南方医科大学学报 ›› 2026, Vol. 46 ›› Issue (1): 150-158.doi: 10.12122/j.issn.1673-4254.2026.01.16
徐嘉艺( ), 杨迪(
), 杨迪( ), 臧开来, 褚孟恩, 赵庆瑶, 李晴, 鲁森, 陈修丽, 李宁(
), 臧开来, 褚孟恩, 赵庆瑶, 李晴, 鲁森, 陈修丽, 李宁( )
)
收稿日期:2025-05-22
出版日期:2026-01-20
发布日期:2026-01-16
通讯作者:
李宁
E-mail:2624309950@qq.com;13792651232@163.com;lining@qdu.edu.cn
作者简介:徐嘉艺,在读本科生,E-mail: 2624309950@qq.com;基金资助:
Jiayi XU( ), Di YANG(
), Di YANG( ), Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI(
), Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI( )
)
Received:2025-05-22
Online:2026-01-20
Published:2026-01-16
Contact:
Ning LI
E-mail:2624309950@qq.com;13792651232@163.com;lining@qdu.edu.cn
Supported by:摘要:
目的 研究跨膜蛋白EVA1A在肝脏脂质代谢及非酒精性脂肪肝病发展过程中的作用及机制。 方法 将8周龄雄性ob/ob小鼠随机分为对照组和实验组,8只/组,实验周期为7周。对照组通过尾静脉注射AAV空载体(AAV-null组),实验组注射重组载体AAV-Eva1a(AAV-Eva1a组)。在人HepG2细胞中使用慢病毒载体LV-EVA1A过表达EVA1A后,使用油酸诱导构建NAFLD细胞模型,设为实验组LV-EVA1A组,对照组为LV-Vector组。采用RT-qPCR、Western blotting、免疫荧光实验测定EVA1A、脂质代谢相关基因以及自噬相关基因的表达情况;采用HE染色、油红O染色以及血脂四项试剂盒检测肝脏、血液和细胞中的脂质积累情况;采用丙氨酸氨基转移酶(ALT)试剂盒、门冬氨酸氨基转移酶(AST)试剂盒、白细胞介素-6(IL-6)试剂盒、白细胞介素-1β(IL-1β)试剂盒以及肿瘤坏死因子-α(TNF-α)试剂盒测定炎症指标;采用透射电镜观察肝脏脂滴自噬情况。 结果 与对照组相比,实验组小鼠肝脏和实验组细胞中EVA1A 的mRNA水平和蛋白水平升高(P<0.05),小鼠肝重和肝脏系数降低(P<0.01),脂质沉积减少,细胞内甘油三酯(TG)含量降低(P<0.01);小鼠血清中的总胆固醇、低密度脂蛋白-胆固醇、高密度脂蛋白-胆固醇和肝脏中的TG水平均降低(P<0.05),血清ALT、AST的水平和炎症相关细胞因子IL-6、TNF-α的水平均降低(P<0.05);实验组小鼠肝脏和实验组细胞中的乙酰辅酶A羧化酶、脂肪酸转运酶、二酰基甘油酰基转移酶的表达均降低(P<0.05),而甘油三酯脂肪酶的表达升高(P<0.05);实验组小鼠肝脏中的脂滴自噬增强,实验组细胞中的自噬体数量增多,实验组小鼠肝脏以及细胞中p62的表达均降低(P<0.05),LC3-Ⅱ和ATG5的表达均升高(P<0.05)。 结论 EVA1A能够改善ob/ob小鼠脂肪肝和炎症,通过抑制脂质摄取和合成相关基因的表达,促进脂质分解相关基因的表达和促进脂滴自噬来清除肝脏积累的脂质。
徐嘉艺, 杨迪, 臧开来, 褚孟恩, 赵庆瑶, 李晴, 鲁森, 陈修丽, 李宁. EVA1A过表达通过调节脂质代谢和促进脂滴自噬改善非酒精性脂肪肝[J]. 南方医科大学学报, 2026, 46(1): 150-158.
Jiayi XU, Di YANG, Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI. EVA1A overexpression improves non-alcoholic fatty liver disease in mice by regulating lipid metabolism and promoting lipophagy[J]. Journal of Southern Medical University, 2026, 46(1): 150-158.
| Gene | Forward primer (5'-3') Reverse primer (5'-3') |
|---|---|
Mus Eva1a Homo EVA1A | CCTTGGCCGCCTTGGTGATGAG TACCATCCTCGCTGTCGCTGCT AGATGGCTTTGCTCAGCAACA GATGCACACGCCAGAAACAA |
| Mus ACC1 | TCGGATCGGTTCCTTTGGGCCT TGTTCGCTGCCACGTAGATGCG |
| Mus CD36 | TGACGTGGCAAAGAACAGCAGCA AGACACAGTGTGGTCCTCGGGG |
| Mus DGAT2 | TCCCAGCAGCTGTGGCCTTACT GCACCACAGGTTGACATCCCGG |
Mus ATGL Mus β-actin Homo β-actin | TCCAAGGGGTGCGCTATGTGGA GTGGAGCTGTCCTGAGGGCAGA CCCGGGCTGTATTCCCCTCCAT CCTCTCTTGCTCTGGGCCTCGT AGGATTCCTATGTGGGCGAC ATAGCACAGCCTGGATAGCAA |
表1 用于RT-qPCR的引物序列
Tab.1 Primers sequences for RT-qPCR
| Gene | Forward primer (5'-3') Reverse primer (5'-3') |
|---|---|
Mus Eva1a Homo EVA1A | CCTTGGCCGCCTTGGTGATGAG TACCATCCTCGCTGTCGCTGCT AGATGGCTTTGCTCAGCAACA GATGCACACGCCAGAAACAA |
| Mus ACC1 | TCGGATCGGTTCCTTTGGGCCT TGTTCGCTGCCACGTAGATGCG |
| Mus CD36 | TGACGTGGCAAAGAACAGCAGCA AGACACAGTGTGGTCCTCGGGG |
| Mus DGAT2 | TCCCAGCAGCTGTGGCCTTACT GCACCACAGGTTGACATCCCGG |
Mus ATGL Mus β-actin Homo β-actin | TCCAAGGGGTGCGCTATGTGGA GTGGAGCTGTCCTGAGGGCAGA CCCGGGCTGTATTCCCCTCCAT CCTCTCTTGCTCTGGGCCTCGT AGGATTCCTATGTGGGCGAC ATAGCACAGCCTGGATAGCAA |
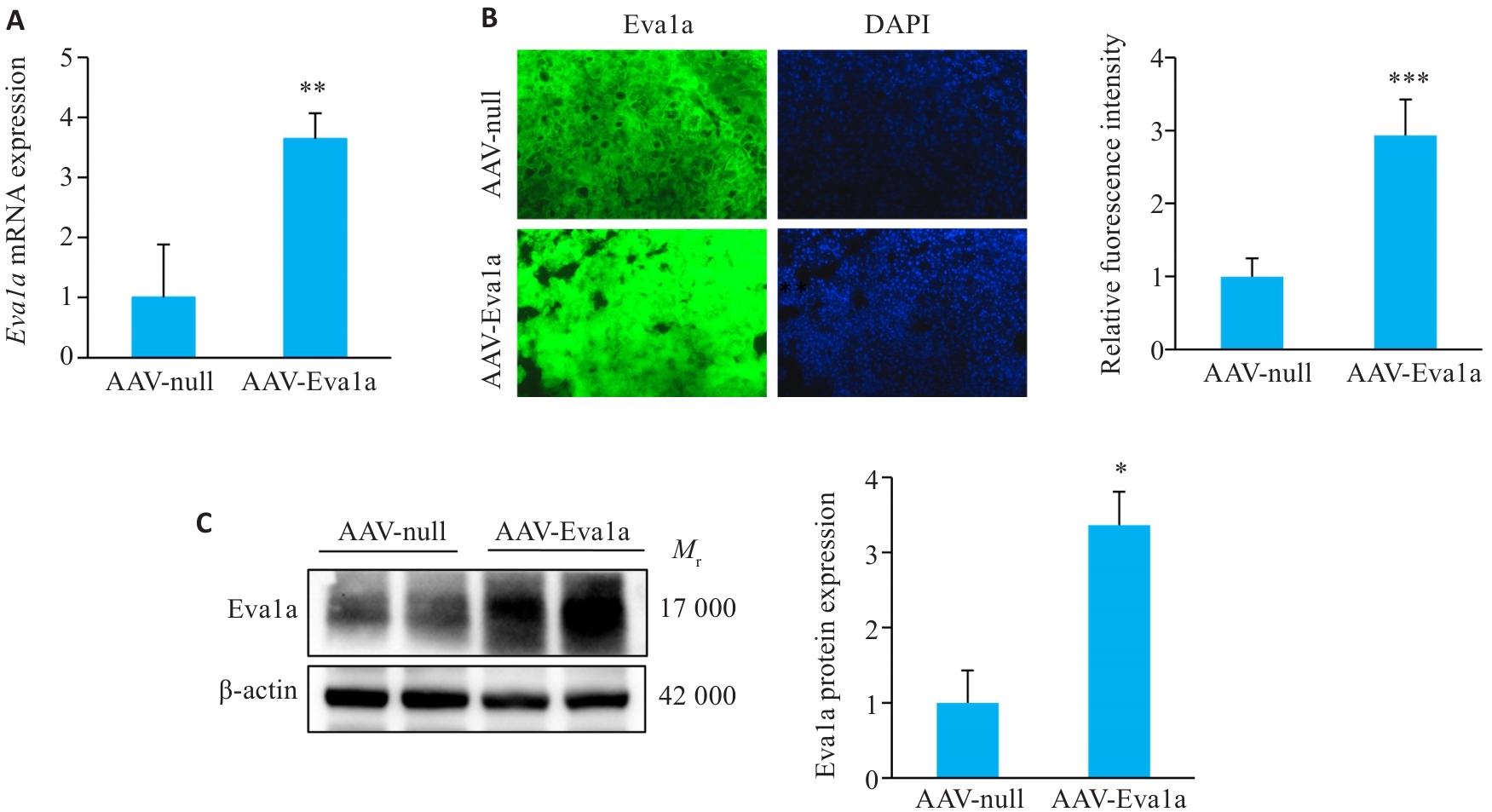
图1 Eva1a在ob/ob小鼠肝脏中的过表达
Fig.1 Overexpression of Eva1a in ob/ob mouse liver. A: Relative hepatic Eva1a mRNA levels. B: Immunofluorescence staining for detecting Eva1a expression in mouse liver tissues (Original magnification: ×200). C: Hepatic Eva1a protein expression levels detected with Western blotting. AAV-Eva1a: Mice overexpressing Eva1a; AAV-null: Control mice. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group.
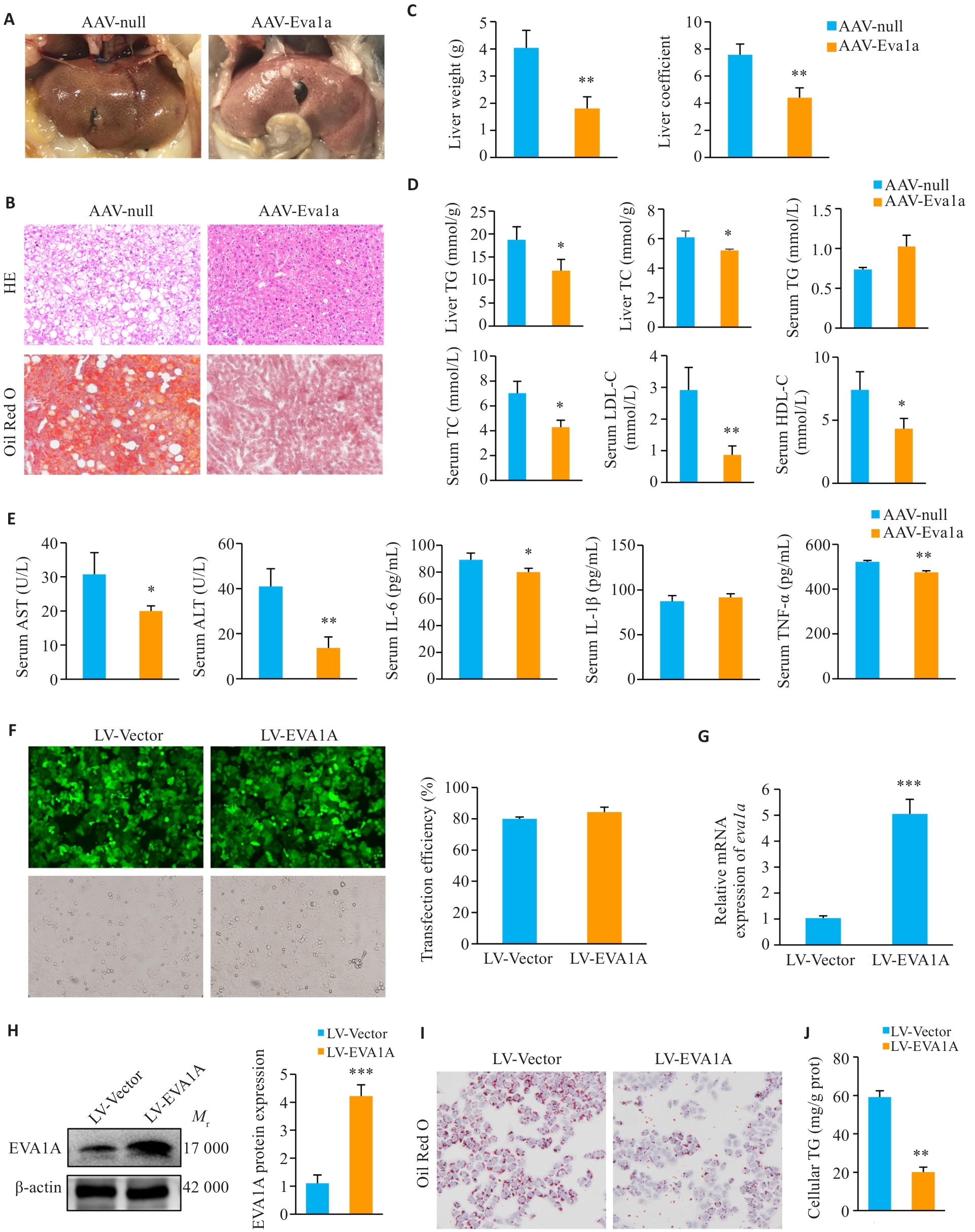
图2 EVA1A过表达对ob/ob小鼠脂肪肝、炎症及OA诱导的NAFLD细胞模型脂质沉积的影响
Fig.2 Effect of EVA1A overexpression on steatosis and inflammation in ob/ob mice and on lipid deposition in OA-induced HepG2 cells. A: Observation of mouse livers. B: Liver tissue HE staining and Oil Red O (ORO) staining (×200). C: Liver weight and liver coefficients of the mice. D: Triglyceride (TG) and total cholesterol (TC) levels in mouse livers, and TG, TC, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels in mouse serum. E: Serum levels of AST, ALT, IL-6, IL-1β, and TNF‑α of the mice. F: Infection efficiency of LV-EVA1A or LV-Vector in HepG2 cells determined by fluorescence microscopy (×200). G: RT-qPCR analysis of Eva1a mRNA levels in HepG2 cells infected with LV-EVA1A or LV-Vector. H: Western blotting of EVA1A protein levels in HepG2 cells infected with LV-EVA1A or LV-Vector. I: ORO staining for lipid droplets in the cells in LV-Vector and LV-EVA1A groups treated with 400 μmol/L OA for 12 h (×400). J: Quantitative analysis of cellular TG contents. AAV-Eva1a: Mice overexpressing Eva1a; AAV-null: Control mice. LV-EVA1A: HepG2 cells overexpressing EVA1A; LV-Vector: Control cells. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
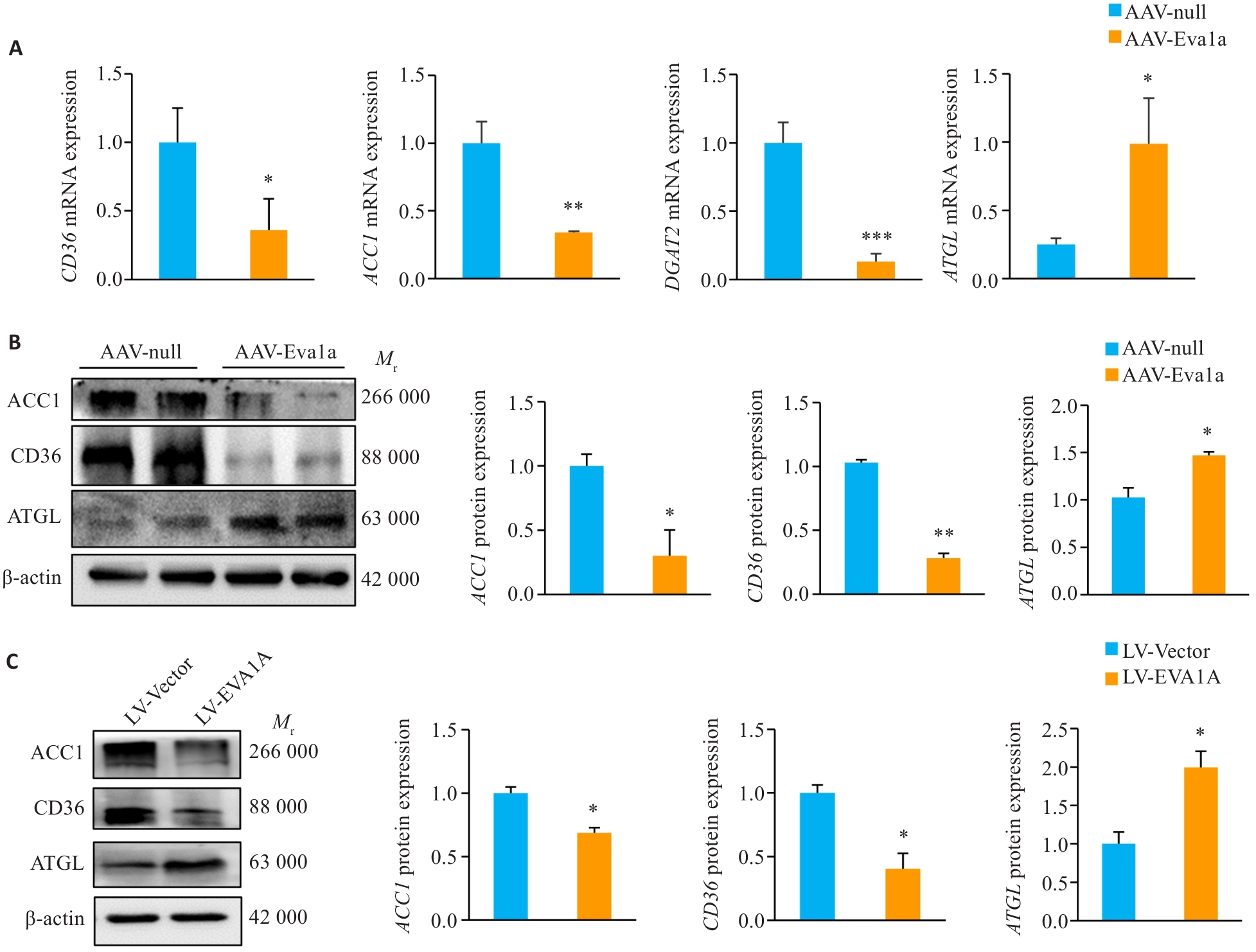
图3 EVA1A过表达对ob/ob小鼠肝脏和OA诱导的NAFLD细胞模型中脂质代谢相关基因表达的影响
Fig.3 Effect of EVA1A overexpression on expression of lipid metabolism-related genes in the liver of ob/ob mice and in HepG2 cells induced by OA. A: Relative hepatic mRNA levels of CD36, ACC1, DGAT2 and ATGL. B: Protein expression levels of ACC1, CD36, and ATGL in the liver of ob/ob mice. C: Protein expression levels of ACC1, CD36, and ATGL in HepG2 cells treated with OA. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
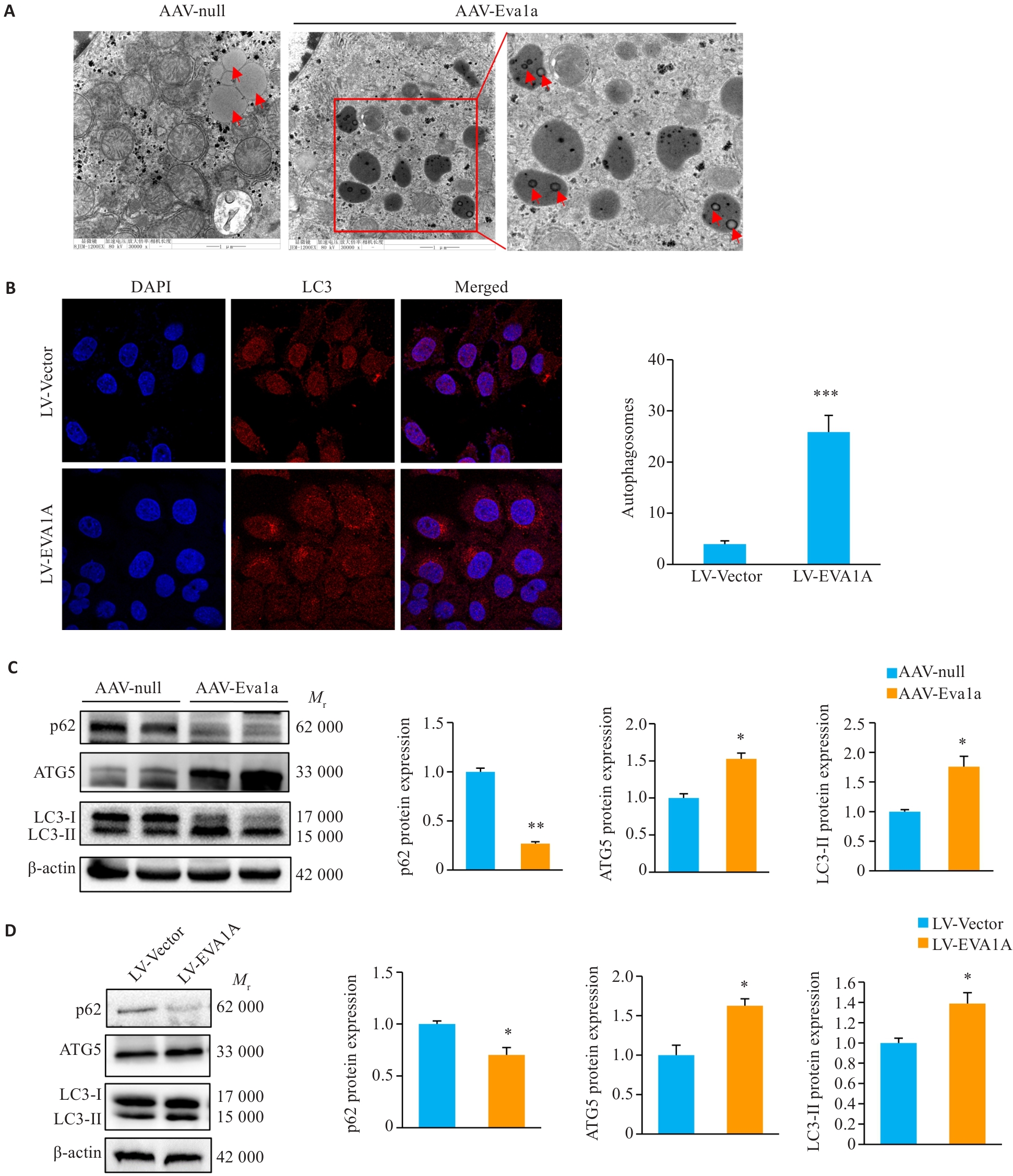
图4 EVA1A过表达对小鼠肝脏及OA诱导的NAFLD细胞模型中脂滴自噬的影响
Fig.4 Effect of Eva1a overexpression on lipophagy in the liver of ob/ob mice and in OA-induced HepG2 cells. A: Lipophagy in mouse liver observed with transmission electron microscopy (×30 000). B: Immunofluorescence staining of LC3-labeled autophagosomes in HepG2 cells treated with OA (×1260). C: Protein expression levels of autophagy-related genes p62, LC3, and ATG5 in ob/ob mouse liver. D: Protein expression levels of autophagy-related genes p62, LC3, and ATG5 in HepG2 cells treated with OA. The red arrows indicate lipid droplets. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
| [1] | Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22(1): 63. doi:10.1186/s12902-022-00980-1 |
| [2] | Shi MR, Zhang H, Wang W, et al. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease[J]. J Diabetes Complicat, 2023, 37(10): 108610. doi:10.1016/j.jdiacomp.2023.108610 |
| [3] | Xu S, Wu X, Wang S, et al. TRIM56 protects against nonalcoholic fatty liver disease by promoting the degradation of fatty acid synthase[J]. J Clin Invest, 2024, 134(5): e166149. doi:10.1172/jci166149 |
| [4] | Wang L, Yu CF, Lu Y, et al. TMEM166, a novel transmembrane protein, regulates cell autophagy and apoptosis[J]. Apoptosis, 2007, 12(8): 1489-502. doi:10.1007/s10495-007-0073-9 |
| [5] | Hu J, Li G, Qu L, et al. TMEM166/EVA1A interacts with ATG16L1 and induces autophagosome formation and cell death[J]. Cell Death Dis, 2016, 7(8): e2323. doi:10.1038/cddis.2016.230 |
| [6] | De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights[J]. Curr Pharm Des, 2013, 19(29): 5239-49. doi:10.2174/13816128130303 |
| [7] | Lu GD, Ang YH, Zhou J, et al. CCAAT/enhancer binding protein α predicts poorer prognosis and prevents energy starvation-induced cell death in hepatocellular carcinoma[J]. Hepatology, 2015, 61(3): 965-78. doi:10.1002/hep.27593 |
| [8] | Xu Q, Liao Z, Gong Z, et al. Down-regulation of EVA1A by miR-103a-3p promotes hepatocellular carcinoma cells proliferation and migration[J]. Cell Mol Biol Lett, 2022, 27(1): 93. doi:10.1186/s11658-022-00388-8 |
| [9] | Yang JJ, Wang B, Xu Q, et al. TMEM166 inhibits cell proliferation, migration and invasion in hepatocellular carcinoma via upregulating TP53[J]. Mol Cell Biochem, 2021, 476(2): 1151-63. doi:10.1007/s11010-020-03979-1 |
| [10] | Zhen Y, Yuan Z, Zhang J, et al. Flubendazole induces mitochondrial dysfunction and DRP1-mediated mitophagy by targeting EVA1A in breast cancer[J]. Cell Death Dis, 2022, 13(4): 375. doi:10.1038/s41419-022-04823-8 |
| [11] | Lin X, Cui M, Xu D, et al. Liver-specific deletion of Eva1a/Tmem166 aggravates acute liver injury by impairing autophagy[J]. Cell Death Dis, 2018, 9(7): 768. doi:10.1038/s41419-018-0800-x |
| [12] | Li MT, Lu G, Hu J, et al. EVA1A/TMEM166 regulates embryonic neurogenesis by autophagy[J]. Stem Cell Rep, 2016, 6(3): 396-410. doi:10.1016/j.stemcr.2016.01.011 |
| [13] | Liu B, Liu B, Zhou Y, et al. EVA1A regulates hematopoietic stem cell regeneration via ER-mitochondria mediated apoptosis[J]. Cell Death Dis, 2023, 14(1): 71. doi:10.1038/s41419-023-05559-9 |
| [14] | Canham L, Sendac S, Diagbouga MR, et al. EVA1A (Eva-1 homolog A) promotes endothelial apoptosis and inflammatory activation under disturbed flow via regulation of autophagy[J]. Arterioscler Thromb Vasc Biol, 2023, 43(4): 547-61. doi:10.1161/atvbaha.122.318110 |
| [15] | Li J, Chen Y, Gao J, et al. Eva1a ameliorates atherosclerosis by promoting re-endothelialization of injured arteries via Rac1/Cdc42/Arpc1b[J]. Cardiovasc Res, 2021, 117(2): 450-61. doi:10.1093/cvr/cvaa011 |
| [16] | Liu XK, Gao X, Yang YL, et al. EVA1A reverses lenvatinib resistance in hepatocellular carcinoma through regulating PI3K/AKT/p53 signaling axis[J]. Apoptosis, 2024, 29(7): 1161-84. doi:10.1007/s10495-024-01967-0 |
| [17] | Li YX, Huang XG, Yang G, et al. CD36 favours fat sensing and transport to govern lipid metabolism[J]. Prog Lipid Res, 2022, 88: 101193. doi:10.1016/j.plipres.2022.101193 |
| [18] | Yang YX, Liu XK, Yang D, et al. Interplay of CD36, autophagy, and lipid metabolism: insights into cancer progression[J]. Metabolism, 2024, 155: 155905. doi:10.1016/j.metabol.2024.155905 |
| [19] | Zeng H, Qin H, Liao M, et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing[J]. Mol Metab, 2022, 57: 101428. doi:10.1016/j.molmet.2021.101428 |
| [20] | Zeng S, Wu F, Chen M, et al. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid β-oxidation by increasing its localization to mitochondria and interaction with long-chain acyl-CoA synthetase 1[J]. Antioxid Redox Signal, 2022, 36(16/17/18): 1081-100. doi:10.1089/ars.2021.0157 |
| [21] | Zhu HL, Zhao TM, Zhao S, et al. O-GlcNAcylation promotes the progression of nonalcoholic fatty liver disease by upregulating the expression and function of CD36[J]. Metabolism, 2024, 156: 155914. doi:10.1016/j.metabol.2024.155914 |
| [22] | Wilson CG, Tran JL, Erion DM, et al. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice[J]. Endocrinology, 2016, 157(2): 570-85. doi:10.1210/en.2015-1866 |
| [23] | Brownsey RW, Zhande R, Boone AN. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions[J]. Biochem Soc Trans, 1997, 25(4): 1232-8. doi:10.1042/bst0251232 |
| [24] | Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, et al. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2[J]. Science, 2001, 291(5513): 2613-6. doi:10.1126/science.1056843 |
| [25] | Akheruzzaman M, Hegde V, Shin AC, et al. Reducing endogenous insulin is linked with protection against hepatic steatosis in mice[J]. Nutr Diabetes, 2020, 10(1): 11. doi:10.1038/s41387-020-0114-9 |
| [26] | Gluchowski NL, Gabriel KR, Chitraju C, et al. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice[J]. Hepatology, 2019, 70(6): 1972-85. doi:10.1002/hep.30765 |
| [27] | Bai R, Rebelo A, Kleeff J, et al. Identification of prognostic lipid droplet-associated genes in pancreatic cancer patients via bioinformatics analysis[J]. Lipids Health Dis, 2021, 20(1): 58. doi:10.1186/s12944-021-01476-y |
| [28] | Fang QH, Shen QL, Li JJ, et al. Inhibition of microRNA-124a attenuates non-alcoholic fatty liver disease through upregulation of adipose triglyceride lipase and the effect of liraglutide intervention[J]. Hepatol Res, 2019, 49(7): 743-57. doi:10.1111/hepr.13330 |
| [29] | Reid BN, Ables GP, Otlivanchik OA, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis[J]. J Biol Chem, 2008, 283(19): 13087-99. doi:10.1074/jbc.m800533200 |
| [30] | Saadati S, Sadeghi A, Mansour A, et al. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial[J]. BMC Gastroenterol, 2019, 19(1): 133. doi:10.1186/s12876-019-1055-4 |
| [31] | Yu HY, Yang F, Zhong WT, et al. Secretory Galectin-3 promotes hepatic steatosis via regulation of the PPARγ/CD36 signaling pathway[J]. Cell Signal, 2021, 84: 110043. doi:10.1016/j.cellsig.2021.110043 |
| [32] | Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice[J]. J Hepatol, 2013, 58(5): 993-9. doi:10.1016/j.jhep.2013.01.011 |
| [33] | Blanchard PG, Festuccia WT, Houde VP, et al. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion [S[J]. J Lipid Res, 2012, 53(6): 1117-25. doi:10.1194/jlr.m021485 |
| [34] | Owen JL, Zhang Y, Bae SH, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase[J]. Proc Natl Acad Sci USA, 2012, 109(40): 16184-9. doi:10.1073/pnas.1213343109 |
| [35] | Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway[J]. Cell, 2011, 146(3): 408-20. doi:10.1016/j.cell.2011.06.034 |
| [36] | Chakrabarti P, English T, Shi J, et al. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage[J]. Diabetes, 2010, 59(4): 775-81. doi:10.2337/db09-1602 |
| [37] | Chakrabarti P, Kim JY, Singh M, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway[J]. Mol Cell Biol, 2013, 33(18): 3659-66. doi:10.1128/mcb.01584-12 |
| [38] | Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nat Cell Biol, 2011, 13(2): 132-41. doi:10.1038/ncb2152 |
| [1] | 乔通, 尹林, 张可妮, 牛民主, 黄菊, 耿志军, 李静, 胡建国. 茯苓新酸A通过调节AMPK/mTOR介导的自噬来减轻葡聚糖硫酸钠诱导的小鼠结肠炎[J]. 南方医科大学学报, 2026, 46(1): 131-140. |
| [2] | 张淑芬, 黄添容, 杨灿洪, 陈家镒, 吕田明, 张嘉发. 莱菔硫烷通过抑制Aβ42寡聚体激活的U87细胞中MAPK/NF-κB信号通路降低反应性星形胶质细胞介导的SH-SY5Y凋亡[J]. 南方医科大学学报, 2026, 46(1): 191-199. |
| [3] | 沙桐, 王文研, 宣佳斌, 吴洁, 石能贤, 何劲, 胡鸿彬, 张耀元. 基于Th1/Th2细胞因子检测的脓毒症免疫状态分型及预后分析:一项回顾性研究[J]. 南方医科大学学报, 2026, 46(1): 6-22. |
| [4] | 杨勤军, 朱虹宇, 高远, 杨程, 刘桐, 张璐, 童佳兵, 李泽庚. 桑麻止咳方改善感染后咳嗽大鼠气道炎症和敏感性:基于TRPV1-SP/CGRP和细胞焦亡途径[J]. 南方医科大学学报, 2025, 45(9): 1830-1839. |
| [5] | 骆碧云, 易欣, 蔡怡静, 张世卿, 王鹏, 李彤, 翁建霖, 周平正. 清心牛黄丸通过改善脂质代谢紊乱缓解小鼠非酒精性脂肪性肝病[J]. 南方医科大学学报, 2025, 45(9): 1840-1849. |
| [6] | 闫爱丽, 罗梦瑶, 常晋瑞, 李新华, 朱娟霞. 橙皮素通过调控AMPK/NLRP3通路减轻阿霉素诱导的小鼠心肌毒性[J]. 南方医科大学学报, 2025, 45(9): 1850-1858. |
| [7] | 罗善玉, 朱强, 闫玉翡, 纪宗红, 邹华杰, 张瑞霞, 巴应贵. 低氧环境下NLRP3信号通路促进非酒精性脂肪性肝炎小鼠的肝细胞焦亡[J]. 南方医科大学学报, 2025, 45(9): 2026-2033. |
| [8] | 刘辰菲, 张玮, 曾尧, 梁艳, 王梦婷, 张明芳, 李新元, 王凤超, 杨燕青. 2,6-二甲氧基-1,4-苯醌通过抑制NLRP3炎症小体活化改善葡聚糖硫酸钠诱导的小鼠溃疡性结肠炎[J]. 南方医科大学学报, 2025, 45(8): 1654-1662. |
| [9] | 尚菲菲, 师晓可, 曾尧, 陶循浅, 李天真, 梁艳, 杨燕青, 宋传旺. Avitinib抑制NLRP3炎症小体活化并改善小鼠感染性休克[J]. 南方医科大学学报, 2025, 45(8): 1697-1705. |
| [10] | 范正媛, 沈子涵, 李亚, 沈婷婷, 李高峰, 李素云. 补肺益肾方对香烟烟雾提取物诱导的人支气管上皮细胞损伤的保护作用及其机制[J]. 南方医科大学学报, 2025, 45(7): 1372-1379. |
| [11] | 王立明, 陈宏睿, 杜燕, 赵鹏, 王玉洁, 田燕歌, 刘新光, 李建生. 益气滋肾方通过抑制PI3K/Akt/NF-κB通路改善小鼠慢性阻塞性肺疾病的炎症反应[J]. 南方医科大学学报, 2025, 45(7): 1409-1422. |
| [12] | 夏冰, 彭进, 丁九阳, 王杰, 唐国伟, 刘国杰, 王沄, 万昌武, 乐翠云. ATF3通过NF-κB信号通路调控动脉粥样硬化斑块内的炎症反应[J]. 南方医科大学学报, 2025, 45(6): 1131-1142. |
| [13] | 王心恒, 邵小涵, 李童童, 张璐, 杨勤军, 叶卫东, 童佳兵, 李泽庚, 方向明. 平喘宁方通过调控HMGB1/Beclin-1轴介导的自噬改善患寒哮证大鼠的气道炎症[J]. 南方医科大学学报, 2025, 45(6): 1153-1162. |
| [14] | 牛民主, 殷丽霞, 乔通, 尹林, 张可妮, 胡建国, 宋传旺, 耿志军, 李静. 旱莲苷A通过调控JAK2/STAT3通路抑制M1型巨噬细胞极化改善葡聚糖硫酸钠诱导的小鼠结肠炎[J]. 南方医科大学学报, 2025, 45(6): 1297-1306. |
| [15] | 李丹丹, 楚佳鑫, 闫妍, 徐文隽, 朱行春, 孙韵, 丁浩峰, 任丽, 朱博. 姜黄素通过下调HIF-1α通路抑制非小细胞肺癌脂质代谢[J]. 南方医科大学学报, 2025, 45(5): 1039-1046. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||