Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (8): 1743-1750.doi: 10.12122/j.issn.1673-4254.2025.08.18
Zejin OU1,2( ), Ying LI3, Shi CHEN3, Ziyi WANG4, Meiyi HE4, Zhicheng CHEN3, Shihao TANG1,2, Xiaojing MENG3, Zhi WANG1,2(
), Ying LI3, Shi CHEN3, Ziyi WANG4, Meiyi HE4, Zhicheng CHEN3, Shihao TANG1,2, Xiaojing MENG3, Zhi WANG1,2( )
)
Received:2025-02-13
Online:2025-08-20
Published:2025-09-05
Contact:
Zhi WANG
E-mail:ouzejin@smu.edu.cn;zhi_wang@outlook.com
Zejin OU, Ying LI, Shi CHEN, Ziyi WANG, Meiyi HE, Zhicheng CHEN, Shihao TANG, Xiaojing MENG, Zhi WANG. Inhibition of ferroptosis alleviates acute kidney injury caused by diquat in zebrafish[J]. Journal of Southern Medical University, 2025, 45(8): 1743-1750.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.08.18
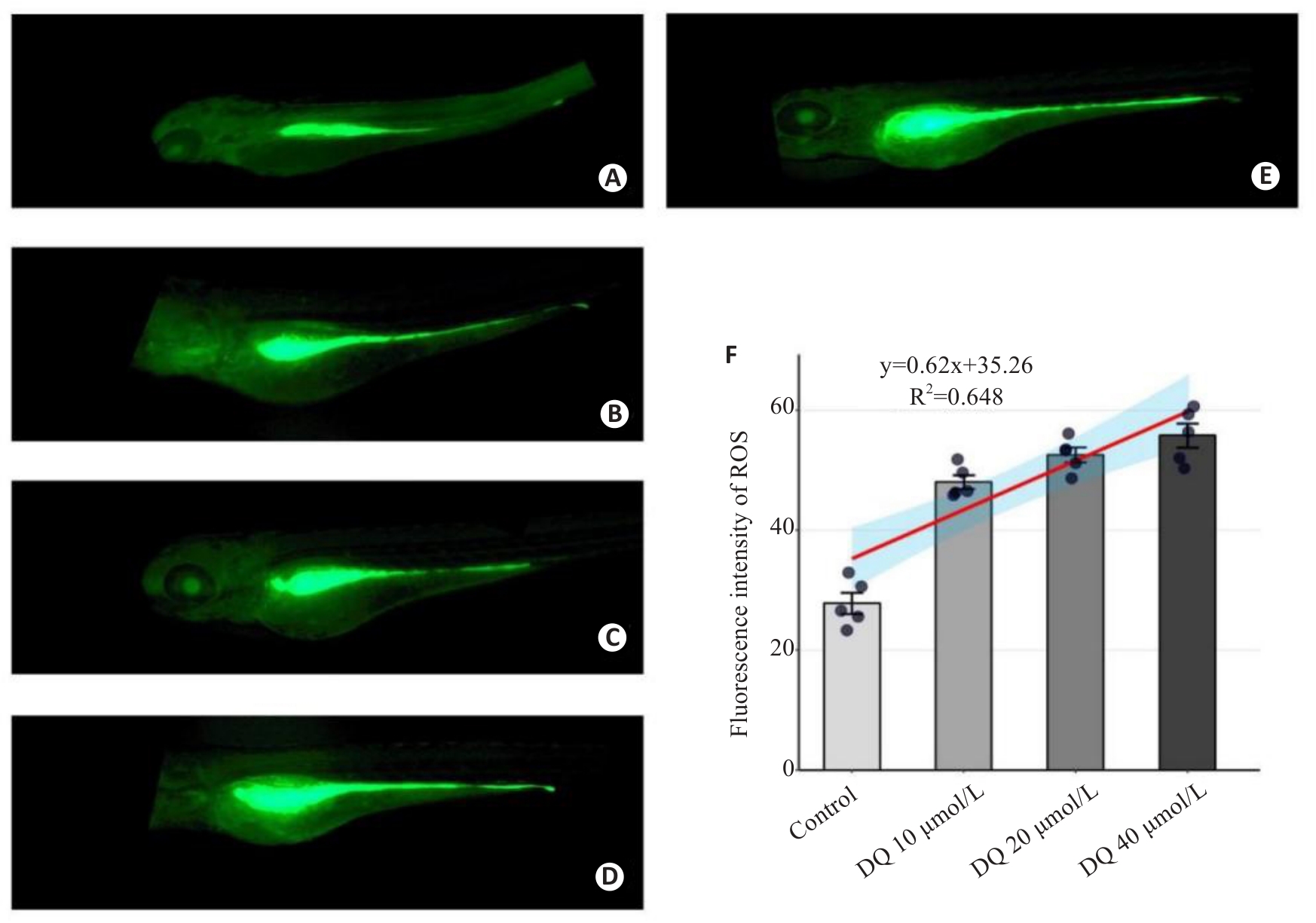
Fig.3 Fluorescence quantitative analysis of the effect of diquat on ROS level in live-stained zebrafish (×40). A: Control group. B: Gentamicin-positive group. C: Diquat 10 μmol/L group. D: Diquat 20 μmol/L group. E: Diquat 40 μmol/L group. F: Results of quantitative analysis.
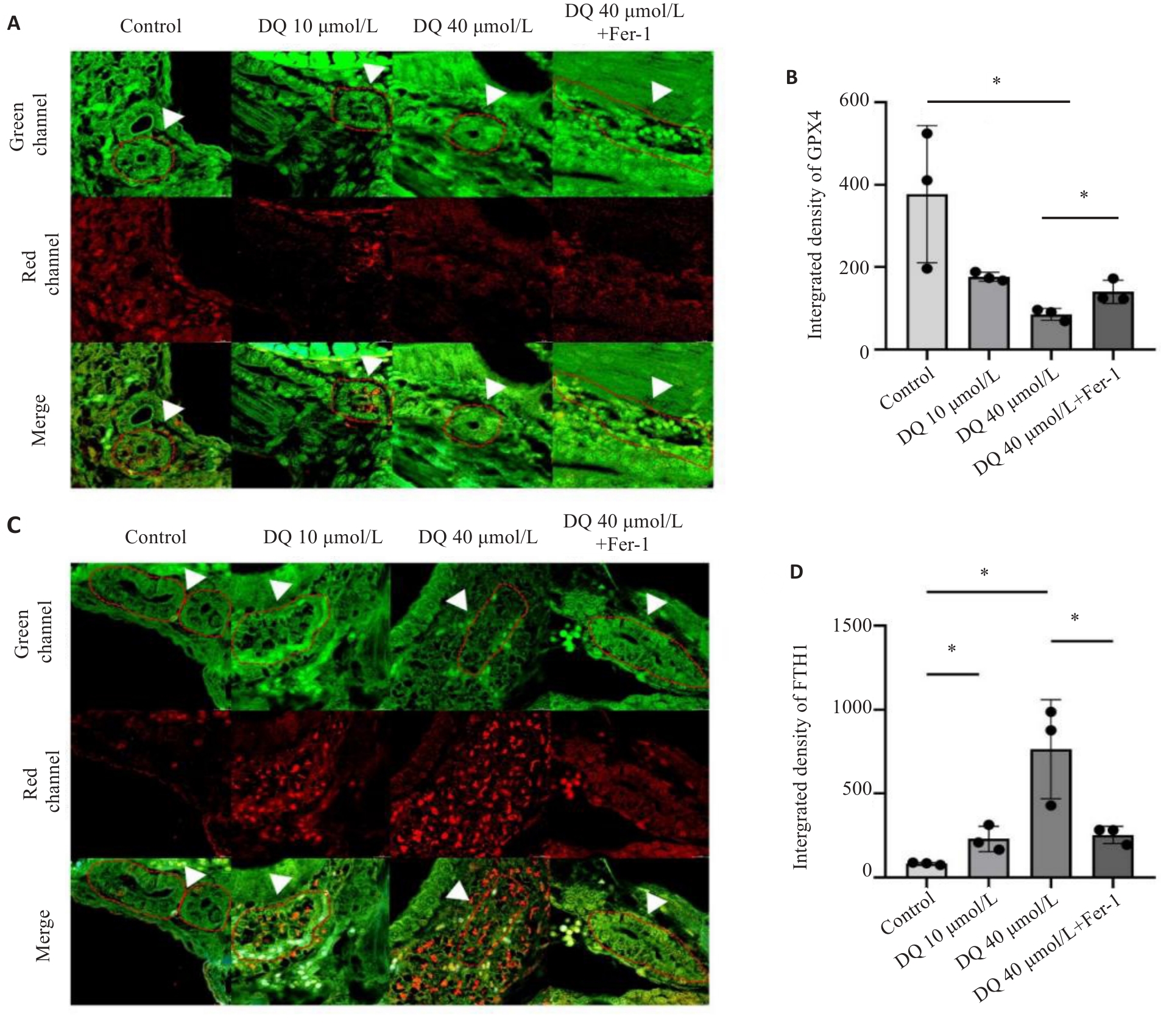
Fig 4 Effect of ferroptosis inhibition on expressions of GPX4 and FTH1 in the kidneys of diquat-exposed zebrafish (×400). A, B: Immunofluorescence and quantitative analysis of GPX4. C,D: Immunofluorescence and quantitative analysis of FTH1. The white arrows indicated the zebrafish kidney tissue. *P<0.05.
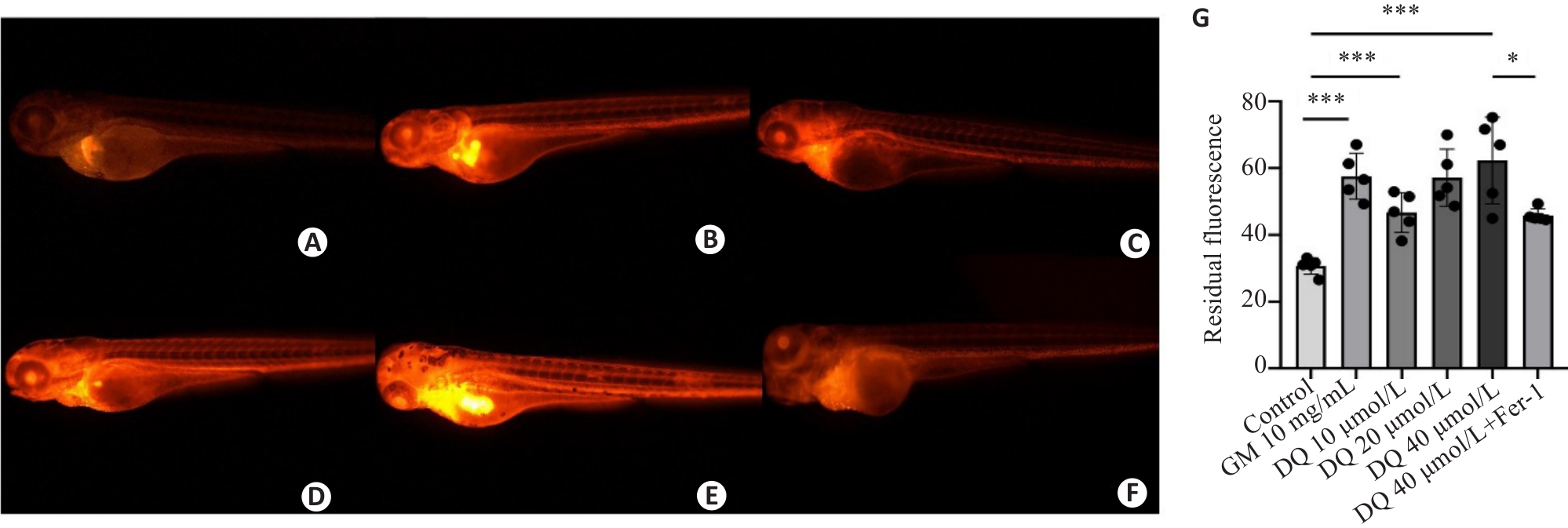
Fig.5 Effect of diquat exposure on glomerular filtration rate in zebrafish (×40). The metabolism of rhodamine B-labeled dextran was detected using fluorescence quantification. A: Control group. B: Gentamicin-positive group. C: Diquat 10 μmol/L group. D: Diquat 20 μmol/L group. E: Diquat 40 μmol/L group. F: Diquat 40 μmol/L group+Fer-1 intervention group. G: The results of quantitative analysis. *P<0.05, ***P<0.001.
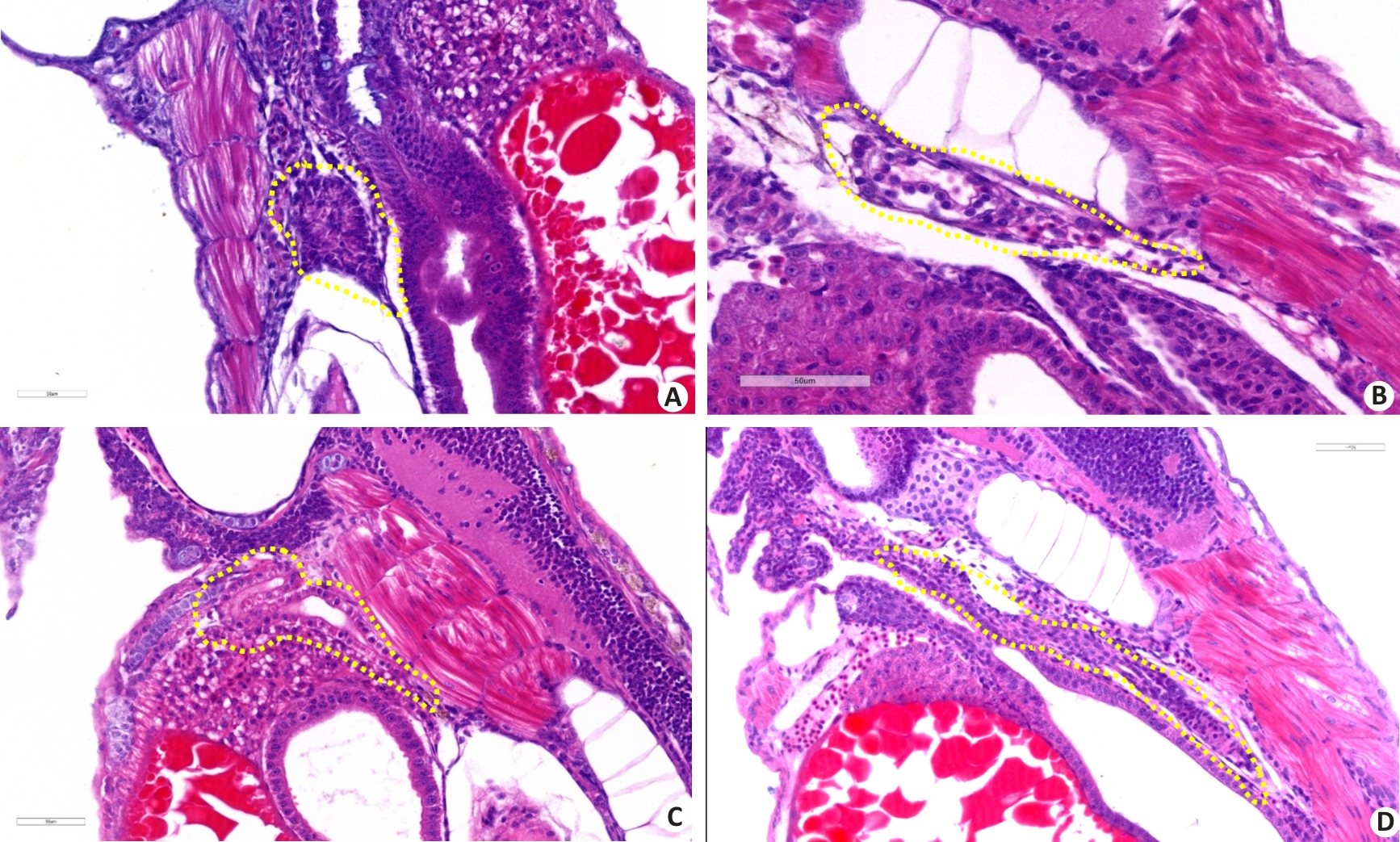
Fig.6 Pathological analysis of the effect of diquat exposure on renal injury in zebrafish (HE staining, ×400). A: Control group. B: Gentamicin-positive group. C: Diquat exposure 40 μmol/L group. D: Diquat 40 μmol/L group+Fer-1 intervention group.
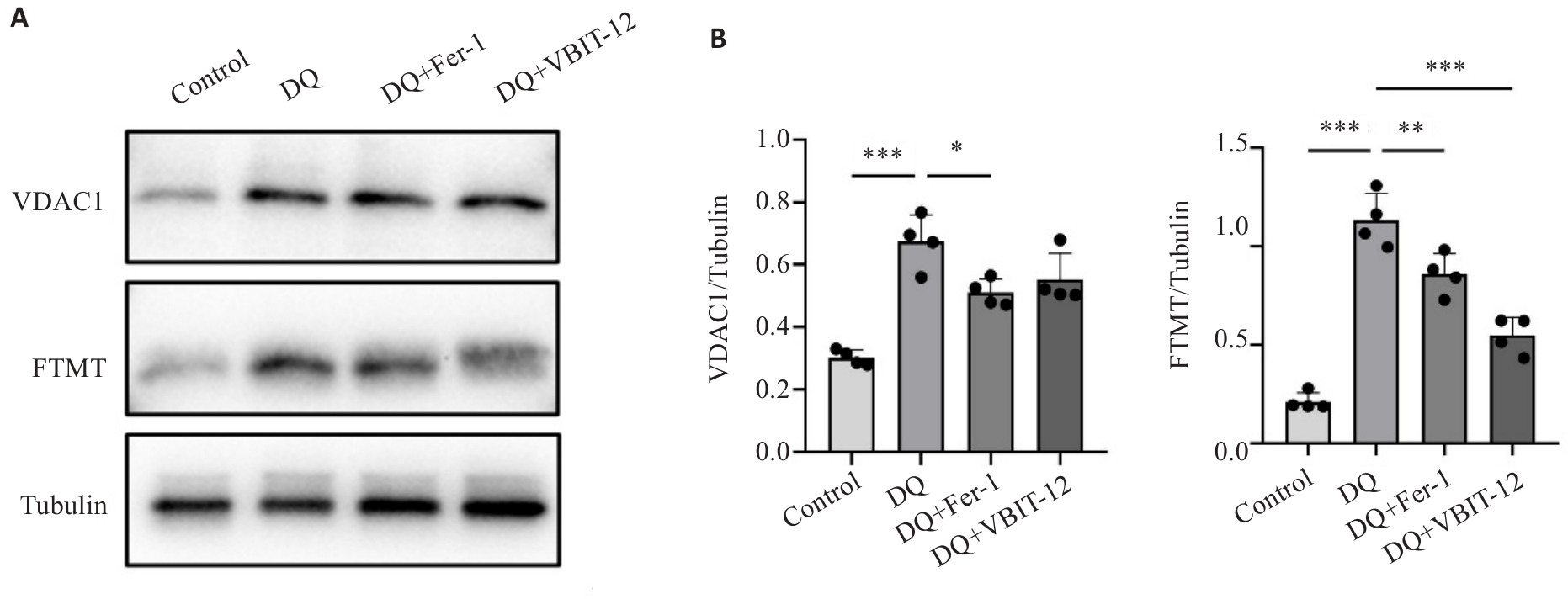
Fig.7 Effect of diquat exposure and drug interventions on protein expressions of VDAC1 and FTMT. A: Protein expressions of VDAC1 and FTMT detected using Western blotting. B: Quantitative analysis of the protein expressions (n=20). *P<0.05, **P<0.01, ***P<0.001. Each experimental repetition involved 20 larvae.
| [1] | Aloise DM, Memon A, Zaldivar A. Diquat herbicide organo-phosphate poisoning and multi-organ failure: a case report[J]. Cureus, 2022, 14(7): e27241. |
| [2] | 孟 娜, 孙艺青, 刘 亮, 等. 急性敌草快中毒86例临床分析[J]. 中华危重病急救医学, 2022(3): 301-5. doi:10.3760/cma.j.cn121430-20220128-00105 |
| [3] | 敌草快中毒诊断与治疗专家共识组. 急性敌草快中毒诊断与治疗专家共识[J]. 中华急诊医学杂志, 2020, 10(29): 1282-302. |
| [4] | Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment[J]. Hum Exp Toxicol, 2018, 37(11): 1131-60. doi:10.1177/0960327118765330 |
| [5] | Zeng DH, Chen XH, Li Y, et al. Clinical and pathological characteristics of acute kidney injury caused by diquat poisoning[J]. Clin Toxicol (Phila), 2023, 61(9): 705-8. doi:10.1080/15563650.2023.2262113 |
| [6] | Vohra R, Salazar A, Cantrell FL, et al. The poison pen: bedside diagnosis of urinary diquat[J]. J Med Toxicol, 2010, 6(1): 35-6. doi:10.1007/s13181-010-0033-6 |
| [7] | Chen YC, Ou ZJ, Zhang RC, et al. Case report: Successful outcome of a young patient with rhabdomyolysis and shock caused by diquat poisoning[J]. Front Med (Lausanne), 2023, 10: 1116912. doi:10.3389/fmed.2023.1116912 |
| [8] | Yin J, Liu MF, Ren WK, et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets[J]. PLoS One, 2015, 10(4): e0122893. doi:10.1371/journal.pone.0122893 |
| [9] | Jović-Stosić J, Babić G, Todorović V. Fatal diquat intoxication[J]. Vojnosanit Pregl, 2009, 66(6): 477-81. doi:10.2298/vsp0906477j |
| [10] | Suleiman SA, Stevens JB. Bipyridylium herbicide toxicity: effects of paraquat and diquat on isolated rat hepatocytes[J]. J Environ Pathol Toxicol Oncol, 1987, 7(3): 73-84. doi:10.3109/08923978709035231 |
| [11] | Lai KM, Wang JJ, Lin SY, et al. Sensing of mitochondrial DNA by ZBP1 promotes RIPK3-mediated necroptosis and ferroptosis in response to diquat poisoning[J]. Cell Death Differ, 2024, 31(5): 635-50. doi:10.1038/s41418-024-01279-5 |
| [12] | von Mässenhausen A, Tonnus W, Himmerkus N, et al. Phenytoin inhibits necroptosis[J]. Cell Death Dis, 2018, 9(3): 359. doi:10.1038/s41419-018-0394-3 |
| [13] | Qin QY, Yu NJ, Gu YX, et al. Inhibiting multiple forms of cell death optimizes ganglion cells survival after retinal ischemia reperfusion injury[J]. Cell Death Dis, 2022, 13(5): 507. doi:10.1038/s41419-022-04911-9 |
| [14] | Wu YZ, Cui SQ, Wang WJ, et al. Kidney and lung injury in rats following acute diquat exposure[J]. Exp Ther Med, 2022, 23(4): 275. doi:10.3892/etm.2022.11201 |
| [15] | Scindia Y, Dey P, Thirunagari A, et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis[J]. J Am Soc Nephrol, 2015, 26(11): 2800-14. doi:10.1681/asn.2014101037 |
| [16] | 黄 奕, 林丽珊, 黄浩华, 等. VDAC1通过诱导气道上皮细胞铁死亡参与屋尘螨诱导的哮喘小鼠气道炎症[J]. 南方医科大学学报, 2023, 43(8): 1333-8. |
| [17] | Kim J, Gupta R, Blanco LP, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease[J]. Science, 2019, 366(6472): 1531-6. doi:10.1126/science.aav4011 |
| [18] | Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome[J]. Nature, 2013, 496(7446): 498-503. |
| [19] | McCampbell KK, Springer KN, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney[J]. J Vis Exp, 2014(90): e51644. doi:10.3791/51644-v |
| [20] | Diep CQ, Peng ZZ, Ukah TK, et al. Development of the zebrafish mesonephros[J]. Genesis, 2015, 53(3/4): 257-69. doi:10.1002/dvg.22846 |
| [21] | Outtandy P, Russell C, Kleta R, et al. Zebrafish as a model for kidney function and disease[J]. Pediatr Nephrol, 2019, 34(5): 751-62. doi:10.1007/s00467-018-3921-7 |
| [22] | Xu X, Wei Y, Hua HW, et al. Glycine alleviated intestinal injury by inhibiting ferroptosis in piglets challenged with diquat[J]. Animals (Basel), 2022, 12(22): 3071. doi:10.3390/ani12223071 |
| [23] | Chen YN, Zhang H, Li Y, et al. Pterostilbene confers protection against diquat-induced intestinal damage with potential regulation of redox status and ferroptosis in broiler chickens[J]. Oxid Med Cell Longev, 2023, 2023: 8258354. doi:10.1155/2023/8258354 |
| [24] | Cheng JY, Yang L, Zhang ZL, et al. Diquat causes mouse testis injury through inducing heme oxygenase-1-mediated ferroptosis in spermatogonia[J]. Ecotoxicol Environ Saf, 2024, 280: 116562. doi:10.1016/j.ecoenv.2024.116562 |
| [25] | Cui SQ, Zhang XX, Wang C, et al. Study on the therapeutic effect of glucocorticoids on acute kidney injury in rats exposed to diquat[J]. Biomed Pharmacother, 2023, 166: 115310. doi:10.1016/j.biopha.2023.115310 |
| [26] | Gorgulho R, Jacinto R, Lopes SS, et al. Usefulness of zebrafish larvae to evaluate drug-induced functional and morphological renal tubular alterations[J]. Arch Toxicol, 2018, 92(1): 411-23. doi:10.1007/s00204-017-2063-1 |
| [27] | Drechsel DA, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in Parkinsonism[J]. Toxicol Sci, 2009, 112(2): 427-34. doi:10.1093/toxsci/kfp223 |
| [28] | Qu J, Pei H, Li XZ, et al. Erythrocyte membrane biomimetic EGCG nanoparticles attenuate renal injury induced by diquat through the NF-κB/NLRP3 inflammasome pathway[J]. Front Pharmacol, 2024, 15: 1414918. doi:10.3389/fphar.2024.1414918 |
| [29] | Huang SF, Lin SR, Zhou SL, et al. Soluble thrombomodulin alleviates Diquat-induced acute kidney injury by inhibiting the HMGB1/IκBα/NF‑κB signalling pathway[J]. Food Chem Toxicol, 2023, 178: 113871. doi:10.1016/j.fct.2023.113871 |
| [30] | Liu Y, Yuan JM, Xi WS, et al. Lactiplantibacillus plantarum ameliorated morphological damage and barrier dysfunction and reduced apoptosis and ferroptosis in the jejunum of oxidatively stressed piglets[J]. Animals (Basel), 2024, 14(22): 3335. doi:10.3390/ani14223335 |
| [31] | Chen KY, Tang YH, Lan LH, et al. Autophagy mediated FTH1 degradation activates gasdermin E dependent pyroptosis contributing to diquat induced kidney injury[J]. Food Chem Toxicol, 2024, 184: 114411. doi:10.1016/j.fct.2023.114411 |
| [32] | Wu LZ, Luo ZW, Luo FL, et al. Edaravone inhibits neuronal ferroptosis and alleviates acute Central nervous system injury induced by diquat via enhancement of METTL14-mediated m6A methylation of Aldh1l1[J]. Free Radic Res, 2025, 59(3): 274-88. doi:10.1080/10715762.2025.2482774 |
| [33] | Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis[J]. Proc Natl Acad Sci USA, 2014, 111(47): 16836-41. doi:10.1073/pnas.1415518111 |
| [34] | Sun MF, Zhu L, Chen X. The role of ferroptosis in renal injury induced by diquat[J]. Chin J Indust Hygiene Occupat Dis, 2025, 43(1): 14-24. |
| [35] | Yang YT, Lin QS, Zhu XY, et al. Activation of lipophagy is required for RAB7 to regulate ferroptosis in sepsis-induced acute kidney injury[J]. Free Radic Biol Med, 2024, 218: 120-31. doi:10.1016/j.freeradbiomed.2024.04.213 |
| [36] | Cao YX, Liu XW, Guo CJ, et al. Biomimetic reactive oxygen/nitrogen nanoscavengers inhibit "ferroptosis storm" and modulate immune targeting for acute kidney injury[J]. J Control Release, 2025, 379: 59-76. doi:10.1016/j.jconrel.2025.01.006 |
| [37] | Karmi O, Marjault HB, Bai F, et al. A VDAC1-mediated NEET protein chain transfers [2Fe-2S] clusters between the mitochondria and the cytosol and impacts mitochondrial dynamics[J]. Proc Natl Acad Sci USA, 2022, 119(7): e2121491119. doi:10.1073/pnas.2121491119 |
| [38] | Shao FY, Han JY, Tian ZY, et al. Synergistic ROS generation and directional overloading of endogenous calcium induce mito-chondrial dysfunction in living cells[J]. Biomaterials, 2023, 301: 122284. doi:10.1016/j.biomaterials.2023.122284 |
| [39] | Niu BL, Lei XH, Xu QL, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury[J]. Cell Biol Toxicol, 2022, 38(3): 505-30. doi:10.1007/s10565-021-09624-x |
| [40] | Yang J, Lu X, Hao JL, et al. VSTM2L protects prostate cancer cells against ferroptosis via inhibiting VDAC1 oligomerization and maintaining mitochondria homeostasis[J]. Nat Commun, 2025, 16(1): 1160. doi:10.1038/s41467-025-56494-6 |
| [41] | Drysdale J, Arosio P, Invernizzi R, et al. Mitochondrial ferritin: a new player in iron metabolism[J]. Blood Cells Mol Dis, 2002, 29(3): 376-83. doi:10.1006/bcmd.2002.0577 |
| [42] | Wang YQ, Chang SY, Wu Q, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis[J]. Front Aging Neurosci, 2016, 8: 308. doi:10.3389/fnagi.2016.00308 |
| [43] | Qian B, Jiang RJ, Song JL, et al. Organophosphorus flame retardant TDCPP induces neurotoxicity via mitophagy-related ferroptosis in vivo and in vitro [J]. Chemosphere, 2022, 308(Pt 2): 136345. doi:10.1016/j.chemosphere.2022.136345 |
| [1] | Xinyuan CHEN, Chengting WU, Ruidi LI, Xueqin PAN, Yaodan ZHANG, Junyu TAO, Caizhi LIN. Shuangshu Decoction inhibits growth of gastric cancer cell xenografts by promoting cell ferroptosis via the P53/SLC7A11/GPX4 axis [J]. Journal of Southern Medical University, 2025, 45(7): 1363-1371. |
| [2] | Mengying ZHANG, Chenling ZHAO, Liwei TIAN, Guofang YU, Wenming YANG, Ting DONG. Gandou Fumu Decoction improves liver steatosis by inhibiting hepatocyte ferroptosis in mice with Wilson's disease through the GPX4/ACSL4/ALOX15 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1471-1478. |
| [3] | Zihao WANG, Lili TAO, Biqing ZOU, Shengli AN. First 24-hour arterial oxygen partial pressure is correlated with mortality in ICU patients with acute kidney injury: an analysis based on MIMIC-IV database [J]. Journal of Southern Medical University, 2025, 45(5): 1056-1062. |
| [4] | Liupan ZHANG, Xiaotong SHI, Lulan LI, Rui SHI, Shengli AN, Zhenhua ZENG. Association between serum albumin levels after albumin infusion and 28-day mortality in critically ill patients with acute kidney injury [J]. Journal of Southern Medical University, 2025, 45(5): 1074-1081. |
| [5] | Anbang ZHANG, Xiuqi SUN, Bo PANG, Yuanhua WU, Jingyu SHI, Ning ZHANG, Tao YE. Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury in rats by inhibiting ferroptosis through the gut-brain axis and the Nrf2/HO-1 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(5): 911-920. |
| [6] | Linluo ZHANG, Changqing LI, Lingling HUANG, Xueping ZHOU, Yuanyuan LOU. Catalpol reduces liver toxicity of triptolide in mice by inhibiting hepatocyte ferroptosis through the SLC7A11/GPX4 pathway: testing the Fuzheng Zhidu theory for detoxification [J]. Journal of Southern Medical University, 2025, 45(4): 810-818. |
| [7] | Chunfei JI, Zongchao ZUO, Jun WANG, Miaonan LI. N-acetylneuraminic acid promotes ferroptosis of H9C2 cardiomyocytes with hypoxia/reoxygenation injury by inhibiting the Nrf2 axis [J]. Journal of Southern Medical University, 2025, 45(1): 72-79. |
| [8] | Kai CHEN, Zhaofei MENG, Jingting MIN, Jiahui WANG, Zhenghong LI, Qin GAO, Junfeng HU. Curcumin alleviates septic lung injury in mice by inhibiting TXNIP/TRX-1/GPX4-mediated ferroptosis [J]. Journal of Southern Medical University, 2024, 44(9): 1805-1813. |
| [9] | Mingzi OUYANG, Jiaqi CUI, Hui WANG, Zheng LIANG, Dajin PI, Liguo CHEN, Qianjun CHEN, Yingchao WU. Kaixinsan alleviates adriamycin-induced depression-like behaviors in mice by reducing ferroptosis in the prefrontal cortex [J]. Journal of Southern Medical University, 2024, 44(8): 1441-1449. |
| [10] | Yinliang ZHANG, Zetan LUO, Rui ZHAO, Na ZHAO, Zhidong XU, Di AO, Guyi CONG, Xinyu LIU, Hailun ZHENG. Sanguinarine induces ferroptosis of colorectal cancer cells by upregulating STUB1 and downregulating GPX4 [J]. Journal of Southern Medical University, 2024, 44(8): 1537-1544. |
| [11] | Yuanguo WANG, Peng ZHANG. Ferroptosis suppressor genes are highly expressed in esophageal cancer to inhibit tumor cell ferroptosis [J]. Journal of Southern Medical University, 2024, 44(7): 1389-1396. |
| [12] | Huaxing HE, Lulin LIU, Yingyin LIU, Nachuan CHEN, Suxia SUN. Sodium butyrate and sorafenib synergistically inhibit hepatocellular carcinoma cells possibly by inducing ferroptosis through inhibiting YAP [J]. Journal of Southern Medical University, 2024, 44(7): 1425-1430. |
| [13] | Zhixian REN, Beixian ZHOU, Linxin WANG, Jing LI, Rongping ZHANG, Xiping PAN. Inhibitory effect of 5-hydroxy-6,7-dimethoxyflavone on H1N1 influenza virus-induced ferroptosis and inflammation in A549 cells and its possible mechanisms [J]. Journal of Southern Medical University, 2024, 44(6): 1070-1078. |
| [14] | Fangyuan ZHANG, Gang LIU. Dexmedetomidine inhibits ferroptosis of human renal tubular epithelial cells by activating the Nrf2/HO-1/GPX4 pathway [J]. Journal of Southern Medical University, 2024, 44(6): 1135-1140. |
| [15] | Nan WANG, Bin SHI, Xiaolan MAN, Weichao WU, Jia CAO. High expression of fragile X mental retardation protein inhibits ferroptosis of colorectal tumor cells by activating the RAS/MAPK signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 885-893. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||