南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (2): 261-268.doi: 10.12122/j.issn.1673-4254.2025.02.07
黄菊1,2( ), 殷丽霞3, 牛民主5, 耿志军1,2, 左芦根2,4, 李静2,3, 胡建国2,3(
), 殷丽霞3, 牛民主5, 耿志军1,2, 左芦根2,4, 李静2,3, 胡建国2,3( )
)
收稿日期:2024-09-09
出版日期:2025-02-20
发布日期:2025-03-03
通讯作者:
胡建国
E-mail:huangju0713@163.com;jghu9200@bbmc.edu.cn
作者简介:黄 菊,硕士,E-mail: huangju0713@163.com
基金资助:
Ju HUANG1,2( ), Lixia YIN3, Minzhu NIU5, Zhijun GENG1,2, Lugen ZUO2,4, Jing LI2,3, Jianguo HU2,3(
), Lixia YIN3, Minzhu NIU5, Zhijun GENG1,2, Lugen ZUO2,4, Jing LI2,3, Jianguo HU2,3( )
)
Received:2024-09-09
Online:2025-02-20
Published:2025-03-03
Contact:
Jianguo HU
E-mail:huangju0713@163.com;jghu9200@bbmc.edu.cn
Supported by:摘要:
目的 探讨天然植物单体紫花前胡苷(Nod)对克罗恩病(CD)样结肠炎的影响及其作用机制。 方法 建立脂多糖和三磷酸腺苷(ATP)联合诱导的结肠类器官焦亡模型,通过检测焦亡关键调节因子、通透性和促炎因子,探讨Nod对细胞焦亡、肠道屏障功能和炎症反应的影响。以2,4,6-三硝基苯磺酸(TNBS)干预小鼠为CD动物模型,通过检测体质量、DAI评分、组织病理学分析、炎症评分、肠屏障功能和肠上皮细胞焦亡,探讨Nod对CD样结肠炎的治疗效果。通过网络药理学和体内、体外实验验证探索Nod保护肠上皮细胞焦亡的潜在机制。 结果 脂多糖和ATP诱导的结肠类器官经Nod干预后显著抑制NLRP3、GSDMD-N、cleaved caspase-1和caspase-11的表达,改善肠道FITC-dextran(FD4,4000)通透性,以及降低IL-1β和IL-18水平(P<0.05)。在TNBS诱导的结肠炎小鼠中,Nod治疗后能缓解小鼠体质量下降幅度、降低DAI评分、改善炎症细胞浸润和炎症评分(P<0.05),并降低血清中FD4、I-FABP的含量与细菌移位至肠系膜淋巴结、脾和肝脏中比例(P<0.05)。Nod可抑制小鼠肠黏膜中NLRP3、GSDMD-N、cleaved caspase-1和caspase-11的表达(P<0.05)。网络药理学预测分析显示,Nod抗结肠炎可能与PI3K/Akt通路有关;体内外实验证实,Nod抑制PI3K/Akt通路的激活,且PI3K/Akt通路的激活剂(IGF-1)逆转了Nod对肠上皮细胞焦亡和肠屏障功能的保护作用(P<0.05)。 结论 Nod至少部分通过抑制PI3K/Akt信号传导拮抗肠上皮细胞的焦亡,从而保护肠屏障功能和改善CD样结肠炎,有望成为一种新的CD治疗药物。
黄菊, 殷丽霞, 牛民主, 耿志军, 左芦根, 李静, 胡建国. 紫花前胡苷通过抑制肠上皮细胞焦亡改善2,4,6-三硝基苯磺酸诱导的小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(2): 261-268.
Ju HUANG, Lixia YIN, Minzhu NIU, Zhijun GENG, Lugen ZUO, Jing LI, Jianguo HU. Nodakenin ameliorates TNBS-induced experimental colitis in mice by inhibiting pyroptosis of intestinal epithelial cells[J]. Journal of Southern Medical University, 2025, 45(2): 261-268.
| Gene | Forward primer (5'→3') | Reverse primer (5'→3') |
|---|---|---|
| IL-1β | ACAGCAAGGCGAAAAAGGATG | TGGTGGACCACTCGGATGA |
| IL-18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
表1 引物序列
Tab.1 Primer sequences for qRT-PCR
| Gene | Forward primer (5'→3') | Reverse primer (5'→3') |
|---|---|---|
| IL-1β | ACAGCAAGGCGAAAAAGGATG | TGGTGGACCACTCGGATGA |
| IL-18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
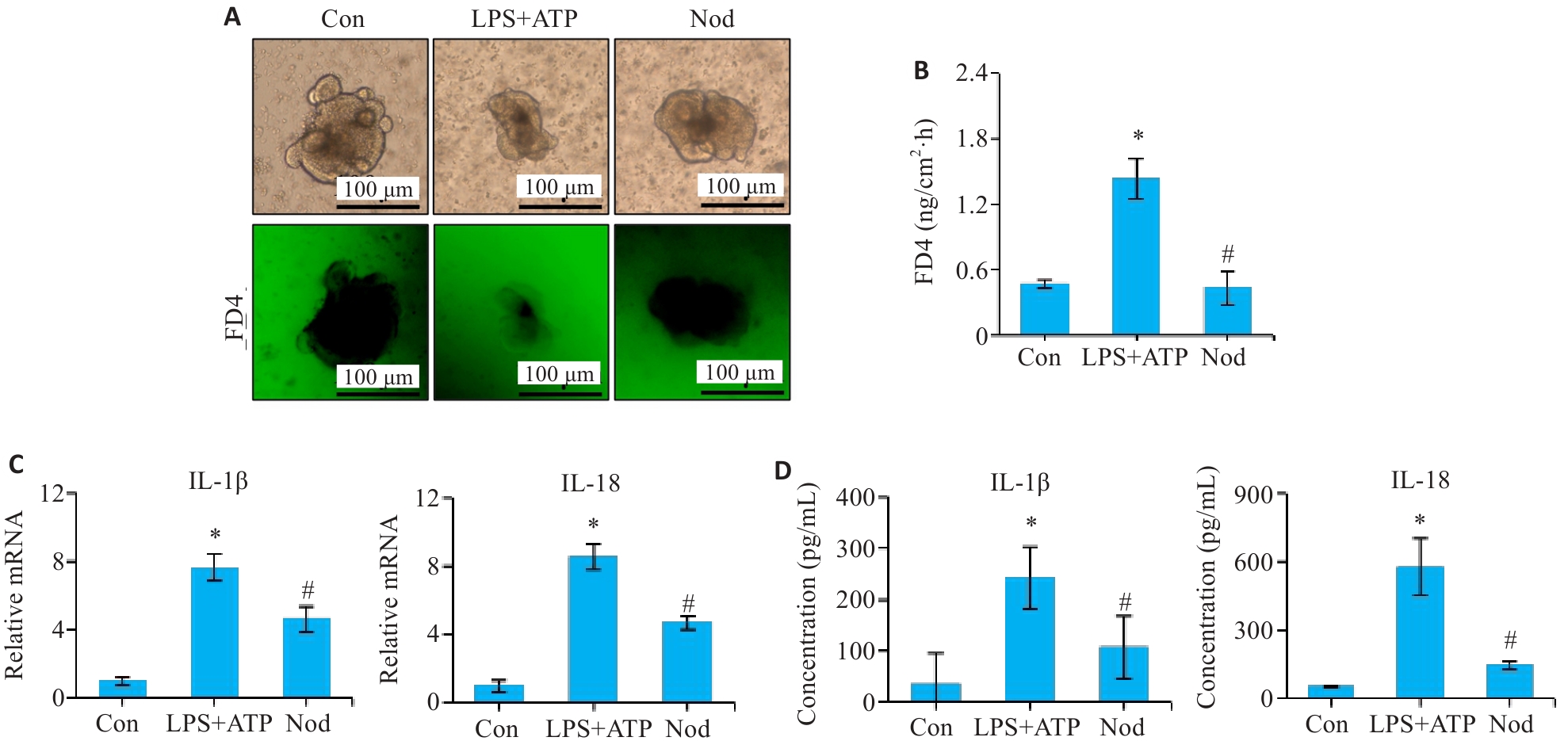
图2 Nod对结肠类器官中通透性和促炎因子表达的影响
Fig.2 Effect of Nod on permeability and pro-inflammatory factor expression in colonic organoids. A, B: Permeability assay of the colonic organoids. C: qRT-PCR analysis of mRNA levels of IL-1β and IL-18 in the colonic organoids. D: ELISA of protein levels of IL-1β and IL-18 in culture supernatants of the colonic organoids.*P<0.05 vs Con group; #P<0.05 vs LPS+ATP group.
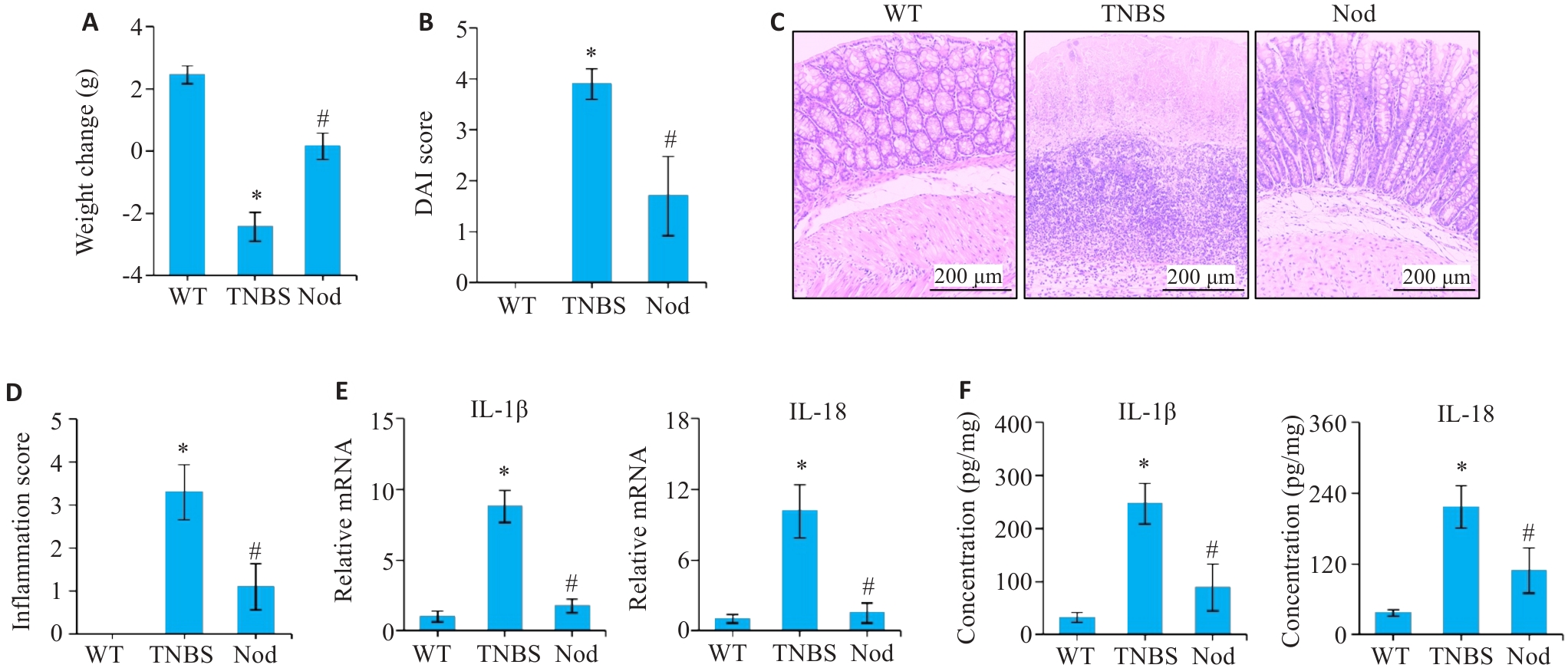
图3 Nod对小鼠TNBS诱导结肠炎的影响
Fig.3 Effect of Nod on TNBS-induced colitis in mice. A: Weight change of the mice. B: DAI score. C: HE staining of the colon tissue. D: Inflammation score of the colon tissue. E: mRNA expression levels of IL-1β and IL-18 analyzed using qRT-PCR. F: Protein expression levels of IL-1β and IL-18 detected by ELISA.*P<0.05 vs WT group; #P<0.05 vs TNBS group.
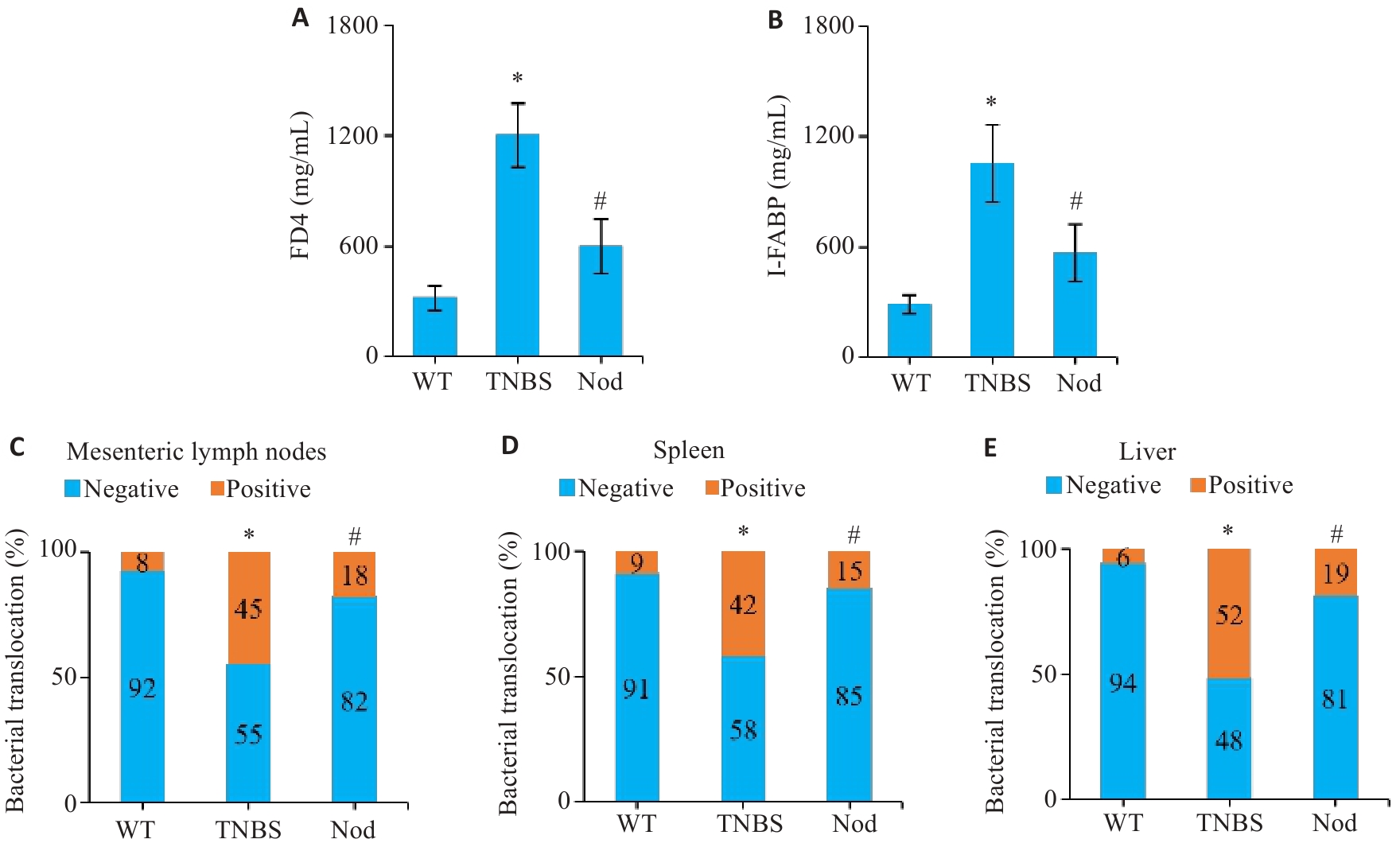
图4 Nod对TNBS诱导小鼠肠屏障的影响
Fig.4 Effect of Nod on intestinal barrier in TNBS-treated mice. A, B: Detection of serum FD4 and I-FABP levels in the mice. C-E: Positive rate of translocation of intestinal bacteria to the mesenteric lymph nodes, spleen, and liver. *P<0.05 vs WT group; #P<0.05 vs TNBS group.
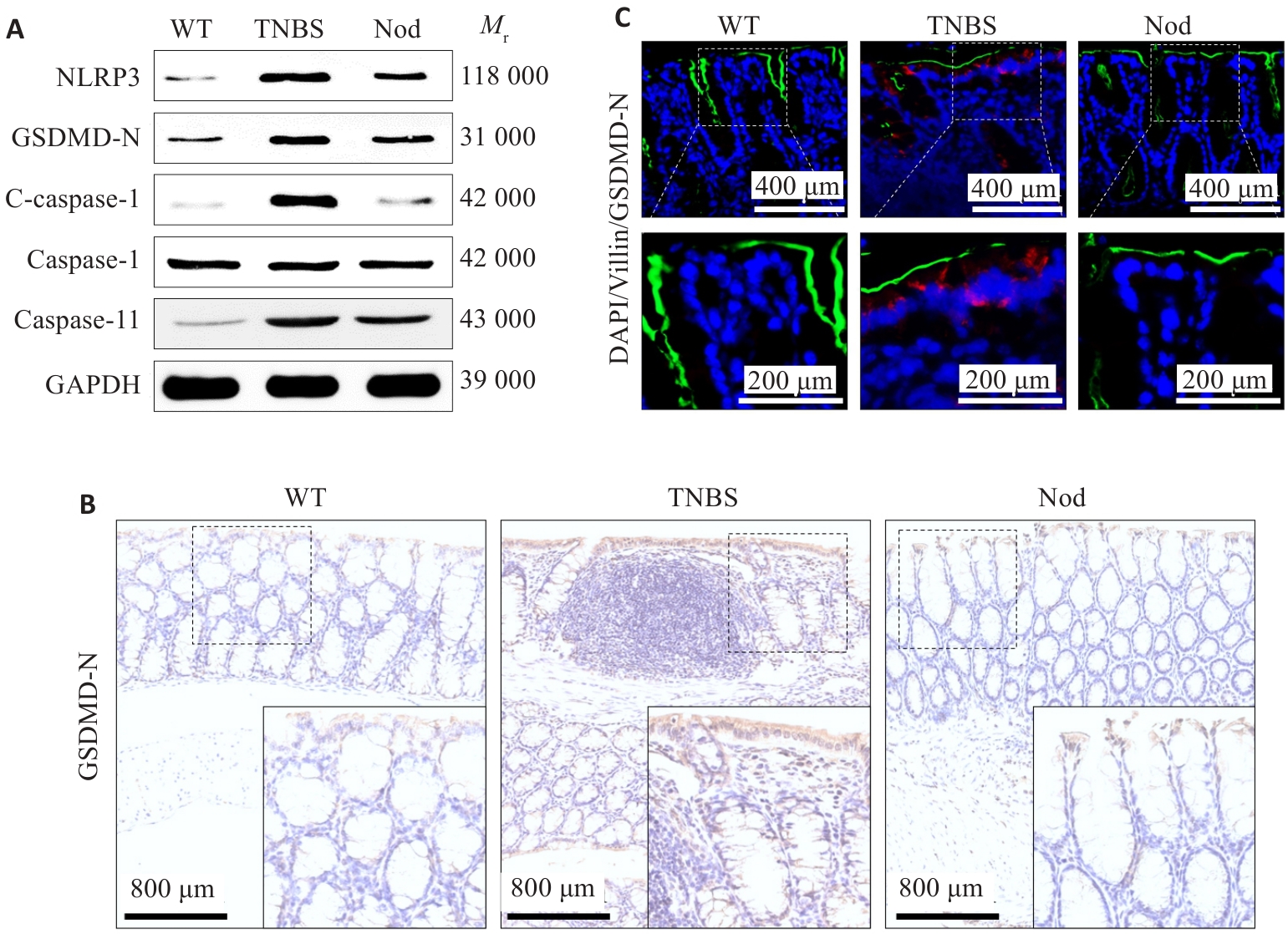
图5 Nod对TNBS诱导小鼠结肠组织中肠上皮细胞焦亡的影响
Fig.5 Effect of Nod on epithelial cell pyroptosis in the colon tissue of TNBS-treated mice. A: Western blotting for detecting NLRP3, GSDMD-N, cleaved caspase-1, caspase-1 and caspase-11 levels. B: Expression of GSDMD-N analyzed by immunohistochemical staining. C: Immunofluorescence co-staining of GSDMD-N and Villin.
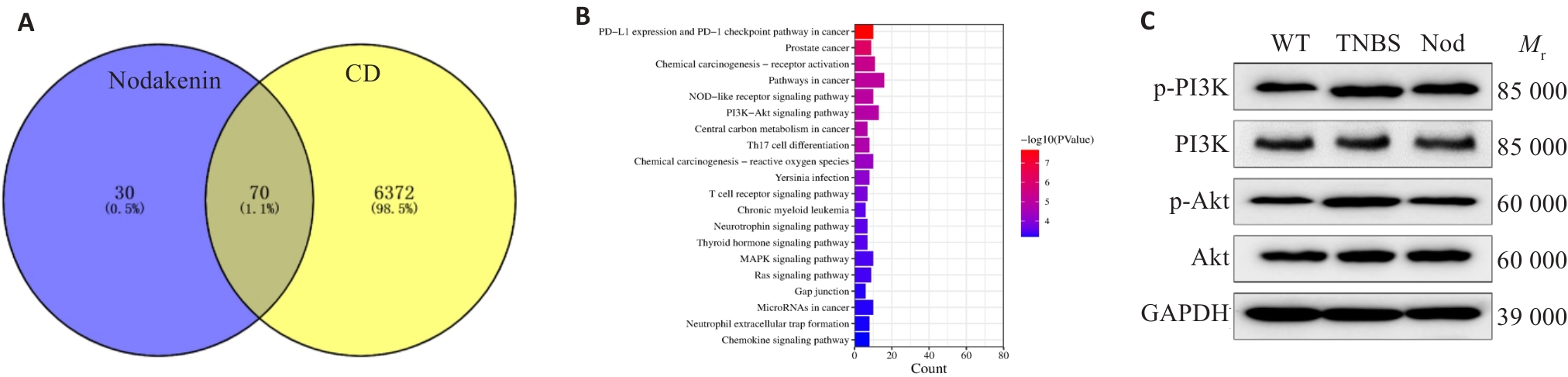
图6 Nod对TNBS诱导小鼠结肠组织中肠上皮细胞焦亡的影响
Fig.6 Effect of Nod on epithelial cell pyroptosis in the colon tissue of TNBS-treated mice and bioinformatics analysis. A: Intersection targets predicted by network pharmacology analysis. B: KEGG enrichment analysis. C: Western blotting analysis of p-PI3K and p-Akt expression levels.
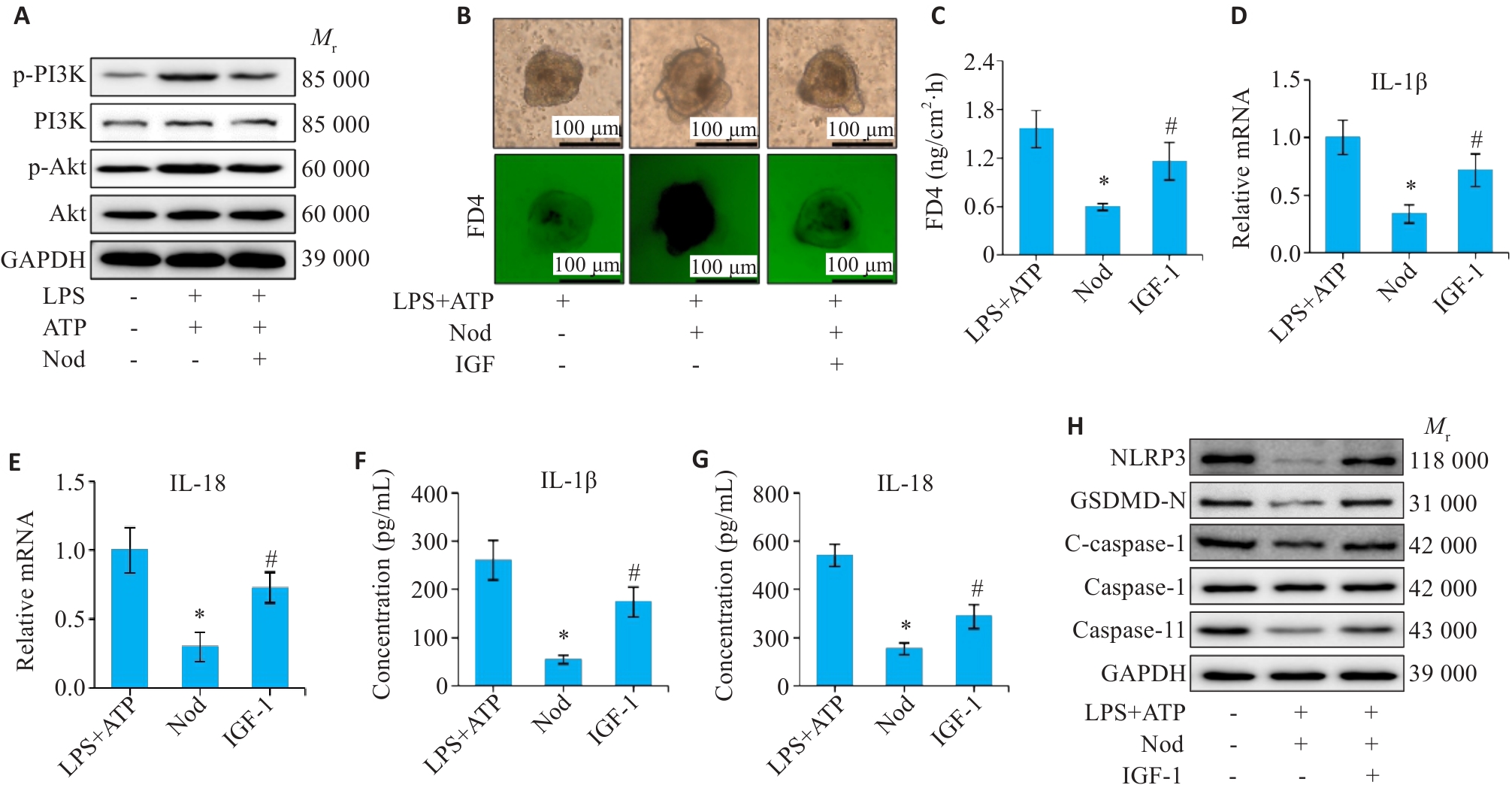
图7 PI3K/Akt参与Nod调控结肠类器官的通透性和促炎因子的表达
Fig.7 PI3K/Akt is involved in Nod-mediated regulation of colon organoid permeability and expression of pro-inflammatory factors. A: Expression levels of p-PI3K and p-Akt analyzed by Western blotting. B, C: Colonic organoid permeability test. D, E: mRNA levels of IL-1β and IL-18 detected by qRT-PCR. F, G: ELISA for determining IL-1β and IL-18 protein levels. H: Western blotting for detecting NLRP3, GSDMD-N, cleaved caspase-1, caspase-1, and caspase-11 levels. *P<0.05 vs LPS+ATP group; #P<0.05 vs Nod group.
| 1 | Palmela C, Chevarin C, Xu Z, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease[J]. Gut, 2018, 67(3): 574-87. |
| 2 | Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases[J]. Gastroenterol, 2011, 140(6): 1785-94. |
| 3 | Tan G, Huang CY, Chen JY, et al. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn's disease by promoting intestinal inflammation[J]. Cell Rep, 2021, 35(11): 109265. |
| 4 | Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation[J]. Nat Rev Immunol, 2020, 20: 143-57. |
| 5 | Cookson BT, Brennan MA. Pro-inflammatory programmed cell death[J]. Trends Microbiol, 2001, 9(3): 113-4. |
| 6 | Shao M, Yan Y, Zhu F, et al. Artemisinin analog SM934 alleviates epithelial barrier dysfunction via inhibiting apoptosis and caspase-1-mediated pyroptosis in experimental colitis[J]. Front Pharmacol, 2022, 13: 849014. |
| 7 | Kim HM, Kim YM. HMGB1: LPS delivery vehicle for caspase-11-mediated pyroptosis[J]. Immunity, 2018, 49(4): 582-4. |
| 8 | Liu W, Chen Y, Meng J, et al. Ablation of caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo [J]. J Neuroinflammation, 2018, 15(1): 48. |
| 9 | Lauro A, D'Amico F, Gondolesi G. The current therapeutic options for Crohn's disease: from medical therapy to intestinal transplan-tation[J]. Expert Rev Gastroenterol Hepatol, 2017, 11(12): 1105-17. |
| 10 | Guo BJ, Bian ZX, Qiu HC, et al. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease[J]. Ann N Y Acad Sci, 2017, 1401(1): 37-48. |
| 11 | Kang JH, Choi S, Jang JE, et al. Wasabia Japonica is a potential functional food to prevent colitis via inhibiting the NF-κB signaling pathway[J]. Food Funct, 2017, 8(8): 2865-74. |
| 12 | Khare T, Palakurthi SS, Shah BM, et al. Natural product-based nanomedicine in treatment of inflammatory bowel disease[J]. Int J Mol Sci, 2020, 21(11): E3956. |
| 13 | Xu P, Elizalde M, Masclee A, et al. Corticosteroid enhances epithelial barrier function in intestinal organoids derived from patients with Crohn's disease[J]. J Mol Med, 2021, 99(6): 805-15. |
| 14 | Zhang X, Zuo L, Geng Z, et al. Vindoline ameliorates intestinal barrier damage in Crohn's disease mice through MAPK signaling pathway[J]. FASEB J, 2022, 36(11): e22589. |
| 15 | Kim DH, Kim DY, Kim YC, et al. Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice[J]. Life Sci, 2007, 80(21): 1944-50. |
| 16 | Liao Y, Lin X, Li J, et al. Nodakenin alleviates renal ischaemia-reperfusion injury via inhibiting reactive oxygen species-induced NLRP3 inflammasome activation[J]. Nephrology: Carlton, 2021, 26(1): 78-87. |
| 17 | Jia YF, Cui RX, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway[J]. Redox Biol, 2020, 32: 101534. |
| 18 | Karmakar M, Minns M, Greenberg EN, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis[J]. Nat Commun, 2020, 11: 2212. |
| 19 | Li S, Sun Y, Song M, et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression[J]. JCI Insight, 2021, 6(23): e146852. |
| 20 | Wang C, Yang T, Xiao J, et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation[J]. Sci Immunol, 2021, 6(64): eabj3859. |
| 21 | Lukonin I, Serra D, Challet Meylan L, et al. Phenotypic landscape of intestinal organoid regeneration[J]. Nature, 2020, 586: 275-80. |
| 22 | Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature, 2009, 459: 262-5. |
| 23 | Chen XL, Liu GL, Yuan YY, et al. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF‑κB signaling[J]. Cell Death Dis, 2019, 10: 906. |
| 24 | Rong L, Li ZD, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomed Pharmacother, 2020, 122: 109726. |
| 25 | Zuo L, Li J, Zhang X, et al. Aberrant mesenteric adipose extracellular matrix remodelling is involved in adipocyte dysfunction in Crohn's disease: the role of TLR-4-mediated macrophages[J]. J Crohns Colitis, 2022, 16(11): 1762-76. |
| 26 | Lim JY, Lee JH, Yun DH, et al. Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice[J]. Phytomedicine, 2021, 81: 153411. |
| 27 | Zuo LG, Li Y, Wang HG, et al. Cigarette smoking is associated with intestinal barrier dysfunction in the small intestine but not in the large intestine of mice[J]. J Crohns Colitis, 2014, 8(12): 1710-22. |
| 28 | Dolinger M, Torres J, Vermeire S. Crohn's disease[J]. Lancet, 2024, 403(10432): 1177-91. |
| 29 | Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor[J]. J Clin Invest, 1999, 104(6): 761-7. |
| 30 | Qu Y, Franchi L, Nunez G, et al. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages[J]. J Immunol, 2007, 179(3): 1913-25. |
| 31 | Fink SL, Cookson BT. Pillars article: caspase-1-dependent pore formation during pyroptosis leads to osmotic Lysis of infected host macrophages. Cell microbiol. 2006.8: 1812-1825[J]. J Immunol, 2019, 202(7): 1913-26. |
| 32 | Liu ZJ, Gan L, Xu YT, et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF‑κB/GSDMD signal in mice adipose tissue[J]. J Pineal Res, 2017, 63(1): e12414. |
| 33 | Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn's disease pathogenesis[J]. Ann N Y Acad Sci, 2012, 1258(1): 159-65. |
| 34 | Liu BH, Tu Y, Ni GX, et al. Total flavones of Abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-dependent m6A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling[J]. Front Pharmacol, 2021, 12: 667644. |
| 35 | Xu S, Wang J, Jiang JY, et al. TLR4 promotes microglial pyroptosis via lncRNA-F630028O10Rik by activating PI3K/AKT pathway after spinal cord injury[J]. Cell Death Dis, 2020, 11: 693. |
| 36 | Xu S, Wang J, Zhong J, et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling[J]. Clin Transl Med, 2021, 11(1): e269. |
| 37 | Zhang CY, Lin TJ, Nie GH, et al. Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells[J]. Environ Pollut, 2021, 272: 116403. |
| 38 | Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function[J]. Gastroenterology, 2007, 132(4): 1359-74. |
| 39 | Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis[J]. Gastroenterology, 2010, 139(3): 869-81. e9. |
| 40 | Liao Y, Xu JH, Qin BY, et al. Advanced oxidation protein products impair autophagic flux in macrophage by inducing lysosomal dysfunction via activation of PI3K-Akt-mTOR pathway in Crohn's disease[J]. Free Radic Biol Med, 2021, 172: 33-47. |
| 41 | Lee NY, Chung KS, Jin JS, et al. The inhibitory effect of nodakenin on mast-cell-mediated allergic inflammation via downregulation of NF-κB and caspase-1 activation[J]. J Cell Biochem, 2017, 118(11): 3993-4001. |
| 42 | Rim HK, Cho W, Sung SH, et al. Nodakenin suppresses lipopo-lysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock[J]. J Pharmacol Exp Ther, 2012, 342(3): 654-64. |
| 43 | Gao QT, Jeon SJ, Jung HA, et al. Nodakenin enhances cognitive function and adult hippocampal neurogenesis in mice[J]. Neurochem Res, 2015, 40(7): 1438-47. |
| [1] | 周海忆, 何斯怡, 韩瑞芳, 关永格, 董丽娟, 宋阳. 艾灸通过调控miR-223-3p/NLRP3焦亡通路修复薄型子宫内膜[J]. 南方医科大学学报, 2025, 45(7): 1380-1388. |
| [2] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [3] | 卞芬兰, 倪诗垚, 赵鹏, 戚毛男星, 唐碧, 王洪巨, 康品方, 刘进军. 积雪草苷通过抑制NLRP3炎症体介导的细胞焦亡减轻大鼠心肌缺血再灌注损伤[J]. 南方医科大学学报, 2025, 45(5): 977-985. |
| [4] | 孙亚磊, 罗萌, 郭长胜, 高静, 苏凯奇, 陈立典, 冯晓东. 穗花杉双黄酮通过抑制细胞焦亡减轻小鼠急性肺损伤[J]. 南方医科大学学报, 2025, 45(4): 692-701. |
| [5] | 朱正望, 王琳琳, 赵静涵, 马瑞雪, 余雨春, 蔡庆春, 王兵, 朱平生, 苗明三. 退黄合剂通过调控法尼醇X受体抑制NLRP3炎症小体改善α-萘异硫氰酸酯诱导的大鼠胆汁淤积[J]. 南方医科大学学报, 2025, 45(4): 718-724. |
| [6] | 陈悦, 肖林雨, 任侣, 宋雪, 李静, 胡建国. 水晶兰苷通过抑制PI3K/AKT信号通路减少神经元凋亡改善脊髓损伤后小鼠的运动功能[J]. 南方医科大学学报, 2025, 45(4): 774-784. |
| [7] | 储菲, 陈孝华, 宋博文, 杨晶晶, 左芦根. 苏荠宁黄酮通过抑制PI3K/AKT信号通路拮抗肠上皮细胞凋亡改善小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(4): 819-828. |
| [8] | 董妍妍, 张可敬, 储俊, 储全根. 抵当汤含药血清通过PI3K/Akt/mTOR信号通路增强高糖诱导的大鼠肾小球内皮细胞自噬[J]. 南方医科大学学报, 2025, 45(3): 461-469. |
| [9] | 殷丽霞, 牛民主, 张可妮, 耿志军, 胡建国, 李江艳, 李静. 升麻素抑制MAPK通路调节辅助性T细胞免疫平衡改善小鼠克罗恩病样结肠炎[J]. 南方医科大学学报, 2025, 45(3): 595-602. |
| [10] | 徐皓男, 张放, 黄钰莹, 姚其盛, 管悦琴, 陈浩. 百蕊草通过调节肠道菌群和调控EGFR/PI3K/Akt信号通路改善小鼠抗生素相关性腹泻[J]. 南方医科大学学报, 2025, 45(2): 285-295. |
| [11] | 裴月娇, 刘慧敏, 昕宇, 刘波. miR-124通过调控PI3K/AKT信号通路改善睡眠剥夺大鼠认知功能[J]. 南方医科大学学报, 2025, 45(2): 340-346. |
| [12] | 展俊平, 黄硕, 孟庆良, 范围, 谷慧敏, 崔家康, 王慧莲. 缺氧微环境下补阳还五汤通过抑制BNIP3-PI3K/Akt通路抑制类风湿关节炎滑膜成纤维细胞的线粒体自噬[J]. 南方医科大学学报, 2025, 45(1): 35-42. |
| [13] | 张玉如, 万磊, 方昊翔, 李方泽, 王丽文, 李柯霏, 闫佩文, 姜辉. miR-155-5p介导PIK3R1负调控PI3K/AKT信号通路促进原发性干燥综合征人唾液腺上皮细胞增殖[J]. 南方医科大学学报, 2025, 45(1): 65-71. |
| [14] | 左涵珺, 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟. 天麻素经PI3K/AKT通路改善新生大鼠缺氧缺血性脑损伤后小胶质细胞介导的炎症反应[J]. 南方医科大学学报, 2024, 44(9): 1712-1719. |
| [15] | 周雪利, 李华, 陈青宇, 靳美娜, 李海波, 白炜, 贾楚璇, 魏翠英. 慢性间歇低氧和复氧对大鼠胰岛素抵抗及骨骼肌miR-27a-3p/PPARγ/IRS1/PI3K/AKT表达的影响[J]. 南方医科大学学报, 2024, 44(9): 1729-1737. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||