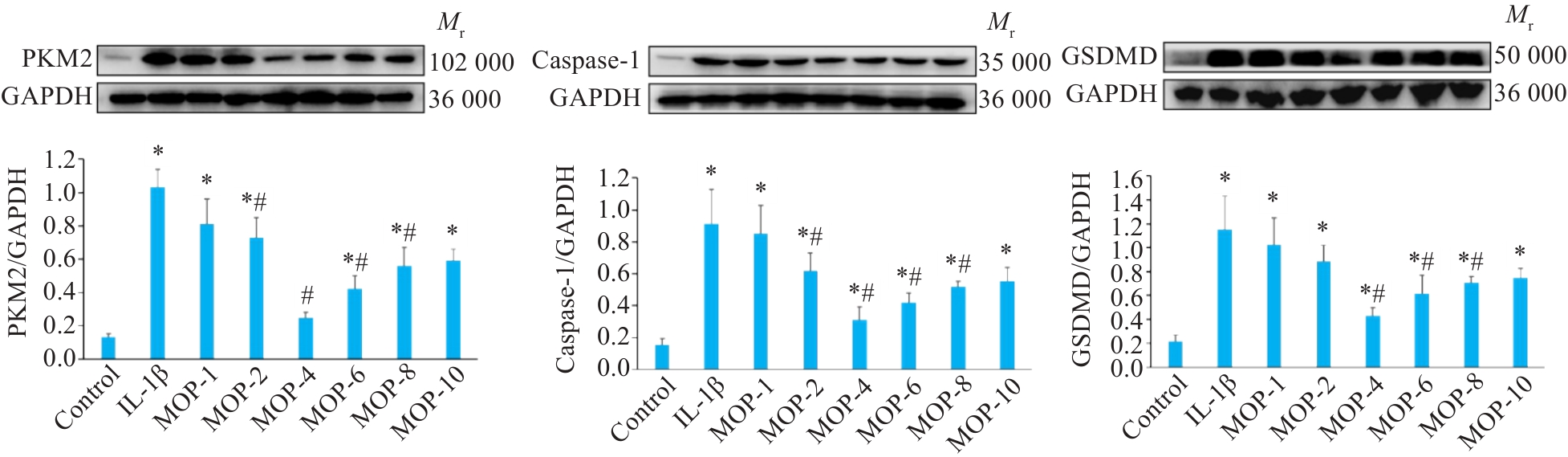
Fig.3 Effect of different concentrations of MOP on protein expressions of PKM2, caspase-1 and GSDMD in mouse chondrocytes. Data are presented as Mean±SD (n=3). *P<0.05 vs Control group, #P<0.05 vs IL-1β group.
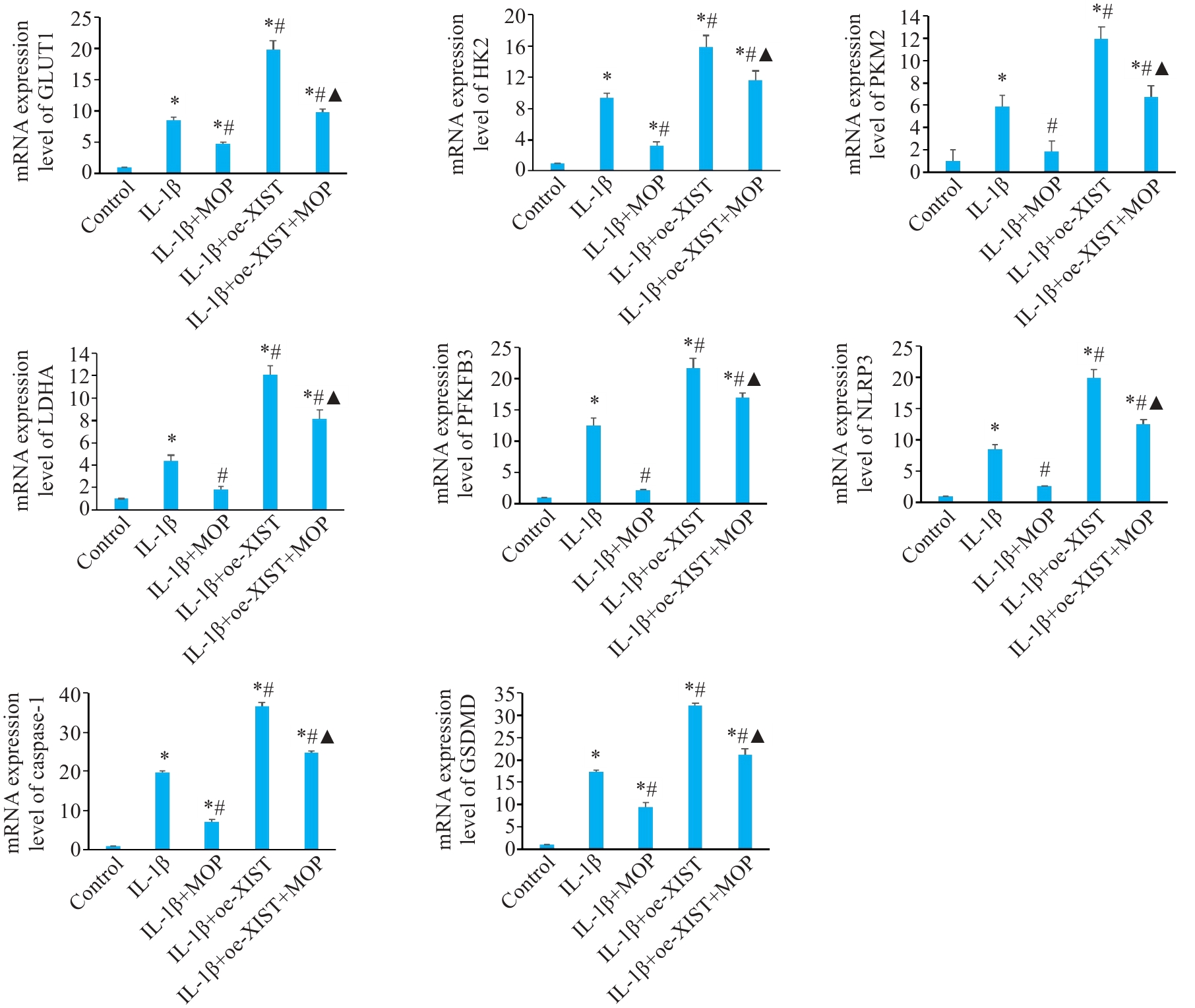
Fig.5 Effect of MOP on mRNA levels of GLUT1, HK2, PKM2, LDHA, PFKFB3, NLRP3, caspase-1, and GSDMD in mouse chondrocytes overexpressing XIST with IL-1β treatment. Data are presented as Mean±SD (n=3). *P<0.05 vs Control group; #P<0.05 vs IL-1β group; ▲P<0.05 vs IL-1β+oe-XIST group.
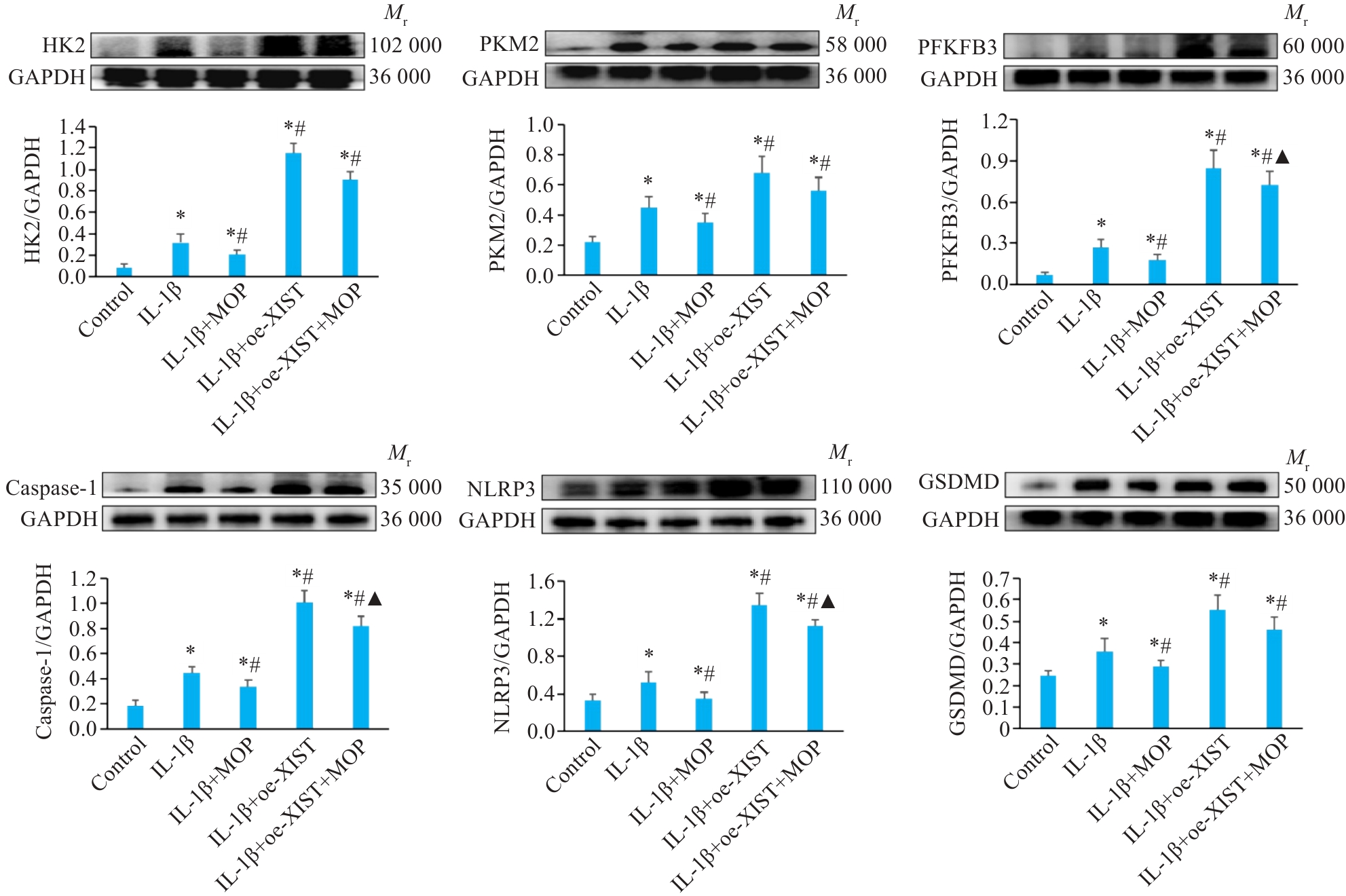
Fig.6 Effect of MOP on protein expressions of HK2, PKM2, PFKFB3, NLRP3, caspase-1, and GSDMD in mouse chondrocytes overexpressing XIST with IL-1β treatment. Data are presented as Mean±SD (n=3). *P<0.05 vs Control group; #P<0.05 vs IL-1β group; ▲P<0.05 vs IL-1β+oe-XIST group.
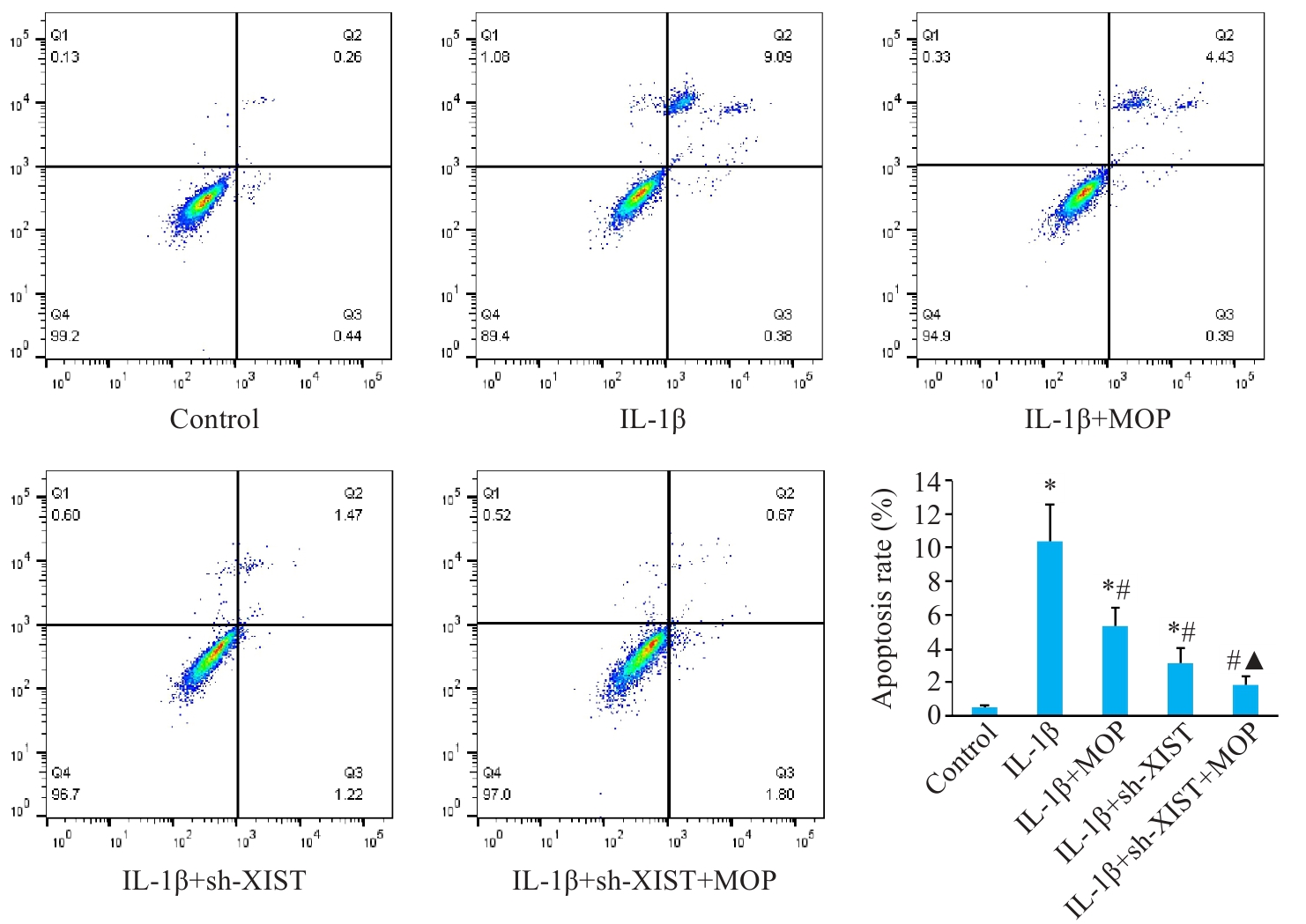
Fig.7 Effect of MOP on apoptosis in mouse chondrocytes with XIST knockout and IL-1β treatment. Data are presented as Mean±SD (n=3). *P<0.05 vs Control group; #P<0.05 vs IL-1β group; ▲P<0.05 vs IL-1β+sh-XIST group.

Fig.9 Effect of MOP on protein expressions of HK2, PKM2, PFKFB3, NLRP3, caspase-1, and GSDMD in mouse chondrocytes with XIST knockout and IL-1β treatment. Data are presented as Mean±SD (n=3). *P<0.05 vs Control group; #P<0.05 vs IL-1β group; ▲P<0.05 vs IL-1β+sh-XIST group.
| [1] | Wu YT, Hu HF, Wang T, et al. Characterizing mitochondrial features in osteoarthritis through integrative multi-omics and machine learning analysis[J]. Front Immunol, 2024, 15: 1414301. doi:10.3389/fimmu.2024.1414301 |
| [2] | Couch JL, King MG, De Oliveira Silva D, et al. Noisy knees-knee crepitus prevalence and association with structural pathology: a systematic review and meta-analysis[J]. Br J Sports Med, 2025, 59(2): 126-32. doi:10.1136/bjsports-2024-108866 |
| [3] | Tang S-A, Zhang CQ, Oo WM, et al. Osteoarthritis[J]. Nat Rev Dis Primers, 2025, 11: 10. doi:10.1038/s41572-025-00594-6 |
| [4] | Fuggle N, Laslop A, Rizzoli R, et al. Treatment of osteoporosis and osteoarthritis in the oldest old[J]. Drugs, 2025, 85(3): 343-60. doi:10.1007/s40265-024-02138-w |
| [5] | Lan WR, Chen XM, Yu H, et al. UGDH lactylation aggravates osteoarthritis by suppressing glycosaminoglycan synthesis and orchestrating nucleocytoplasmic transport to activate MAPK signaling[J]. Adv Sci (Weinh), 2025, 12(20): e2413709. doi:10.1002/advs.202413709 |
| [6] | Zhou YK, Li MZ, Lin S, et al. Mechanical sensing protein PIEZO1 controls osteoarthritis via glycolysis mediated mesenchymal stem cells-Th17 cells crosstalk[J]. Cell Death Dis, 2025, 16(1): 231. doi:10.1038/s41419-025-07577-1 |
| [7] | Dong YH, Zhou XM, Zhang ZZ, et al. cGAS-STING aggravates cartilage degradation by promoting glycolysis in temporo-mandibular joint osteoarthritis[J]. J Bone Miner Res, 2025, 40(5): 699-709. doi:10.1093/jbmr/zjaf029 |
| [8] | Meng JZ, Lu JF, Jiang CC, et al. Collagen hydrogel-driven pyroptosis suppression and combined microfracture technique delay osteoarthritis progression[J]. Biomaterials, 2025, 314: 122817. doi:10.1016/j.biomaterials.2024.122817 |
| [9] | Ma J, Yang P, Zhou ZB, et al. GYY4137-induced p65 sulfhydration protects synovial macrophages against pyroptosis by improving mitochondrial function in osteoarthritis development[J]. J Adv Res, 2025, 71: 173-88. doi:10.1016/j.jare.2024.05.033 |
| [10] | Bao CC, Zhu SY, Song KP, et al. HK2: a potential regulator of osteoarthritis via glycolytic and non-glycolytic pathways[J]. Cell Commun Signal, 2022, 20(1): 132. doi:10.1186/s12964-022-00943-y |
| [11] | Bao CC, Zhu SY, Pang DJ, et al. Hexokinase 2 suppression alleviates the catabolic properties in osteoarthritis via HMGA2 and contributes to pulsed electromagnetic field-mediated cartilage protection[J]. Int J Biol Sci, 2025, 21(4): 1459-77. doi:10.7150/ijbs.101597 |
| [12] | Liu JB, Jia SS, Yang Y, et al. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF‑κB and NLRP3/caspase-1/GSDMD signaling[J]. Biomed Pharmacother, 2023, 158: 114118. doi:10.1016/j.biopha.2022.114118 |
| [13] | 付长龙, 罗 雁, 许佳佳, 等. 透骨消痛胶囊调控circFOXO3缓解骨关节炎软骨细胞糖酵解代谢紊乱的作用机制[J]. 中国中药杂志, 2025, 50(16): 4641-8. |
| [14] | 金灵璐, 付长龙, 涂海水, 等. 乌头汤对膝骨关节炎大鼠软骨细胞焦亡相关基因表达的影响[J]. 风湿病与关节炎, 2023, 12(3): 1-4, 12. |
| [15] | 林 晴, 潘丹虹, 李 路, 等. 荣筋拈痛方调控软骨细胞NLRP3/caspase-1/GSDMD通路改善骨关节炎炎性病变的机制[J]. 中华中医药杂志, 2022, 37(10): 5653-8. |
| [16] | You XJ, Xie YJ, Tan QY, et al. Glycolytic reprogramming governs crystalline silica-induced pyroptosis and inflammation through promoting lactylation modification[J]. Ecotoxicol Environ Saf, 2024, 283: 116952. doi:10.1016/j.ecoenv.2024.116952 |
| [17] | Liu RX, Xiao Y, Huang SH, et al. LncRNA XIST inhibits mitophagy and increases mitochondrial dysfunction by promoting BNIP3 promoter methylation to facilitate the progression of KBD[J]. Mol Immunol, 2025, 182: 62-75. doi:10.1016/j.molimm.2025.03.016 |
| [18] | Fu CL, Lin YM, Lin Q, et al. Protective mechanism of prim-O-glucosylcimifugin in the treatment of osteoarthritis: based on lncRNA XIST regulation of Nav1.7[J]. Biomed Pharmacother, 2024, 181: 117597. doi:10.1016/j.biopha.2024.117597 |
| [19] | Yang F, Zhang J, Zhao ZJ, et al. Artemisinin suppresses aerobic glycolysis in thyroid cancer cells by downregulating HIF-1a, which is increased by the XIST/miR-93/HIF-1a pathway[J]. PLoS One, 2023, 18(4): e0284242. doi:10.1371/journal.pone.0284242 |
| [20] | Zhang Y, Yang L, Mu HT, et al. CVB3 regulates Treg cell pyroptosis through the lncRNA XIST/miR-195-5p/caspase-1 molecular axis[J]. Immunobiology, 2025, 230(2): 152882. doi:10.1016/j.imbio.2025.152882 |
| [21] | 付长龙, 林艳铭, 兰书洁, 等. 透骨消痛胶囊调控Malat1与miR-16-5p的ceRNA减轻骨关节炎软骨细胞“胆固醇-铁” 代谢紊乱的机制[J]. 中国中药杂志, 2025, 50(15): 4363-71. |
| [22] | 付长龙, 林艳铭, 兰书洁, 等. 透骨消痛胶囊调控Nav1.7减轻膝骨关节炎小鼠软骨细胞退变[J]. 南方医科大学学报, 2024, 44(11): 2074-81. doi:10.12122/j.issn.1673-4254.2024.11.03 |
| [23] | Liu MY, Wang C, Zhang HW, et al. A systematic review on polysaccharides from Morinda officinalis How: Advances in the preparation, structural characterization and pharmacological activities[J]. J Ethnopharmacol, 2024, 328: 118090. doi:10.1016/j.jep.2024.118090 |
| [24] | Zhao DF, Xing SQ, Qi J, et al. Alleviating the IL-1β-stimulated extracellular matrix degradation in osteoarthritis, and chondrocyte inflammation by Morinda officinalis polysaccharide via the SIRT6/NF-κB pathway[J]. Biomol Biomed, 2025, 25(7): 1610-20. doi:10.17305/bb.2024.11437 |
| [25] | 黄艳峰, 陈 俊, 林 洁, 等. 荣筋拈痛方对白细胞介素-1β诱导大鼠退变软骨细胞增殖的影响[J]. 中华中医药杂志, 2021, 36(4): 2077-82. |
| [26] | 王 群, 熊益亮, 赵希睿, 等. 先秦两汉简帛医书中的“痹” 与“痿” 探析[J]. 中医杂志, 2019, 60(9): 730-3. |
| [27] | 李西海, 刘献祥. 骨关节炎的核心病机: 本痿标痹[J]. 中医杂志, 2014, 55(14): 1248-9, 1252. |
| [28] | 刘献祥. 基于陈可冀学术思想之骨性关节炎研究[J]. 康复学报, 2016, 26(1): 2-5. |
| [29] | 刘献祥, 郑春松, 叶蕻芝, 等. 透骨消痛胶囊防治骨性关节炎的化学空间分析[J]. 福建中医学院学报, 2010, 20(2): 16-8, 27. doi:10.3969/j.issn.1004-5627.2010.02.006 |
| [30] | 朱海波, 何忠斌. 透骨消痛胶囊治疗骨质疏松性骨关节炎的临床疗效[J]. 内蒙古中医药, 2019, 38(8): 25-6. |
| [31] | 洪昆达, 万 甜, 李 俐, 等. 温针合透骨消痛胶囊内服治疗疼痛性膝骨性关节炎30例[J]. 中医药通报, 2010, 9(3): 55-6. doi:10.3969/j.issn.1671-2749.2010.03.017 |
| [32] | 陈 鸿, 洪昆达. 透骨消痛胶囊治疗疼痛性膝骨性关节炎30例[J]. 福建中医药, 2015, 46(2): 21-2. |
| [33] | 郑春松, 林珠灿, 许惠风, 等. 透骨消痛胶囊治疗骨性关节炎的多向药理学研究[J]. 福建中医药大学学报, 2011(1): 43-7. |
| [34] | 杨绍春, 瞿广城, 帅 焘. 《黄帝内经》“精神内守” 探究[J]. 云南中医中药杂志, 2025, 46(6): 27-9. |
| [1] | Shanyu LUO, Qiang ZHU, Yufei YAN, Zonghong JI, Huajie ZOU, Ruixia ZHANG, Yinggui BA. NLRP3 signaling pathway promotes hepatocyte pyroptosis in mice with nonalcoholic steatohepatitis in hypoxic environment [J]. Journal of Southern Medical University, 2025, 45(9): 2026-2033. |
| [2] | Haiyi ZHOU, Siyi HE, Ruifang HAN, Yongge GUAN, Lijuan DONG, Yang SONG. Moxibustion promotes endometrial repair in rats with thin endometrium by inhibiting the NLRP3/pyroptosis axis via upregulating miR-223-3p [J]. Journal of Southern Medical University, 2025, 45(7): 1380-1388. |
| [3] | Fenlan BIAN, Shiyao NI, Peng ZHAO, Maonanxing QI, Bi TANG, Hongju WANG, Pinfang KANG, Jinjun LIU. Asiaticoside alleviates myocardial ischemia-reperfusion injury in rats by inhibiting NLRP3 inflammasome-mediated pyroptosis [J]. Journal of Southern Medical University, 2025, 45(5): 977-985. |
| [4] | Yalei SUN, Meng LUO, Changsheng GUO, Jing GAO, Kaiqi SU, Lidian CHEN, Xiaodong FENG. Amentoflavone alleviates acute lung injury in mice by inhibiting cell pyroptosis [J]. Journal of Southern Medical University, 2025, 45(4): 692-701. |
| [5] | Zhengwang ZHU, Linlin WANG, Jinghan ZHAO, Ruixue MA, Yuchun YU, Qingchun CAI, Bing WANG, Pingsheng ZHU, Mingsan MIAO. Tuihuang Mixture improves α‑naphthylisothiocyanate-induced cholestasis in rats by inhibiting NLRP3 inflammasomes via regulating farnesoid X receptor [J]. Journal of Southern Medical University, 2025, 45(4): 718-724. |
| [6] | Liping FU, Lixia YUAN, Jie WANG, Xuelan CHEN, Guizhi KE, Yu HUANG, Xinyi YANG, Gang LIU. Advances of low-intensity pulsed ultrasound for treatment of musculoskeletal disorders in the past decade [J]. Journal of Southern Medical University, 2025, 45(3): 661-668. |
| [7] | Ju HUANG, Lixia YIN, Minzhu NIU, Zhijun GENG, Lugen ZUO, Jing LI, Jianguo HU. Nodakenin ameliorates TNBS-induced experimental colitis in mice by inhibiting pyroptosis of intestinal epithelial cells [J]. Journal of Southern Medical University, 2025, 45(2): 261-268. |
| [8] | Shicheng XIA, Huifang WEI, Weican HONG, Yuming ZHANG, Feiyang YIN, Yixin ZHANG, Linlin ZHANG, Qin GAO, Hongwei YE. Protective effect of Lonicerae Japonicae Flos extract against doxorubicin-induced myocardial injury in mice and the possible mechanisms [J]. Journal of Southern Medical University, 2025, 45(12): 2527-2540. |
| [9] | Yongxin MAI, Shuting ZHOU, Ruijia WEN, Jinfang ZHANG, Dongxiang ZHAN. Aucubin alleviates knee osteoarthritis in mice by suppressing the NF‑κB signaling pathway [J]. Journal of Southern Medical University, 2025, 45(10): 2104-2110. |
| [10] | Siyu ZHANG, Linwu RAN, Jin ZENG, Yujiong WANG. Clostridium perfringens Beta1 toxin induces macrophage pyroptosis and ferroptosis through the purinergic receptor P2X7-Ca2+ axis [J]. Journal of Southern Medical University, 2025, 45(10): 2126-2134. |
| [11] | Hui LU, Bowen SONG, Jinran SHI, Shunyin WANG, Xiaohua CHEN, Jingjing YANG, Sitang GE, Lugen ZUO. SF3B3 overexpression promotes proliferation of gastric cancer cells and correlates with poor patient prognosis [J]. Journal of Southern Medical University, 2025, 45(10): 2240-2249. |
| [12] | Songqi HE, Yang LIU, Mengchen QIN, Chunyu HE, Wentao JIANG, Yiqin WANG, Sirui TAN, Haiyan SUN, Haitao SUN. Traditional Chinese medicine for regulating glycolysis to remodel the tumor immune microenvironment: research progress and future prospects [J]. Journal of Southern Medical University, 2025, 45(10): 2277-2284. |
| [13] | Xiaohui WEN, Shiya HUANG, Xuehong LIU, Kunyin LI, Yongge GUAN. Role of Notch 1 signaling and glycolysis in the pathogenic mechanism of adenomyosis [J]. Journal of Southern Medical University, 2024, 44(8): 1599-1604. |
| [14] | Mengdong ZHENG, Yan LIU, Jiaojiao LIU, Qiaozhen KANG, Ting WANG. Effect of deletion of protein 4.1R on proliferation, apoptosis and glycolysis of hepatocyte HL-7702 cells [J]. Journal of Southern Medical University, 2024, 44(7): 1355-1360. |
| [15] | Yiming SUN, Rong ZHANG, Ying MENG, Lei ZHU, Mingqiang LI, Zhe LIU. Coenzyme Q10 alleviates depression-like behaviors in mice with chronic restraint stress by down-regulating the pyroptosis signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 810-817. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||