南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (2): 379-386.doi: 10.12122/j.issn.1673-4254.2025.02.19
• • 上一篇
唐天威1( ), 李路安2, 陈源汉2, 张丽2, 徐丽霞2, 李志莲2, 冯仲林2, 张辉林3, 华瑞芳3, 叶智明2, 梁馨苓2, 李锐钊1(
), 李路安2, 陈源汉2, 张丽2, 徐丽霞2, 李志莲2, 冯仲林2, 张辉林3, 华瑞芳3, 叶智明2, 梁馨苓2, 李锐钊1( )
)
收稿日期:2024-08-17
出版日期:2025-02-20
发布日期:2025-03-03
通讯作者:
李锐钊
E-mail:tw122666@163.com;liruizhao1979@126.com
作者简介:唐天威,在读硕士研究生,E-mail: tw122666@163.com
基金资助:
Tianwei TANG1( ), Luan LI2, Yuanhan CHEN2, Li ZHANG2, Lixia XU2, Zhilian LI2, Zhonglin FENG2, Huilin ZHANG3, Ruifang HUA3, Zhiming YE2, Xinling LIANG2, Ruizhao LI1(
), Luan LI2, Yuanhan CHEN2, Li ZHANG2, Lixia XU2, Zhilian LI2, Zhonglin FENG2, Huilin ZHANG3, Ruifang HUA3, Zhiming YE2, Xinling LIANG2, Ruizhao LI1( )
)
Received:2024-08-17
Online:2025-02-20
Published:2025-03-03
Contact:
Ruizhao LI
E-mail:tw122666@163.com;liruizhao1979@126.com
摘要:
目的 探讨血清胱抑素C(CysC)水平评估IgA肾病(IgAN)患者肾脏预后的价值。 方法 回顾性收集2014年1月~2018年12月在广东省人民医院通过肾穿刺活检诊断为IgAN患者的临床资料。根据基线血清CysC值将患者分为高血清CysC组(CysC>1.03 mg/L)和正常血清CysC组(CysC≤1.03 mg/L)。估算肾小球滤过率(eGFR)下降≥50%,和/或进入终末期肾病(ESRD)作为肾脏不良预后的随访复合终点事件。采用lasso回归和多因素Cox回归筛选独立危险因素,并基于这些独立危险因素构建多因素Cox回归预测模型。采用Kaplan⁃Meier生存分析比较两组之间的肾脏生存率差异。平滑曲线拟合及阈值效应探究血清CysC水平与结局之间的关系。通过Bootstrap法内部验证预测模型并使用一致性指数、校正曲线、受试者工作特征(ROC)曲线及其曲线下面积(AUC)对模型预测效能进行评价,并通过列线图可视化。 结果 本研究共纳入356例IgAN患者,平均随访时间为(4.65±0.93)年,74例发生肾脏不良预后的复合终点事件。高血清CysC被筛选为IgAN肾脏不良预后的独立危险因素(HR=2.142,95% CI:1.222~3.755),且血清CysC水平高的患者肾脏生存率较低(Log-rank检验χ2=47.970,P<0.001)。阈值效应分析显示,当患者血清CysC≤2.12 mg/L时,血清CysC水平越高,肾脏不良预后风险越大(β=3.487,95% CI:2.561~4.413,P<0.001);当患者的血清CysC>2.12 mg/L时,肾脏不良预后的发生风险仍有上升但差异无统计学意义(β=0.676,95% CI:-0.642~1.995,P=0.315)。基于血清 CysC及其他3个独立危险因素构建的多因素Cox回归预测模型经内部验证表现良好,其一致性指数为0.873(95% CI:0.839~0.907),AUC为0.909(95% CI:0.873~0.945)。 结论 血清CysC水平与IgAN患者肾脏预后相关,高血清CysC是IgA肾病不良预后的独立危险因素。
唐天威, 李路安, 陈源汉, 张丽, 徐丽霞, 李志莲, 冯仲林, 张辉林, 华瑞芳, 叶智明, 梁馨苓, 李锐钊. 高血清胱抑素C水平是IgA肾病不良预后的独立危险因素[J]. 南方医科大学学报, 2025, 45(2): 379-386.
Tianwei TANG, Luan LI, Yuanhan CHEN, Li ZHANG, Lixia XU, Zhilian LI, Zhonglin FENG, Huilin ZHANG, Ruifang HUA, Zhiming YE, Xinling LIANG, Ruizhao LI. High serum cystatin C is an independent risk factor for poor renal prognosis in IgA nephropathy[J]. Journal of Southern Medical University, 2025, 45(2): 379-386.
| Pathological parameters and score | Content |
|---|---|
| Mesangial hypercellularity (M) | |
| M0 | Mesangial score ≤0.5 |
| M1 | Mesangial score >0.5 |
| Endocapillary hypercellularity (E) | |
| E0 | Absent |
| E1 | Present |
| Segmental glomerulosclerosis (S) | |
| S0 | Absent |
| S1 | Present |
| Tubular atrophy/interstitial fibrosis (T) | |
| T0 | 0-25% |
| T1 | 26%-50% |
| T2 | >50% |
| Cellular/fibrocellular crescents (C) | |
| C0 | Absent |
| C1 | Crescents in a least 1 glomerulus and <25% of glomeruli |
| C2 | Crescents in ≥25% of glomeruli |
表1 IgAN牛津分型(MEST-C评分)
Tab.1 Oxford Classification of IgA nephropathy (MEST-C score)
| Pathological parameters and score | Content |
|---|---|
| Mesangial hypercellularity (M) | |
| M0 | Mesangial score ≤0.5 |
| M1 | Mesangial score >0.5 |
| Endocapillary hypercellularity (E) | |
| E0 | Absent |
| E1 | Present |
| Segmental glomerulosclerosis (S) | |
| S0 | Absent |
| S1 | Present |
| Tubular atrophy/interstitial fibrosis (T) | |
| T0 | 0-25% |
| T1 | 26%-50% |
| T2 | >50% |
| Cellular/fibrocellular crescents (C) | |
| C0 | Absent |
| C1 | Crescents in a least 1 glomerulus and <25% of glomeruli |
| C2 | Crescents in ≥25% of glomeruli |
| Variables | Overall (n=356) | Normal serum CysC group (n=130) | High serum CysC group (n=226) | t/Z/χ2 | P |
|---|---|---|---|---|---|
| Age (year) | 36 (29, 45) | 32 (26, 39) | 39 (32, 47) | -5.245 | <0.001 |
| Male [n (%)] | 149 (41.9) | 33(25.4) | 116 (51.3) | 21.770 | <0.001 |
| Hypertension [n (%)] | 139 (39.0) | 28 (21.5) | 111 (49.1) | 25.224 | <0.001 |
| Hyperuricaemia [n (%)] | 31 ( 8.7) | 5 (3.8) | 26 (11.5) | 5.163 | 0.023 |
| Mean arterial pressure (mmHg) | 100.34 (91, 112.34) | 94.67 (87.33, 104.33) | 105 (94.67, 115.25) | -5.989 | <0.001 |
| Weight (kg) | 59.8 (52.39, 68) | 58 (51, 65.81) | 61.48 (53.78, 69.48) | -2.766 | 0.006 |
| Serum uric acid (μmol/L) | 423.5 (342, 517.5) | 345 (293, 409) | 470.1 (392.25, 565) | -9.559 | <0.001 |
| Serum sodium (mmol/L) | 139 (137.7, 140.5) | 138.9 (137.7, 140.2) | 139.1 (137.8, 140.7) | -0.794 | 0.427 |
| Serum potassium (mmol/L) | 3.72 (3.49, 3.95) | 3.58 (3.4, 3.77) | 3.84 (3.58, 4.07) | -6.489 | <0.001 |
| Serum albumin (g/L) | 36.7 (33.78, 39.8) | 37.35 (34.53, 40.78) | 36.3 (32.4, 39.1) | 3.049 | 0.002 |
| Triglyceride (mmol/L) | 1.59 (1.11, 2.49) | 1.38 (0.98, 2.02) | 1.78 (1.25, 2.57) | -3.577 | <0.001 |
| Cholesterol (mmol/L) | 5.3 (4.4, 6.04) | 5.15 (4.25, 5.68) | 5.36 (4.56, 6.29) | -2.544 | 0.011 |
| Low density lipoprotein cholesterol (mmol/L) | 3.19 (2.62, 3.89) | 3.1 (2.49, 3.53) | 3.27 (2.65, 4.04) | -2.777 | 0.005 |
| High density lipoprotein cholesterol (mmol/L) | 1.15 (0.96, 1.41) | 1.23 (1.01, 1.48) | 1.13 (0.93, 1.38) | 2.306 | 0.021 |
| Transferrin (g/L) | 1.96 (1.76, 2.25) | 2.11 (1.85, 2.36) | 1.91 (1.73, 2.17) | 4.300 | <0.001 |
| Serum IgA (g/L) | 3.4 (2.5, 4.09) | 3.23 (2.49, 3.79) | 3.47 (2.55, 4.45) | -1.908 | 0.056 |
| Serum IgM (g/L) | 1.16 (0.83, 1.68) | 1.21 (0.88, 1.67) | 1.14 (0.8, 1.68) | 1.154 | 0.249 |
| Serum IgG (g/L) | 11.5 (9.74, 13.1) | 11.4 (10.1, 12.6) | 11.55 (9.6, 13.5) | -0.551 | 0.582 |
| Complement C3 (mg/L) | 878 (773.75, 1010) | 889 (769.25, 1010) | 868 (776.5, 1000) | 0.592 | 0.555 |
| Complement C4 (mg/L) | 226 (178, 275) | 215.5 (166.25, 250.5) | 233 (184.5, 284) | -2.971 | 0.003 |
| Hemoglobin (g/L) | 124.56±18.02 | 126.68±16.16 | 123.34±18.92 | 1.765 | 0.079 |
| White blood cell (×109/L) | 7.48 (6.41, 8.69) | 7.37 (6.31, 8.59) | 7.59 (6.51, 8.75) | -1.049 | 0.295 |
| Platelet (×109/L) | 251 (209, 289.25) | 256.5 (218, 296) | 249 (204, 285.75) | 1.685 | 0.092 |
| Fibrinogen (g/L) | 3.56 (3.08, 4.31) | 3.31 (2.91, 3.85) | 3.73 (3.16, 4.54) | -4.052 | <0.001 |
| D-Dimer(ng/mL) | 330 (270, 472.5) | 290 (270, 390) | 350 (270, 520) | -3.168 | 0.001 |
| Hematuria [n (%)] | 151 (42.4) | 78 (60.0) | 73 (32.3) | 24.802 | <0.001 |
| 24 h urine protein (g) | 1.14 (0.51, 2.16) | 0.70 (0.33, 1.40) | 1.38 (0.69, 2.67) | -5.977 | <0.001 |
| eGFR[mL·min-1·(1.73 m2)-1] | 65.62 (39.43, 100.51) | 106.39 (88.53, 119.9) | 44.84 (31.57, 64.36) | 13.664 | <0.001 |
| Serum IgA/Complement C3 | 3.68 (2.83, 4.76) | 3.61 (2.75, 4.34) | 3.8 (2.86, 5.09) | -1.785 | 0.074 |
| RAAS-inhibitor [n (%)] | 294 (82.6) | 125 (96.2) | 169 (74.8) | 24.752 | <0.001 |
| Immunosuppressive agents [n (%)] | 217 (61.0) | 64 (49.2) | 153 (67.7) | 11.064 | 0.001 |
| Percentage of global glomerulosclerosis | 0.23 (0.09, 0.46) | 0.1 (0.03, 0.18) | 0.36 (0.17, 0.55) | -9.405 | <0.001 |
| M1 [n (%)] | 291 (81.7) | 110 (84.6) | 181 (80.1) | 0.850 | 0.357 |
| E1 [n (%)] | 54 (15.2) | 22 (16.9) | 32 (14.2) | 0.299 | 0.585 |
| S1 [n (%)] | 167 (46.9) | 49 (37.7) | 118 (52.2) | 6.416 | 0.011 |
| T [n (%)] | 92.316 | <0.001 | |||
| 0 | 209 (58.7) | 119 (91.5) | 90 (39.8) | ||
| 1 | 91 (25.6) | 10 (7.7) | 81 (35.8) | ||
| 2 | 56 (15.7) | 1 (0.8) | 55 (24.3) | ||
| C [n (%)] | 7.860 | 0.020 | |||
| 0 | 152 (42.7) | 49 (37.7) | 103 (45.6) | ||
| 1 | 168 (47.2) | 73 (56.2) | 95 (42.0) | ||
| 2 | 36 (10.1) | 8 (6.2) | 28 (12.4) |
表2 两组患者的基线资料
Tab.2 Baseline data of the patients with normal and high serum CysC levels
| Variables | Overall (n=356) | Normal serum CysC group (n=130) | High serum CysC group (n=226) | t/Z/χ2 | P |
|---|---|---|---|---|---|
| Age (year) | 36 (29, 45) | 32 (26, 39) | 39 (32, 47) | -5.245 | <0.001 |
| Male [n (%)] | 149 (41.9) | 33(25.4) | 116 (51.3) | 21.770 | <0.001 |
| Hypertension [n (%)] | 139 (39.0) | 28 (21.5) | 111 (49.1) | 25.224 | <0.001 |
| Hyperuricaemia [n (%)] | 31 ( 8.7) | 5 (3.8) | 26 (11.5) | 5.163 | 0.023 |
| Mean arterial pressure (mmHg) | 100.34 (91, 112.34) | 94.67 (87.33, 104.33) | 105 (94.67, 115.25) | -5.989 | <0.001 |
| Weight (kg) | 59.8 (52.39, 68) | 58 (51, 65.81) | 61.48 (53.78, 69.48) | -2.766 | 0.006 |
| Serum uric acid (μmol/L) | 423.5 (342, 517.5) | 345 (293, 409) | 470.1 (392.25, 565) | -9.559 | <0.001 |
| Serum sodium (mmol/L) | 139 (137.7, 140.5) | 138.9 (137.7, 140.2) | 139.1 (137.8, 140.7) | -0.794 | 0.427 |
| Serum potassium (mmol/L) | 3.72 (3.49, 3.95) | 3.58 (3.4, 3.77) | 3.84 (3.58, 4.07) | -6.489 | <0.001 |
| Serum albumin (g/L) | 36.7 (33.78, 39.8) | 37.35 (34.53, 40.78) | 36.3 (32.4, 39.1) | 3.049 | 0.002 |
| Triglyceride (mmol/L) | 1.59 (1.11, 2.49) | 1.38 (0.98, 2.02) | 1.78 (1.25, 2.57) | -3.577 | <0.001 |
| Cholesterol (mmol/L) | 5.3 (4.4, 6.04) | 5.15 (4.25, 5.68) | 5.36 (4.56, 6.29) | -2.544 | 0.011 |
| Low density lipoprotein cholesterol (mmol/L) | 3.19 (2.62, 3.89) | 3.1 (2.49, 3.53) | 3.27 (2.65, 4.04) | -2.777 | 0.005 |
| High density lipoprotein cholesterol (mmol/L) | 1.15 (0.96, 1.41) | 1.23 (1.01, 1.48) | 1.13 (0.93, 1.38) | 2.306 | 0.021 |
| Transferrin (g/L) | 1.96 (1.76, 2.25) | 2.11 (1.85, 2.36) | 1.91 (1.73, 2.17) | 4.300 | <0.001 |
| Serum IgA (g/L) | 3.4 (2.5, 4.09) | 3.23 (2.49, 3.79) | 3.47 (2.55, 4.45) | -1.908 | 0.056 |
| Serum IgM (g/L) | 1.16 (0.83, 1.68) | 1.21 (0.88, 1.67) | 1.14 (0.8, 1.68) | 1.154 | 0.249 |
| Serum IgG (g/L) | 11.5 (9.74, 13.1) | 11.4 (10.1, 12.6) | 11.55 (9.6, 13.5) | -0.551 | 0.582 |
| Complement C3 (mg/L) | 878 (773.75, 1010) | 889 (769.25, 1010) | 868 (776.5, 1000) | 0.592 | 0.555 |
| Complement C4 (mg/L) | 226 (178, 275) | 215.5 (166.25, 250.5) | 233 (184.5, 284) | -2.971 | 0.003 |
| Hemoglobin (g/L) | 124.56±18.02 | 126.68±16.16 | 123.34±18.92 | 1.765 | 0.079 |
| White blood cell (×109/L) | 7.48 (6.41, 8.69) | 7.37 (6.31, 8.59) | 7.59 (6.51, 8.75) | -1.049 | 0.295 |
| Platelet (×109/L) | 251 (209, 289.25) | 256.5 (218, 296) | 249 (204, 285.75) | 1.685 | 0.092 |
| Fibrinogen (g/L) | 3.56 (3.08, 4.31) | 3.31 (2.91, 3.85) | 3.73 (3.16, 4.54) | -4.052 | <0.001 |
| D-Dimer(ng/mL) | 330 (270, 472.5) | 290 (270, 390) | 350 (270, 520) | -3.168 | 0.001 |
| Hematuria [n (%)] | 151 (42.4) | 78 (60.0) | 73 (32.3) | 24.802 | <0.001 |
| 24 h urine protein (g) | 1.14 (0.51, 2.16) | 0.70 (0.33, 1.40) | 1.38 (0.69, 2.67) | -5.977 | <0.001 |
| eGFR[mL·min-1·(1.73 m2)-1] | 65.62 (39.43, 100.51) | 106.39 (88.53, 119.9) | 44.84 (31.57, 64.36) | 13.664 | <0.001 |
| Serum IgA/Complement C3 | 3.68 (2.83, 4.76) | 3.61 (2.75, 4.34) | 3.8 (2.86, 5.09) | -1.785 | 0.074 |
| RAAS-inhibitor [n (%)] | 294 (82.6) | 125 (96.2) | 169 (74.8) | 24.752 | <0.001 |
| Immunosuppressive agents [n (%)] | 217 (61.0) | 64 (49.2) | 153 (67.7) | 11.064 | 0.001 |
| Percentage of global glomerulosclerosis | 0.23 (0.09, 0.46) | 0.1 (0.03, 0.18) | 0.36 (0.17, 0.55) | -9.405 | <0.001 |
| M1 [n (%)] | 291 (81.7) | 110 (84.6) | 181 (80.1) | 0.850 | 0.357 |
| E1 [n (%)] | 54 (15.2) | 22 (16.9) | 32 (14.2) | 0.299 | 0.585 |
| S1 [n (%)] | 167 (46.9) | 49 (37.7) | 118 (52.2) | 6.416 | 0.011 |
| T [n (%)] | 92.316 | <0.001 | |||
| 0 | 209 (58.7) | 119 (91.5) | 90 (39.8) | ||
| 1 | 91 (25.6) | 10 (7.7) | 81 (35.8) | ||
| 2 | 56 (15.7) | 1 (0.8) | 55 (24.3) | ||
| C [n (%)] | 7.860 | 0.020 | |||
| 0 | 152 (42.7) | 49 (37.7) | 103 (45.6) | ||
| 1 | 168 (47.2) | 73 (56.2) | 95 (42.0) | ||
| 2 | 36 (10.1) | 8 (6.2) | 28 (12.4) |
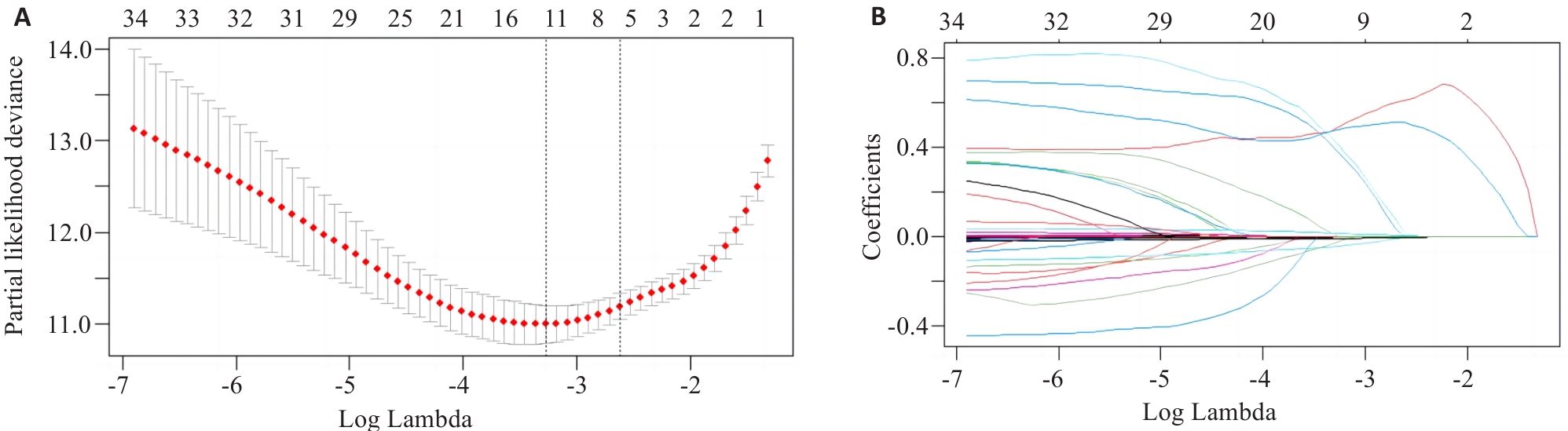
图1 Lasso回归模型筛选变量
Fig.1 Variable selection by Lasso regression model. A: Log Lambda versus partial likelihood deviance. B: Log Lambda versus coefficients.
| Variables | Lasso regression coefficients | Multivariate Cox regression | |
|---|---|---|---|
| HR (95% CI) | P | ||
| Mean arterial pressure (mmHg) | 4.23×10-3 | 1.015 (1.000-1.031) | 0.048 |
| Serum CysC (mg/L) | 6.10×10-1 | 2.142 (1.222-3.755) | 0.008 |
| Serum albumin (g/L) | -4.57×10-3 | 0.934 (0.892-0.977) | 0.003 |
| 24-hour urine protein (g) | 2.88×10-5 | 1.000 (0.999-1.000) | 0.638 |
| eGFR[mL·min-1·(1.73 m2)-1] | -5.43×10-3 | 0.984 (0.965-1.003) | 0.107 |
| Percentage of global glomerulosclerosis | 1.81×10-2 | 2.077 (0.518-8.327) | 0.302 |
| T [n (%)] | 5.10×10-1 | 1.000 (Ref) | |
| T1 | 3.416 (1.424-8.192) | 0.006 | |
| T2 | 3.408 (1.144-10.150) | 0.028 | |
表3 Lasso回归及多因素Cox回归分析IgAN肾脏不良预后的危险因素
Tab.3 Lasso regression and multivariate Cox regression analysis of the risk factors for poor renal prognosis in IgAN
| Variables | Lasso regression coefficients | Multivariate Cox regression | |
|---|---|---|---|
| HR (95% CI) | P | ||
| Mean arterial pressure (mmHg) | 4.23×10-3 | 1.015 (1.000-1.031) | 0.048 |
| Serum CysC (mg/L) | 6.10×10-1 | 2.142 (1.222-3.755) | 0.008 |
| Serum albumin (g/L) | -4.57×10-3 | 0.934 (0.892-0.977) | 0.003 |
| 24-hour urine protein (g) | 2.88×10-5 | 1.000 (0.999-1.000) | 0.638 |
| eGFR[mL·min-1·(1.73 m2)-1] | -5.43×10-3 | 0.984 (0.965-1.003) | 0.107 |
| Percentage of global glomerulosclerosis | 1.81×10-2 | 2.077 (0.518-8.327) | 0.302 |
| T [n (%)] | 5.10×10-1 | 1.000 (Ref) | |
| T1 | 3.416 (1.424-8.192) | 0.006 | |
| T2 | 3.408 (1.144-10.150) | 0.028 | |
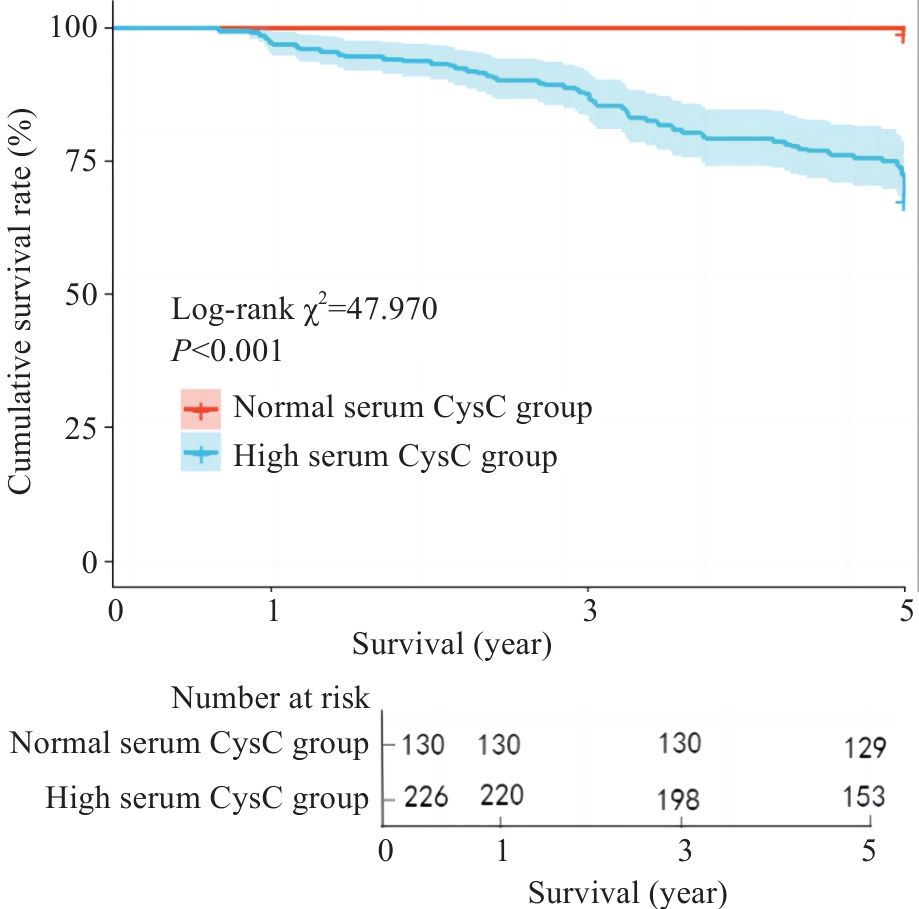
图2 Kaplan⁃Meier生存曲线分析血清CysC对IgAN患者5年肾脏生存率的影响
Fig.2 Kaplan-Meier survival curves for analyzing the effect of serum CysC on 5-year renal survival rate of IgAN patients.
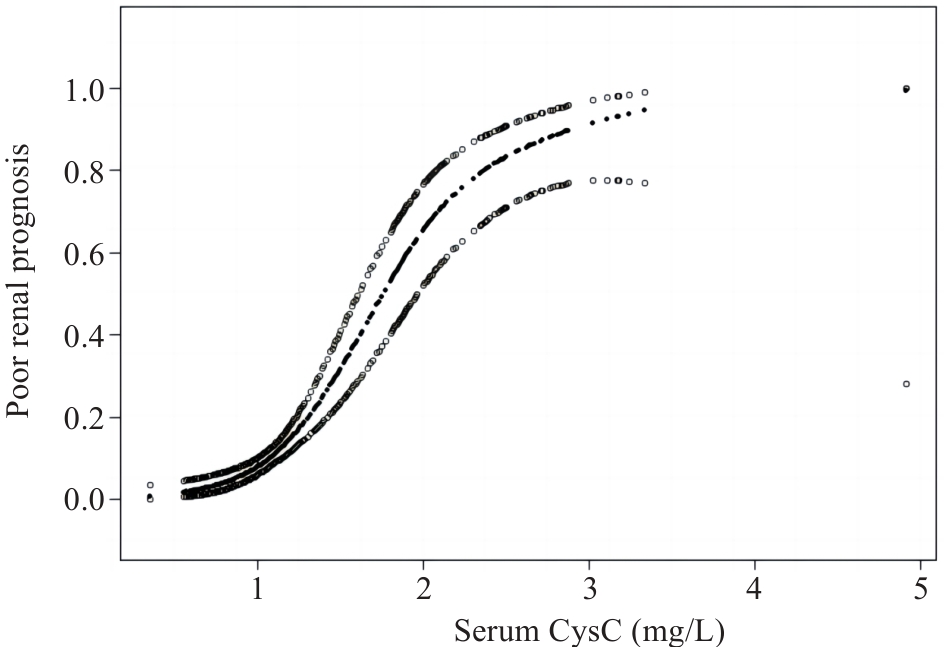
图3 广义加性模型描述血清CysC水平与IgAN肾脏不良预后之间的关系
Fig.3 Generalized additive models demonstrate the relationship between serum CysC level and poor renal prognosis of IgAN.
| Threshold effect | β (95% CI) | P |
|---|---|---|
| Fitting by GAMs | ||
| Serum CysC | 2.637 (2.034, 3.240) | <0.001 |
| Fitting by two-piecewise GAMs | ||
| Inflection point | 2.12 | |
| Serum CysC≤2.12 | 3.487 (2.561, 4.413) | <0.001 |
| Serum CysC>2.12 | 0.676 (-0.642, 1.995) | 0.315 |
| Log likelihood ratio | 0.008 |
表4 血清CysC水平与IgAN肾脏不良预后的阈值效应分析
Tab.4 Threshold effect analysis of serum CysC level and poor renal prognosis of IgAN
| Threshold effect | β (95% CI) | P |
|---|---|---|
| Fitting by GAMs | ||
| Serum CysC | 2.637 (2.034, 3.240) | <0.001 |
| Fitting by two-piecewise GAMs | ||
| Inflection point | 2.12 | |
| Serum CysC≤2.12 | 3.487 (2.561, 4.413) | <0.001 |
| Serum CysC>2.12 | 0.676 (-0.642, 1.995) | 0.315 |
| Log likelihood ratio | 0.008 |
| 1 | Lai KN, Tang SC, Schena FP, et al. IgA nephropathy[J]. Nat Rev Dis Primers, 2016, 2: 16001. |
| 2 | DiseaseKidney: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases[J]. Kidney Int, 2021, 100(4s): S1-276. |
| 3 | Spencer S, Desborough R, Bhandari S. Should cystatin C eGFR become routine clinical practice[J]. Biomolecules, 2023, 13(7): 1075. |
| 4 | Feng BY, Lu Y, Ye L, et al. Mendelian randomization study supports the causal association between serum cystatin C and risk of diabetic nephropathy[J]. Front Endocrinol, 2022, 13: 1043174. |
| 5 | Chen SH, Tang YZ, Zhou XY. Cystatin C for predicting all-cause mortality and rehospitalization in patients with heart failure: a meta-analysis[J]. Biosci Rep, 2019, 39(2): BSR20181761. |
| 6 | Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race[J]. N Engl J Med, 2021, 385(19): 1737-49. |
| 7 | Trimarchi H, Barratt J, Cattran DC, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group[J]. Kidney Int, 2017, 91(5): 1014-21. |
| 8 | Dejenie TA, Abebe EC, Mengstie MA, et al. Dyslipidemia and serum cystatin C levels as biomarker of diabetic nephropathy in patients with type 2 diabetes mellitus[J]. Front Endocrinol, 2023, 14: 1124367. |
| 9 | Séronie-Vivien S, Delanaye P, Piéroni L, et al. Cystatin C: current position and future prospects[J]. Clin Chem Lab Med, 2008, 46(12): 1664-86. |
| 10 | Zou LX, Sun L, Nicholas SB, et al. Comparison of bias and accuracy using cystatin C and creatinine in CKD-EPI equations for GFR estimation[J]. Eur J Intern Med, 2020, 80: 29-34. |
| 11 | Liao XL, Zhu Y, Xue C. Diagnostic value of serum cystatin C for diabetic nephropathy: a meta-analysis[J]. BMC Endocr Disord, 2022, 22(1): 149. |
| 12 | 曾思权. 血清胱抑素C测定在原发性高血压早期肾损伤中的诊断价值[J]. 中国医学工程, 2016, 24(9): 40-2. |
| 13 | 蔡 萍. CysC水平变化在早期诊断急性肾损伤中的价值[J]. 四川医学, 2016, 37(4): 438-40. |
| 14 | Wali U, Hussain MM, Wali N, et al. Comparison of serum levels of Cystatin-C and traditional renal biomarkers for the early detection of pre-hypertensive nephropathy[J]. J Pak Med Assoc, 2019, 69(3): 313-9. |
| 15 | 蔡小凡, 符欣漪, 蔡秀峰, 等. 血清胱抑素C水平与IgA肾病临床及病理特征的相关性[J]. 中华检验医学杂志, 2022, 45(9): 957-62. |
| 16 | 陆 放, 张承宁, 段俗言, 等. 血清胱抑素C水平评估2型糖尿病患者肾病预后的价值[J]. 中华检验医学杂志, 2023, 46(4): 375-84. |
| 17 | Chen TY, Li X, Li YX, et al. Prediction and risk stratification of kidney outcomes in IgA nephropathy[J]. Am J Kidney Dis, 2019, 74(3): 300-9. |
| 18 | Xie JY, Lv JC, Wang WM, et al. Kidney failure risk prediction equations in IgA nephropathy: a multicenter risk assessment study in Chinese patients[J]. Am J Kidney Dis, 2018, 72(3): 371-80. |
| 19 | Zi MT, Xu YK. Involvement of cystatin C in immunity and apoptosis[J]. Immunol Lett, 2018, 196: 80-90. |
| 20 | Xu YK, Ding Y, Li XC, et al. Cystatin C is a disease-associated protein subject to multiple regulation[J]. Immunol Cell Biol, 2015, 93(5): 442-51. |
| 21 | 郭唯一, 安秀平, 孙丽君, 等. 伴肾小动脉硬化病变的IgA肾病的临床病理特点及预后[J]. 中华肾脏病杂志, 2023, 39(3): 209-14. |
| 22 | Sugiura N, Moriyama T, Miyabe Y, et al. Severity of arterial and/or arteriolar sclerosis in IgA nephropathy and the effects of renin-angiotensin system inhibitors on its prognosis[J]. J Pathol Clin Res, 2021, 7(6): 616-23. |
| 23 | Peng W, Tang Y, Tan L, et al. Crescents and global glomerulosclerosis in Chinese IgA nephropathy patients: a five-year follow-up[J]. Kidney Blood Press Res, 2019, 44(1): 103-12. |
| 24 | Lee K, Shin J, Park J, et al. First-year GFR slope and long-term renal outcome in IgA nephropathy[J]. Eur J Clin Invest, 2018, 48(6): e12936. |
| 25 | Le WB, Liang SS, Hu YL, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population[J]. Nephrol Dial Transplant, 2012, 27(4): 1479-85. |
| 26 | Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy[J]. Am J Kidney Dis, 2001, 38(4): 728-35. |
| 27 | 袁燕红, 王 琴, 张敏芳, 等. 血清白蛋白对IgA肾病患者肾脏预后的预测价值[J]. 中华肾脏病杂志, 2015, 31(2): 102-8. |
| 28 | 卢清梅, 潘 玲, 莫曼秋, 等. 同型半胱氨酸预测IgA肾病患者肾脏预后不良的价值[J]. 中华肾脏病杂志, 2022, 38(8): 718-21. |
| 29 | 唐天威, 叶智明, 李锐钊. IgA肾病预后的影响因素及相关评估模型的研究进展[J]. 中华肾脏病杂志, 2024, 40(6): 499-505. |
| 30 | Xie JY, Kiryluk K, Wang WM, et al. Predicting progression of IgA nephropathy: new clinical progression risk score[J]. PLoS One, 2012, 7(6): e38904. |
| 31 | Zhu XJ, Li HQ, Liu YX, et al. Tubular atrophy/interstitial fibrosis scores of Oxford classification combinded with proteinuria level at biopsy provides earlier risk prediction in lgA nephropathy[J]. Sci Rep, 2017, 7(1): 1100. |
| 32 | Coppo R, Troyanov S, Bellur S, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments[J]. Kidney Int, 2014, 86(4): 828-36. |
| [1] | 宋雪, 陈悦, 张敏, 张诺, 左芦根, 李静, 耿志军, 张小凤, 王月月, 王炼, 胡建国. GPSM2在胃癌组织中高表达并通过促进肿瘤细胞的增殖影响患者预后[J]. 南方医科大学学报, 2025, 45(2): 229-238. |
| [2] | 陈晓睿, 魏青政, 张宗亮, 原江水, 宋卫青. 过表达带电多泡体蛋白2B基因抑制肾透明细胞癌细胞的增殖[J]. 南方医科大学学报, 2025, 45(1): 126-136. |
| [3] | 王耀彬, 陈柳燕, 罗伊凌, 申继清, 周素芳. NUF2对泛癌的预后和免疫治疗效果的预测价值[J]. 南方医科大学学报, 2025, 45(1): 137-149. |
| [4] | 周超, 张晶晶, 唐巧, 付双楠, 张宁, 何召云, 张瑾, 张田义, 刘鹏程, 宫嫚. 血清色氨酸用于乙肝相关慢加急性肝衰竭90 d死亡风险分层管理的潜在价值:一项多中心回顾性研究[J]. 南方医科大学学报, 2025, 45(1): 59-64. |
| [5] | 陈孝华, 鲁辉, 王子良, 王炼, 夏勇生, 耿志军, 张小凤, 宋雪, 王月月, 李静, 胡建国, 左芦根. ABI2在胃癌进展和预后中的作用及其调控机制[J]. 南方医科大学学报, 2024, 44(9): 1653-1661. |
| [6] | 叶梦楠, 武鸿美, 梅琰, 张庆玲. CREM在胃癌中高表达并与患者的不良预后相关[J]. 南方医科大学学报, 2024, 44(9): 1776-1782. |
| [7] | 纪凯, 于冠宇, 周乐其, 张天帅, 凌潜龙, 满文江, 朱冰, 张卫. HNRNPA1基因在结直肠癌组织中高表达及其潜在的诊断和治疗价值[J]. 南方医科大学学报, 2024, 44(9): 1685-1695. |
| [8] | 刘鹏程, 娄丽娟, 刘霞, 王建, 姜颖. M2巨噬细胞特征基因风险评分能准确预测HBV相关肝细胞癌患者的预后[J]. 南方医科大学学报, 2024, 44(5): 827-840. |
| [9] | 杨晶晶, 殷丽霞, 段婷, 牛民主, 何震东, 陈心蕊, 张小凤, 李静, 耿志军, 左芦根. 胃癌组织中高表达ATP5A1与患者术后的不良预后和肿瘤细胞的糖代谢有关[J]. 南方医科大学学报, 2024, 44(5): 974-980. |
| [10] | 高志强, 林 洁, 洪 鹏, 胡再宏, 董军君, 石秦林, 田小毛, 刘 丰, 魏光辉. 基于高通量 RNA 测序分析 Wilms 瘤中关键基因对预后及免疫应答的影响[J]. 南方医科大学学报, 2024, 44(4): 727-738. |
| [11] | 沈梦迪, 赵 娜, 邓晓晶, 邓 敏. COX6B2在胃癌组织中高表达并影响患者的远期预后:基于抑制p53信号调控胃癌细胞的增殖及细胞周期[J]. 南方医科大学学报, 2024, 44(2): 289-297. |
| [12] | 张 诺, 张 震, 张雨路, 宋 雪, 张小凤, 李 静, 左芦根, 胡建国. PCID2在胃癌组织中高表达并通过调控细胞周期进程和增殖影响患者预后[J]. 南方医科大学学报, 2024, 44(2): 324-332. |
| [13] | 张文静, 张 诺, 杨 子, 张小凤, 孙奥飞, 王 炼, 宋 雪, 耿志军, 李 静, 胡建国. BZW1 高表达促进胃癌细胞的侵袭和转移:基于调控Wnt//β-catenin通路和促进上皮间质转化[J]. 南方医科大学学报, 2024, 44(2): 354-362. |
| [14] | 谭茹雪, 包晓樟, 韩亮, 李朝晖, 田男. 基于HOXA9 DNA甲基化的两位点联合预测模型可用于脑膜瘤进展风险的早期筛查[J]. 南方医科大学学报, 2024, 44(11): 2110-2120. |
| [15] | 张震, 鲁辉, 陈孝华, 王炼, 王子良, 王月月, 葛思堂, 左芦根. CEP192过表达可作为胃癌患者不良预后的生物标志物并通过调控G2/M期关键蛋白的表达影响肿瘤细胞恶性增殖[J]. 南方医科大学学报, 2024, 44(11): 2137-2145. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||