Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (5): 913-919.doi: 10.12122/j.issn.1673-4254.2024.05.13
• Clinical Research • Previous Articles Next Articles
Yongsheng XIA1,2( ), Lian WANG1,2, Xiaohua CHEN1,2, Yulu ZHANG3, Aofei SUN3, Deli CHEN1(
), Lian WANG1,2, Xiaohua CHEN1,2, Yulu ZHANG3, Aofei SUN3, Deli CHEN1( )
)
Received:2024-01-23
Online:2024-05-20
Published:2024-06-06
Contact:
Deli CHEN
E-mail:xiayongsheng0818@163.com;13965295950@139.com
Yongsheng XIA, Lian WANG, Xiaohua CHEN, Yulu ZHANG, Aofei SUN, Deli CHEN. TSR2 overexpression inhibits proliferation and invasion of gastric cancer cells by downregulating the PI3K/AKT signaling pathway[J]. Journal of Southern Medical University, 2024, 44(5): 913-919.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.05.13
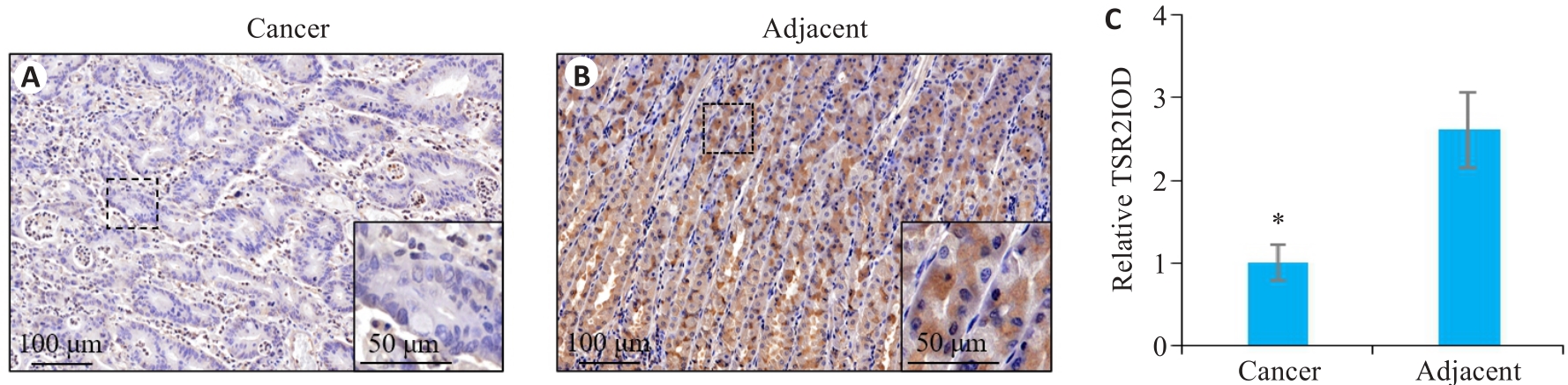
Fig.1 Expression of TSR2 in gastric cancer and adjacent tissues. A: TSR2 is lowly expressed in gastric cancer tissue. B: TSR2 is highly expressed in adjacent tissue. C: Relative IOD value of TSR2 (n=105). *P<0.05 vs adjacent tissues.
| Factors | n | TSR2 expression | χ 2 | P | |
|---|---|---|---|---|---|
| Low (n=53) | High (n=52) | ||||
| Gender | 0.023 | 0.880 | |||
| Male | 74 | 37 (50.0%) | 37 (50.0%) | ||
| Female | 31 | 16 (51.6%) | 15 (48.4%) | ||
| Age (year) | 0.015 | 0.903 | |||
| <60 | 41 | 21 (51.2%) | 20 (48.8%) | ||
| ≥60 | 64 | 32 (50.0%) | 32 (50.0%) | ||
| CEA (μg/L) | 6.940 | 0.008 | |||
| <5 | 49 | 18 (36.7%) | 31 (63.3%) | ||
| ≥5 | 56 | 35 (62.5%) | 21 (37.5%) | ||
| CA19-9 (kU/L) | 17.720 | <0.001 | |||
| <37 | 47 | 13 (27.7%) | 34 (72.3%) | ||
| ≥37 | 58 | 40 (69.0%) | 18 (31.0%) | ||
| Tumor size (cm) | 2.134 | 0.144 | |||
| <5 | 49 | 21 (42.9%) | 28 (57.1%) | ||
| ≥5 | 56 | 32 (57.1%) | 24 (42.9%) | ||
| Histological type | 1.177 | 0.278 | |||
| Adenocarcinoma | 66 | 36 (54.5%) | 30 (45.5%) | ||
| Other | 39 | 17 (43.6%) | 22 (56.4%) | ||
| T stage | 11.675 | 0.001 | |||
| 1-2 | 53 | 18 (34.0%) | 35 (66.0%) | ||
| 3-4 | 52 | 35 (67.3%) | 17 (32.7%) | ||
| N stage | 13.414 | <0.001 | |||
| 0-1 | 60 | 21 (35.0%) | 39 (65.0%) | ||
| 2-3 | 45 | 32 (71.1%) | 13 (28.9%) | ||
Tab.1 Correlation between TSR2 expression and progression of gastric cancer [n (%)]
| Factors | n | TSR2 expression | χ 2 | P | |
|---|---|---|---|---|---|
| Low (n=53) | High (n=52) | ||||
| Gender | 0.023 | 0.880 | |||
| Male | 74 | 37 (50.0%) | 37 (50.0%) | ||
| Female | 31 | 16 (51.6%) | 15 (48.4%) | ||
| Age (year) | 0.015 | 0.903 | |||
| <60 | 41 | 21 (51.2%) | 20 (48.8%) | ||
| ≥60 | 64 | 32 (50.0%) | 32 (50.0%) | ||
| CEA (μg/L) | 6.940 | 0.008 | |||
| <5 | 49 | 18 (36.7%) | 31 (63.3%) | ||
| ≥5 | 56 | 35 (62.5%) | 21 (37.5%) | ||
| CA19-9 (kU/L) | 17.720 | <0.001 | |||
| <37 | 47 | 13 (27.7%) | 34 (72.3%) | ||
| ≥37 | 58 | 40 (69.0%) | 18 (31.0%) | ||
| Tumor size (cm) | 2.134 | 0.144 | |||
| <5 | 49 | 21 (42.9%) | 28 (57.1%) | ||
| ≥5 | 56 | 32 (57.1%) | 24 (42.9%) | ||
| Histological type | 1.177 | 0.278 | |||
| Adenocarcinoma | 66 | 36 (54.5%) | 30 (45.5%) | ||
| Other | 39 | 17 (43.6%) | 22 (56.4%) | ||
| T stage | 11.675 | 0.001 | |||
| 1-2 | 53 | 18 (34.0%) | 35 (66.0%) | ||
| 3-4 | 52 | 35 (67.3%) | 17 (32.7%) | ||
| N stage | 13.414 | <0.001 | |||
| 0-1 | 60 | 21 (35.0%) | 39 (65.0%) | ||
| 2-3 | 45 | 32 (71.1%) | 13 (28.9%) | ||
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank χ2 | P | HR | 95% CI | P | |
| Gender (male vs female) | 1.451 | 0.228 | - | - | - |
| Age (≥60 years vs <60 years) | 1.356 | 0.244 | - | - | - |
| TSR2 expression (low vs high) | 31.840 | <0.001 | 0.418 | 0.201-0.871 | 0.020 |
| CEA (≥5μg/L vs <5 μg/L) | 18.367 | <0.001 | 2.173 | 1.126-4.192 | 0.021 |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 25.491 | <0.001 | 3.144 | 1.559-6.342 | 0.001 |
| Tumor size (≥5 cm vs <5 cm) | 2.163 | 0.141 | - | - | - |
| Histological type(adenocarcinoma vs other) | 0.001 | 0.982 | - | - | - |
| T stage (T3-T4vs T1-T2) | 21.658 | <0.001 | 1.991 | 1.034-3.832 | 0.039 |
| N stage (N2-N3vs N0-N1) | 26.362 | <0.001 | 2.106 | 1.090-4.066 | 0.027 |
Tab.2 Risk factors affecting prognosis of patients with gastric cancer
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank χ2 | P | HR | 95% CI | P | |
| Gender (male vs female) | 1.451 | 0.228 | - | - | - |
| Age (≥60 years vs <60 years) | 1.356 | 0.244 | - | - | - |
| TSR2 expression (low vs high) | 31.840 | <0.001 | 0.418 | 0.201-0.871 | 0.020 |
| CEA (≥5μg/L vs <5 μg/L) | 18.367 | <0.001 | 2.173 | 1.126-4.192 | 0.021 |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 25.491 | <0.001 | 3.144 | 1.559-6.342 | 0.001 |
| Tumor size (≥5 cm vs <5 cm) | 2.163 | 0.141 | - | - | - |
| Histological type(adenocarcinoma vs other) | 0.001 | 0.982 | - | - | - |
| T stage (T3-T4vs T1-T2) | 21.658 | <0.001 | 1.991 | 1.034-3.832 | 0.039 |
| N stage (N2-N3vs N0-N1) | 26.362 | <0.001 | 2.106 | 1.090-4.066 | 0.027 |
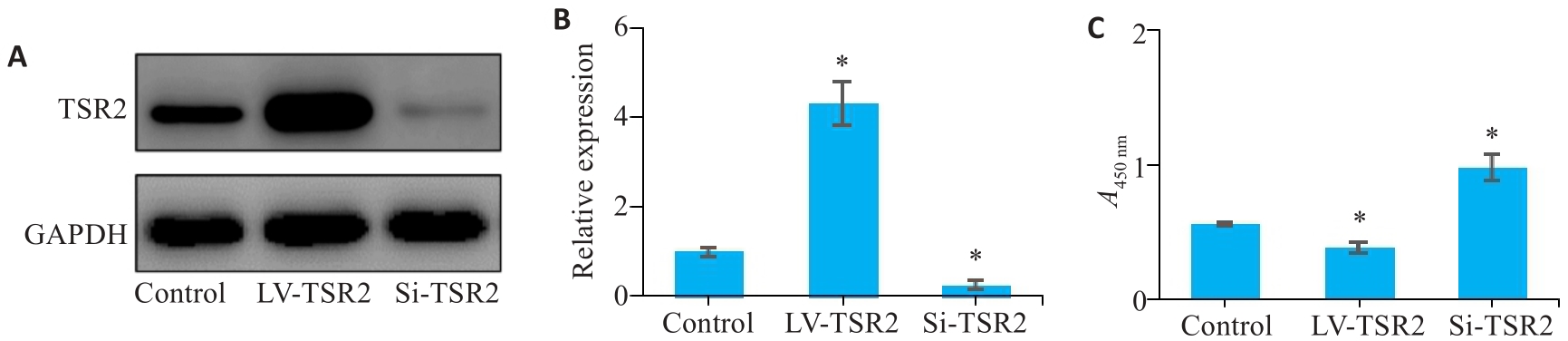
Fig.4 Effect of TSR2 overexpression and knockdown on proliferation of MGC-803 cells. A, B: Lentivirus-mediated TSR2 overexpression and knockdown in MGC803 cells. C: TSR2 overexpression inhibits proliferation of gastric cancer cells (n=3). LV: Overexpression; Si: SiRNA. *P<0.05 vs Control.
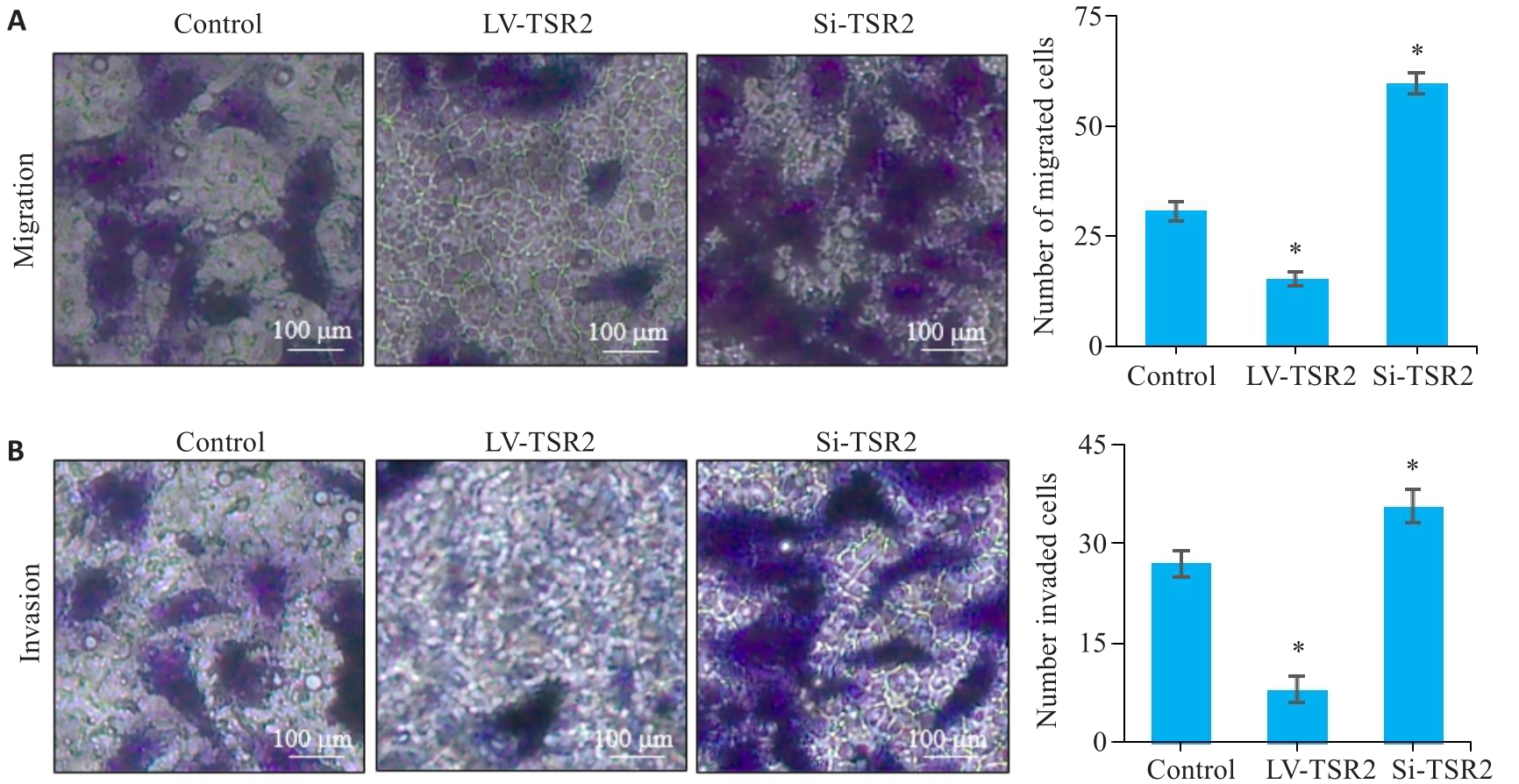
Fig.5 Effects of TSR2 overexpression and knockdown on migration (A) and invasion (B) of MGC-803 cells detected by Transwell assay (n=3). *P<0.05 vs Control.
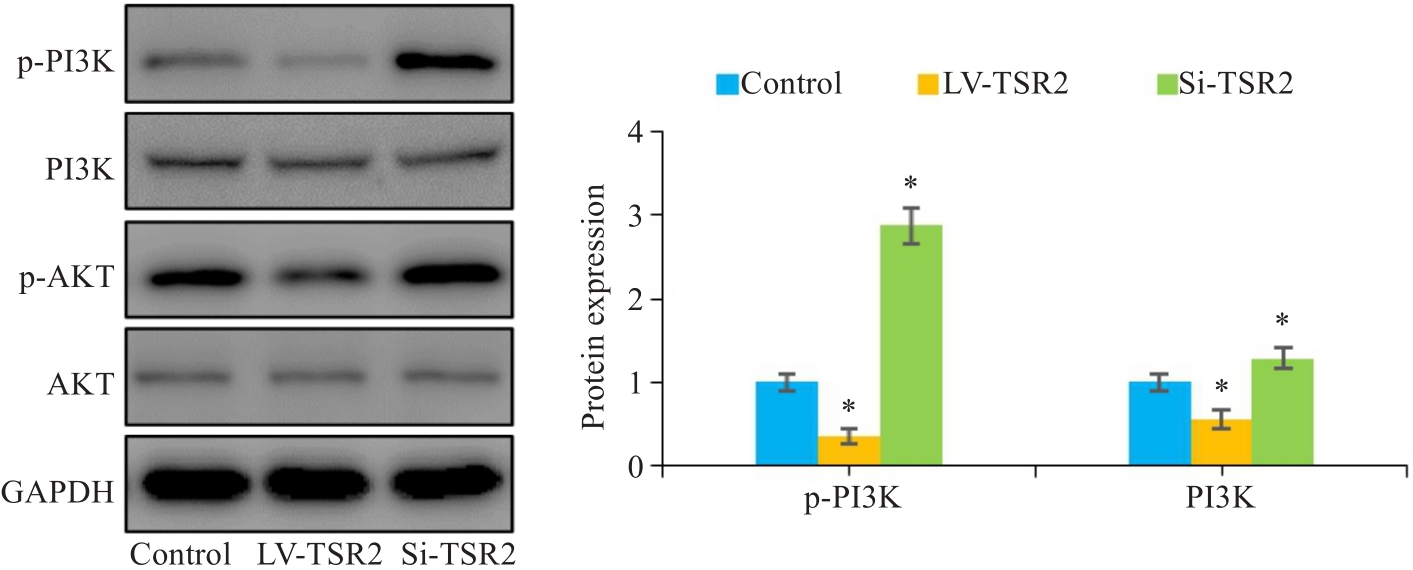
Fig.6 Western blotting for assessing the effect of TSR2 overexpression and knockdown on expression of p-PI3K and p-AKT in MGC803 cells (n=3). *P<0.05 vs Control.
| 1 | Mülder DT, Hahn AI, Huang RJ, et al. Prevalence of gastric precursor lesions in countries with differential gastric cancer burden: a systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2024: S1542-S3565(24)00227-1. DOI: 10.1016/j.cgh.2024.02.023 |
| 2 | Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492 |
| 3 | Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives[J]. World J Gastroenterol, 2016, 22(8): 2403-14. DOI: 10.3748/wjg.v22.i8.2403 |
| 4 | Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer[J]. Lancet, 2020, 396(10251): 635-48. DOI: 10.1016/s0140-6736(20)31288-5 |
| 5 | 朱正纲. 胃癌外科综合治疗的若干进展与展望[J]. 外科理论与实践, 2023, 28(1): 1-6. |
| 6 | Song ZY, Wu YY, Yang JB, et al. Progress in the treatment of advanced gastric cancer[J]. Tumour Biol, 2017, 39(7): 101042831771462. DOI: 10.1177/1010428317714626 |
| 7 | Brisinda G, Chiarello MM, Crocco A, et al. Postoperative mortality and morbidity after D2 lymphadenectomy for gastric cancer: a retrospective cohort study[J]. World J Gastroenterol, 2022, 28(3): 381-98. DOI: 10.3748/wjg.v28.i3.381 |
| 8 | Feng F, Tian YZ, Xu GH, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer[J]. BMC Cancer, 2017, 17(1): 737. DOI: 10.1186/s12885-017-3738-y |
| 9 | de Manzoni G, Yang HK. Gastric cancer surgery[J]. Updat Surg, 2018, 70(2):155. DOI: 10.1007/s13304-018-0551-3 |
| 10 | 贾 祺, 咸小红, 李泱润, 等. MAGE-A家族在胃癌中作用的研究进展[J]. 中南大学学报: 医学版, 2023, 48(2): 260-7. |
| 11 | Zhao QF, Cao L, Guan LL, et al. Immunotherapy for gastric cancer: dilemmas and prospect[J]. Brief Funct Genomics, 2019, 18(2): 107-12. DOI: 10.1093/bfgp/ely019 |
| 12 | Zheng CH, Xu YC, Zhao G, et al. Outcomes of laparoscopic total gastrectomy combined with spleen-preserving hilar lymphadenectomy for locally advanced proximal gastric cancer: a nonrandomized clinical trial[J]. JAMA Netw Open, 2021, 4(12): e2139992. DOI: 10.1001/jamanetworkopen.2021.39992 |
| 13 | Lin XL, Han T, Xia Q, et al. CHPF promotes gastric cancer tumorigenesis through the activation of E2F1[J]. Cell Death Dis, 2021, 12(10): 876. DOI: 10.1038/s41419-021-04148-y |
| 14 | Feng YY, Zhao M, Wang LJ, et al. The heterogeneity of signaling pathways and drug responses in intrahepatic cholangiocarcinoma with distinct genetic mutations[J]. Cell Death Dis, 2024, 15(1): 34. DOI: 10.1038/s41419-023-06406-7 |
| 15 | He HJ, Bing H, Liu GJ. TSR2 Induces laryngeal cancer cell apoptosis through inhibiting NF‑κB signaling pathway[J]. Laryngoscope, 2018, 128(4): E130-4. DOI: 10.1002/lary.27035 |
| 16 | He HJ, Zhu D, Sun J, et al. The Novel protein TSR2 inhibits the transcriptional activity of nuclear factor‑κB and induces apoptosis[J]. Mol Biol, 2011, 45(3): 451-7. DOI: 10.1134/s0026893311020099 |
| 17 | Zhao QX, Rangan R, Weng SN, et al. Inhibition of ribosome biogenesis in the epidermis is sufficient to trigger organism-wide growth quiescence independently of nutritional status in C. elegans[J]. PLoS Biol, 2023, 21(8): e3002276. DOI: 10.1371/journal.pbio.3002276 |
| 18 | Bu DC, Luo HT, Huo PP, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis[J]. Nucleic Acids Res, 2021, 49(W1): W317-25. DOI: 10.1093/nar/gkab447 |
| 19 | Sun XZ, Chen PX, Chen X, et al. KIF4A enhanced cell proliferation and migration via Hippo signaling and predicted a poor prognosis in esophageal squamous cell carcinoma[J]. Thorac Cancer, 2021, 12(4): 512-24. DOI: 10.1111/1759-7714.13787 |
| 20 | Thrift AP, El-Serag HB. Burden of gastric cancer[J]. Clin Gastroenterol Hepatol, 2020, 18(3): 534-42. DOI: 10.1016/j.cgh.2019.07.045 |
| 21 | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors[J]. J Surg Oncol, 2013, 107(3): 230-6. DOI: 10.1002/jso.23262 |
| 22 | Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention[J]. Cancer Epidemiol Biomarkers Prev, 2014, 23(5): 700-13. DOI: 10.1158/1055-9965.epi-13-1057 |
| 23 | Maddineni G, Xie JJ, Brahmbhatt B, et al. Diet and carcinogenesis of gastric cancer[J]. Curr Opin Gastroenterol, 2022, 38(6): 588-91. DOI: 10.1097/mog.0000000000000875 |
| 24 | Yusefi AR, Bagheri Lankarani K, Bastani P, et al. Risk factors for gastric cancer: a systematic review[J]. Asian Pac J Cancer Prev, 2018, 19(3): 591-603. |
| 25 | Ansari S, Gantuya B, Tuan VP, et al. Diffuse gastric cancer: a summary of analogous contributing factors for its molecular pathogenicity[J]. Int J Mol Sci, 2018, 19(8): 2424. DOI: 10.3390/ijms19082424 |
| 26 | Guo ZJ, Guo L. Abnormal activation of RFC3, A YAP1/TEAD downstream target, promotes gastric cancer progression[J]. Int J Clin Oncol, 2024, 29(4): 442-55. DOI: 10.1007/s10147-024-02478-3 |
| 27 | Rong L, Li ZD, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomed Pharmacother, 2020, 122: 109726. DOI: 10.1016/j.biopha.2019.109726 |
| 28 | Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and metastasis[J]. Cell Metab, 2021, 33(7): 1307-21. DOI: 10.1016/j.cmet.2021.04.002 |
| 29 | Wan GQ, Liu YH, Zhu J, et al. SLFN5 suppresses cancer cell migration and invasion by inhibiting MT1-MMP expression via AKT/GSK-3β/β-catenin pathway[J]. Cell Signal, 2019, 59: 1-12. DOI: 10.1016/j.cellsig.2019.03.004 |
| 30 | Yang YM, Karbstein K. The chaperone Tsr2 regulates Rps26 release and reincorporation from mature ribosomes to enable a reversible, ribosome-mediated response to stress[J]. Sci Adv, 2022, 8(8): eabl4386. DOI: 10.1126/sciadv.abl4386 |
| 31 | Yang YM, Jung Y, Abegg D, et al. Chaperone-directed ribosome repair after oxidative damage[J]. Mol Cell, 2023, 83(9): 1527-37, e5. DOI: 10.1016/j.molcel.2023.03.030 |
| 32 | Fernández-Parejo N, Lorenzo-Martín LF, García-Pedrero JM, et al. VAV2 orchestrates the interplay between regenerative proliferation and ribogenesis in both keratinocytes and oral squamous cell carcinoma[J]. Sci Rep, 2024, 14(1): 4060. DOI: 10.1038/s41598-024-54808-0 |
| 33 | Zang Y, Ran X, Yuan J, et al. Genomic hallmarks and therapeutic targets of ribosome biogenesis in cancer[J]. Brief Bioinform, 2024, 25(2): bbae023. DOI: 10.1093/bib/bbae023 |
| 34 | An Y, Xia YC, Wang ZY, et al. Clinical significance of ribosome production factor 2 homolog in hepatocellular carcinoma[J]. Clin Res Hepatol Gastroenterol, 2024, 48(3): 102289. DOI: 10.1016/j.clinre.2024.102289 |
| 35 | Miricescu D, Totan A, Stanescu-Spinu II, et al. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects[J]. Int J Mol Sci, 2020, 22(1): 173. DOI: 10.3390/ijms22010173 |
| 36 | Sun TS, Bi FF, Liu ZN, et al. TMEM119 facilitates ovarian cancer cell proliferation, invasion, and migration via the PDGFRB/PI3K/AKT signaling pathway[J]. J Transl Med, 2021, 19(1): 111. DOI: 10.1186/s12967-021-02781-x |
| 37 | Fresno Vara JA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer[J]. Cancer Treat Rev, 2004, 30(2): 193-204. DOI: 10.1016/j.ctrv.2003.07.007 |
| 38 | Maiello D, Varone M, Vicidomini R, et al. Dyskerin downregulation can induce ER stress and promote autophagy via AKT-mTOR signaling deregulation[J]. Biomedicines, 2022, 10(5): 1092. DOI: 10.3390/biomedicines10051092 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||