Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (12): 2585-2597.doi: 10.12122/j.issn.1673-4254.2025.12.06
Xiaoyu LU1( ), Zhihui LIU1, Ye LIU2, Tianxiao PANG2, Rong BIAN2, Ling GUO2(
), Zhihui LIU1, Ye LIU2, Tianxiao PANG2, Rong BIAN2, Ling GUO2( ), Xuehong HE2(
), Xuehong HE2( )
)
Received:2025-06-30
Online:2025-12-20
Published:2025-12-22
Contact:
Ling GUO, Xuehong HE
E-mail:dfs1107@163.com;vivi_gling@163.com;lnzysnk@163.com
Xiaoyu LU, Zhihui LIU, Ye LIU, Tianxiao PANG, Rong BIAN, Ling GUO, Xuehong HE. Shenqi Xiezhuo Decoction alleviates renal fibrosis in rats by ameliorating oxidative stress and inflammation through the Rap1/MAPK/FoxO3a signaling pathway[J]. Journal of Southern Medical University, 2025, 45(12): 2585-2597.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.12.06
| Time (min) | Aqueous phase proportion(%) | Organic phase proportion(%) |
|---|---|---|
| 1 | 98 | 2 |
| 5 | 80 | 20 |
| 10 | 50 | 50 |
| 15 | 20 | 80 |
| 20 | 5 | 95 |
| 27 | 5 | 95 |
| 28 | 98 | 2 |
| 30 | 98 | 2 |
Tab.1 Chromatographic gradient
| Time (min) | Aqueous phase proportion(%) | Organic phase proportion(%) |
|---|---|---|
| 1 | 98 | 2 |
| 5 | 80 | 20 |
| 10 | 50 | 50 |
| 15 | 20 | 80 |
| 20 | 5 | 95 |
| 27 | 5 | 95 |
| 28 | 98 | 2 |
| 30 | 98 | 2 |
| Gene | Primer sequences (5'-3') | Fragment length (bp) |
|---|---|---|
| α-SMA | F:ACCATCGGGAATGAACGCTT | 191 |
| R:CTGTCAGCAATGCCTGGGTA | ||
| Col1a1 | F:CGTGGAAACCTGATGTATGCTTG | 169 |
| R:CCTATGACTTCTGCGTCTGGTGA | ||
| NAKED2 | F:CGCCTCTGTCAATCATTCCTC | 205 |
| R:ATCTGTGTTGGGCTTCCTGCTATA | ||
| Rap1 | F:GGATTGAAGGCACCAACCAT | 144 |
| R:AGTAATTGAAGTGCTCCTTGCCG | ||
| Raf-1 | F:GATGCTGTCTACTCGGATTGGC | 116 |
| R:GAAGTTGCTCTGGAGTTGGGTC | ||
| B-raf | F:CGCAAGATGTGGTGTAACGG | 201 |
| R:AAGTTGTGGGTTGTCAGAGGAA | ||
| MEK | F:CGTGATGTCAAACCCTCCAAC | 135 |
| R:AGGAGCCATATAGGCAGCACAG | ||
| MEK3 | F:TGAAGATGTGCGACTTTGGC | 141 |
| R:CATCAGACTTGACGTTGTAGCCC | ||
| p38MAPK | F:ACCACGACCCTGATGATGAGC | 94 |
| R:TAGGTCAGGCTCTTCCATTCGT | ||
| ERK | F:GAGACATCCTCAGAGCACCCA | 216 |
| R:TGTTGATAAGCAGATTGGAGGG | ||
| Foxo3a | F:AACAGTACCGTGTTCGGACC | 119 |
| R:AGTGTCTGGTTGCCGTAGTG | ||
| MnSOD | F:TCTGGACAAACCTGAGCCCTAA | 133 |
| R:GAACCTTGGACTCCCACAGACA | ||
| GAPDH | F:CTGGAGAAACCTGCCAAGTATG | 138 |
| R:GGTGGAAGAATGGGAGTTGCT |
Tab.2 Primer sequences
| Gene | Primer sequences (5'-3') | Fragment length (bp) |
|---|---|---|
| α-SMA | F:ACCATCGGGAATGAACGCTT | 191 |
| R:CTGTCAGCAATGCCTGGGTA | ||
| Col1a1 | F:CGTGGAAACCTGATGTATGCTTG | 169 |
| R:CCTATGACTTCTGCGTCTGGTGA | ||
| NAKED2 | F:CGCCTCTGTCAATCATTCCTC | 205 |
| R:ATCTGTGTTGGGCTTCCTGCTATA | ||
| Rap1 | F:GGATTGAAGGCACCAACCAT | 144 |
| R:AGTAATTGAAGTGCTCCTTGCCG | ||
| Raf-1 | F:GATGCTGTCTACTCGGATTGGC | 116 |
| R:GAAGTTGCTCTGGAGTTGGGTC | ||
| B-raf | F:CGCAAGATGTGGTGTAACGG | 201 |
| R:AAGTTGTGGGTTGTCAGAGGAA | ||
| MEK | F:CGTGATGTCAAACCCTCCAAC | 135 |
| R:AGGAGCCATATAGGCAGCACAG | ||
| MEK3 | F:TGAAGATGTGCGACTTTGGC | 141 |
| R:CATCAGACTTGACGTTGTAGCCC | ||
| p38MAPK | F:ACCACGACCCTGATGATGAGC | 94 |
| R:TAGGTCAGGCTCTTCCATTCGT | ||
| ERK | F:GAGACATCCTCAGAGCACCCA | 216 |
| R:TGTTGATAAGCAGATTGGAGGG | ||
| Foxo3a | F:AACAGTACCGTGTTCGGACC | 119 |
| R:AGTGTCTGGTTGCCGTAGTG | ||
| MnSOD | F:TCTGGACAAACCTGAGCCCTAA | 133 |
| R:GAACCTTGGACTCCCACAGACA | ||
| GAPDH | F:CTGGAGAAACCTGCCAAGTATG | 138 |
| R:GGTGGAAGAATGGGAGTTGCT |
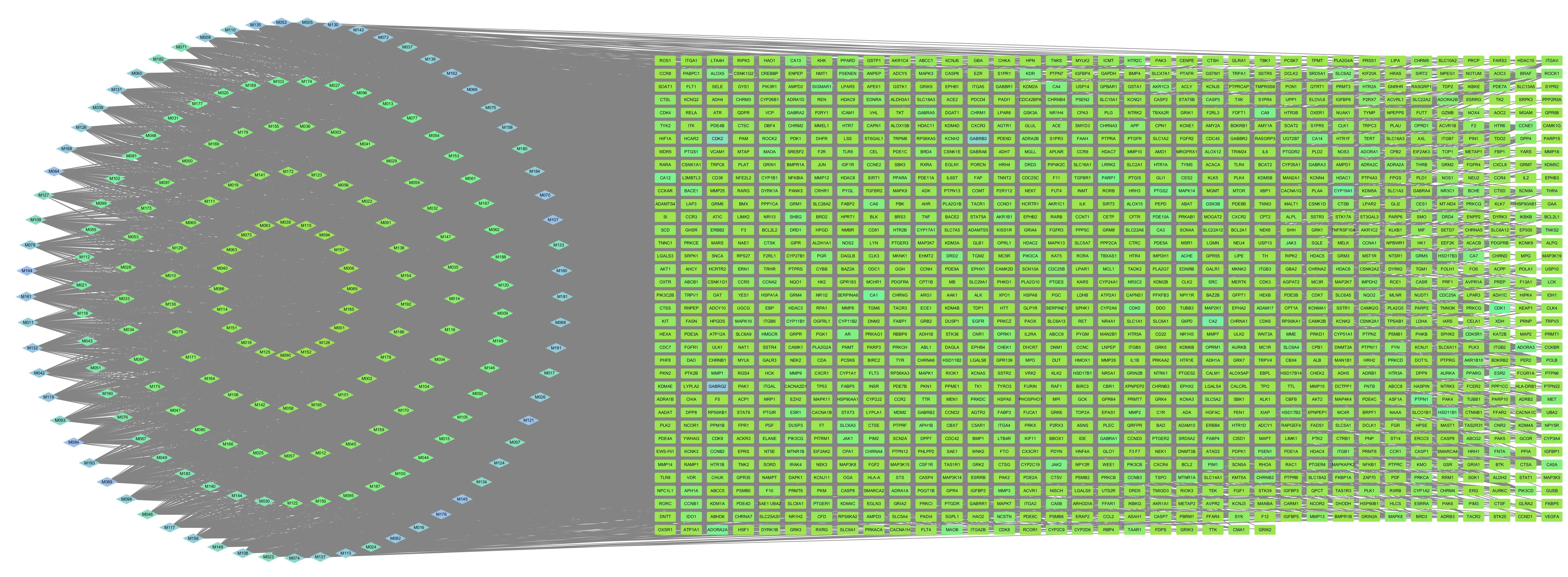
Fig.3 "Component-Target-Disease" network diagram. Diamonds represent active components, and rectangles represent disease targets with a total of 1475 nodes and 11 241 edges.

Fig.4 PPI network diagram of the targets of SQXZD for delaying RF. Node color intensity corresponds to the Degree value, and darker colors indicate higher Degree values (169 nodes and 3682 edges).
| Group | BUN (mmol/L) | Cr (μmol/L) |
|---|---|---|
| Control | 4.94±0.29 | 47.48±2.62 |
| Sham | 7.18±0.32 | 55.48±1.10 |
| Model | 28.08±2.35** | 196.11±12.88** |
| Losartan | 18.97±1.01# | 166.99±4.07# |
| SQXZD low-dose | 23.49±1.09 | 178.97±6.42 |
| SQXZD medium-dose | 16.79±0.55▲ | 149.92±2.57▲ |
| SQXZD high-dose | 19.03±1.01# | 171.32±3.68# |
Tab.3 Effects of SQXZD on serum BUN and Cr levels in rats with unilateral ureteral obstruction (UUO) (Mean±SD, n=5)
| Group | BUN (mmol/L) | Cr (μmol/L) |
|---|---|---|
| Control | 4.94±0.29 | 47.48±2.62 |
| Sham | 7.18±0.32 | 55.48±1.10 |
| Model | 28.08±2.35** | 196.11±12.88** |
| Losartan | 18.97±1.01# | 166.99±4.07# |
| SQXZD low-dose | 23.49±1.09 | 178.97±6.42 |
| SQXZD medium-dose | 16.79±0.55▲ | 149.92±2.57▲ |
| SQXZD high-dose | 19.03±1.01# | 171.32±3.68# |
| Group | SOD (U/mL) | MDA (μmol/L) | GSH-px (U/mL) |
|---|---|---|---|
| Control | 325.42±6.59 | 2.03±0.14 | 2582.35±54.22 |
| Sham | 257.27±16.71 | 2.36±0.05 | 2238.14±105.60 |
| Model | 87.01±2.08** | 8.92±0.94** | 668.21±6.81** |
| Losartan | 212.38±5.19# | 4.26±0.38# | 2048.18±45.04# |
| SQXZD low-dose | 183.70±5.04# | 7.50±0.52 | 1516.04±66.02# |
| SQXZD medium-dose | 270.46±5.87▲ | 2.90±0.11▲ | 2218.58±51.31▲ |
| SQXZD high-dose | 209.35±5.60# | 5.42±0.20# | 1684.99±52.96# |
Tab.4 Effects of SQXZD on serum SOD, MDA and GSH-px levels in UUO rats (Mean±SD, n=5)
| Group | SOD (U/mL) | MDA (μmol/L) | GSH-px (U/mL) |
|---|---|---|---|
| Control | 325.42±6.59 | 2.03±0.14 | 2582.35±54.22 |
| Sham | 257.27±16.71 | 2.36±0.05 | 2238.14±105.60 |
| Model | 87.01±2.08** | 8.92±0.94** | 668.21±6.81** |
| Losartan | 212.38±5.19# | 4.26±0.38# | 2048.18±45.04# |
| SQXZD low-dose | 183.70±5.04# | 7.50±0.52 | 1516.04±66.02# |
| SQXZD medium-dose | 270.46±5.87▲ | 2.90±0.11▲ | 2218.58±51.31▲ |
| SQXZD high-dose | 209.35±5.60# | 5.42±0.20# | 1684.99±52.96# |
| Group | IL-6 | TNF-α |
|---|---|---|
| Control | 0.159±0.001 | 0.038±0.0003 |
| Sham | 0.177±0.005 | 0.040±0.0004 |
| Model | 0.309±0.010** | 0.056±0.0011** |
| Losartan | 0.243±0.004# | 0.051±0.0004# |
| SQXZD low-dose | 0.273±0.006 | 0.053±0.0006 |
| SQXZD medium-dose | 0.228±0.002▲ | 0.049±0.0003▲ |
| SQXZD high-dose | 0.252±0.005# | 0.051±0.0005# |
Tab.5 Effects of SQXZD on serum IL-6 and TNF‑α levels in UUO rats (Mean±SD, n=5)
| Group | IL-6 | TNF-α |
|---|---|---|
| Control | 0.159±0.001 | 0.038±0.0003 |
| Sham | 0.177±0.005 | 0.040±0.0004 |
| Model | 0.309±0.010** | 0.056±0.0011** |
| Losartan | 0.243±0.004# | 0.051±0.0004# |
| SQXZD low-dose | 0.273±0.006 | 0.053±0.0006 |
| SQXZD medium-dose | 0.228±0.002▲ | 0.049±0.0003▲ |
| SQXZD high-dose | 0.252±0.005# | 0.051±0.0005# |
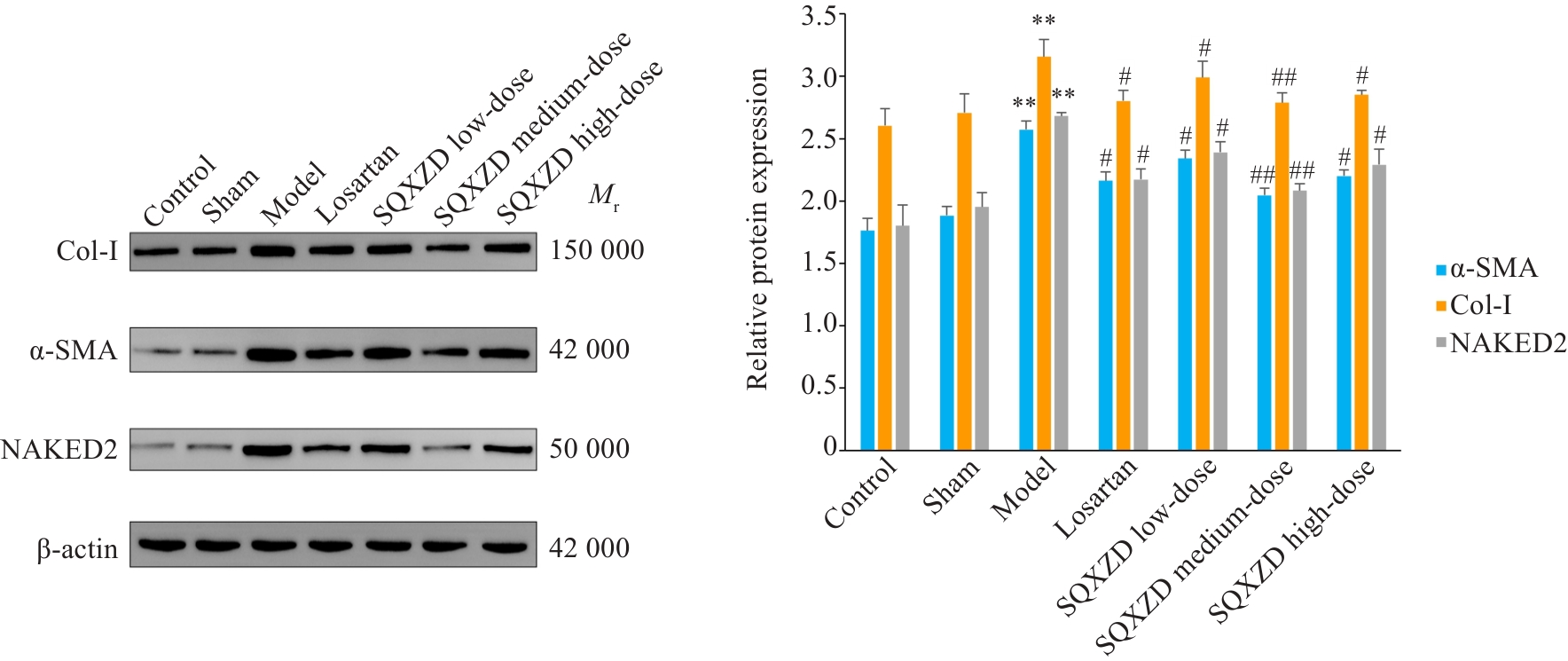
Fig.9 Effects of SQXZD on expressions of α-SMA, Col-I and NAKED2 in the surgical-side kidneys of UUO rats. **P<0.01 vs Sham group; ##P<0.01, #P<0.05 vs Model group.
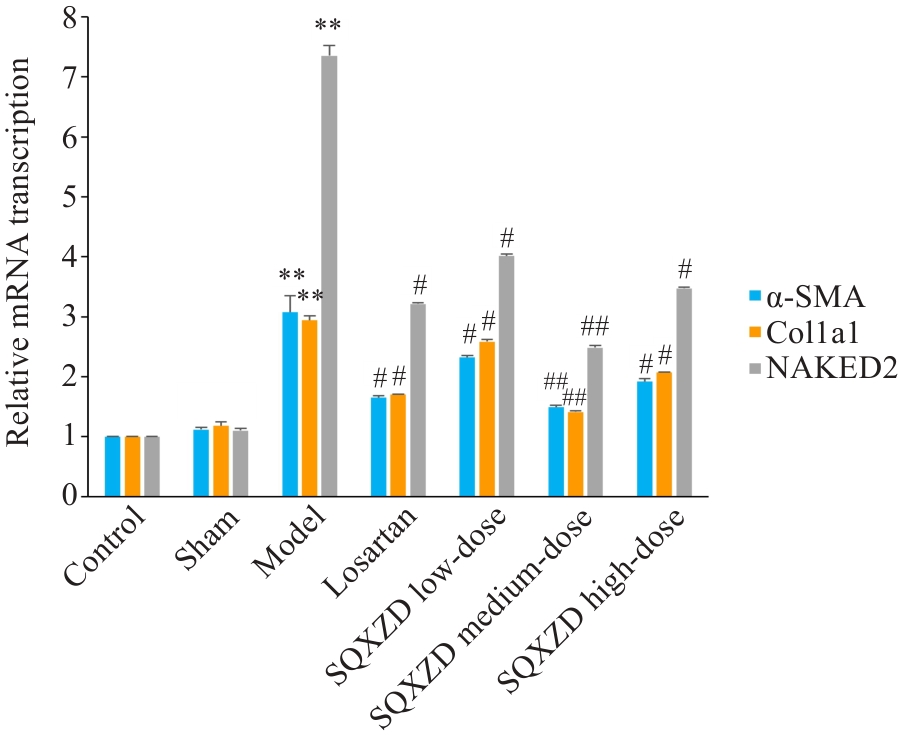
Fig.10 Effects of SQXZD on mRNA transcription levels of α-SMA, Col1a1 and NAKED2 in the surgical-side kidneys of UUO rats. **P<0.01 vs Sham group; ##P<0.01, #P<0.05 vs Model group.
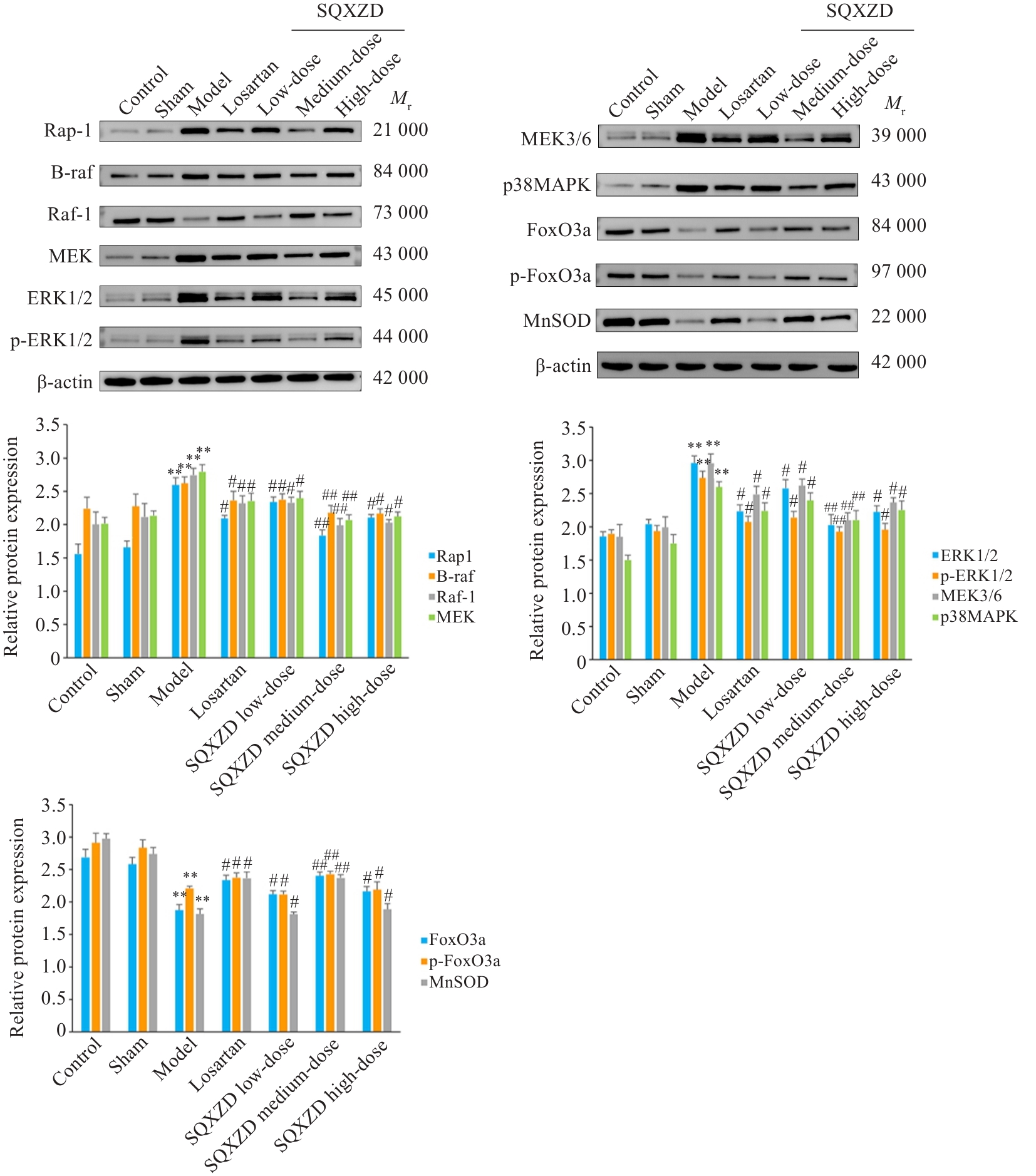
Fig.11 Effects of SQXZD on expressions of Rap1, B-raf, Raf-1, MEK3/6, p38MAPK, MEK, ERK1/2, p-ERK1/2, FoxO3a, p-FoxO3a and MnSOD in surgical-side kidneys of UUO rats. **P<0.01 vs Sham group; ##P<0.01, #P<0.05 vs Model group.
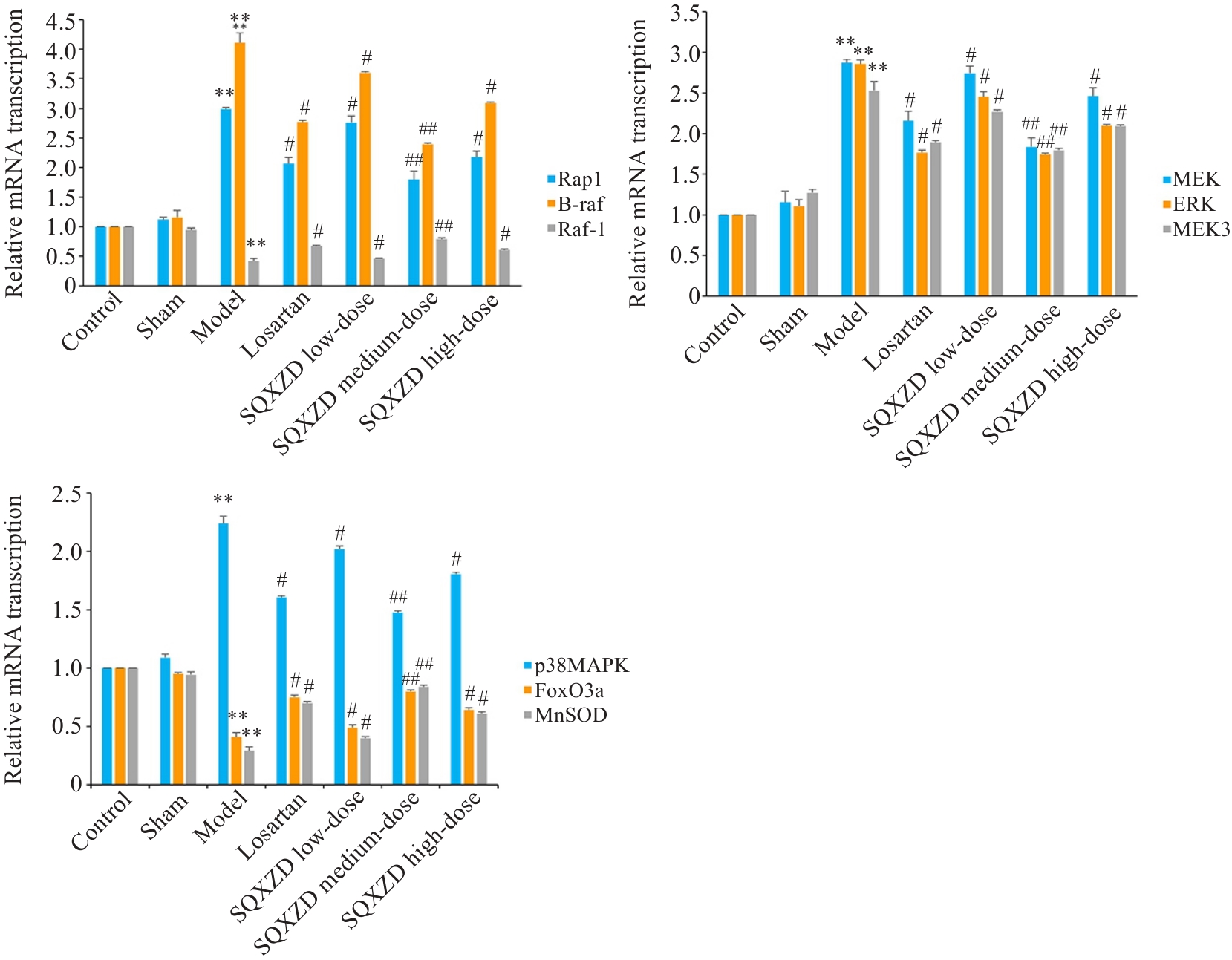
Fig.12 Effects of SQXZD on mRNA transcription levels of Rap1, B-raf, Raf-1, MEK3, p38MAPK, MEK, ERK, FoxO3a and MnSOD in surgical-side kidneys of UUO rats.**P<0.01 vs Sham group; ##P<0.01, #P<0.05 vs Model group.
| [1] | Liu Y. Cellular and molecular mechanisms of renal fibrosis[J]. Nat Rev Nephrol, 2011, 7(12): 684-96. doi:10.1038/nrneph.2011.149 |
| [2] | Conway B, Hughes J. Cellular orchestrators of renal fibrosis[J]. Qjm, 2012, 105(7): 611-5. doi:10.1093/qjmed/hcr235 |
| [3] | Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis[J]. J Am Soc Nephrol, 2010, 21(11): 1819-34. doi:10.1681/asn.2010080793 |
| [4] | 卞帅博, 张 亮, 杨 璇. 1990~2021年中国慢性肾脏病疾病负担变化趋势及发病预测分析[J].实用临床医药杂志, 2025, 29(6): 89-93, 98. |
| [5] | Kramann R, DiRocco DP, Maarouf OH, et al. Matrix-producing cells in chronic kidney disease: origin, regulation, and activation[J]. Curr Pathobiol Rep, 2013, 1(4): 301-11. doi:10.1007/s40139-013-0026-7 |
| [6] | Su H, Wan C, Song A, et al. Oxidative stress and renal fibrosis: mechanisms and therapies[J]. Adv Exp Med Biol, 2019, 1165: 585-604. doi:10.1007/978-981-13-8871-2_29 |
| [7] | Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease[J]. Cells, 2019, 8(11): E1383. doi:10.3390/cells8111383 |
| [8] | Kao MP, Ang DS, Pall A, et al. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options[J]. J Hum Hypertens, 2010, 24(1): 1-8. doi:10.1038/jhh.2009.70 |
| [9] | Davì G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress[J]. JAMA, 2002, 288(16): 2008-14. doi:10.1001/jama.288.16.2008 |
| [10] | Duni A, Liakopoulos V, Roumeliotis S, et al. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne's thread[J]. Int J Mol Sci, 2019, 20(15): E3711. doi:10.3390/ijms20153711 |
| [11] | Lee OYA, Wong ANN, Ho CY, et al. Potentials of natural antioxidants in reducing inflammation and oxidative stress in chronic kidney disease[J]. Antioxidants: Basel, 2024, 13(6): 751. doi:10.3390/antiox13060751 |
| [12] | 梁 亮.何学红教授学术思想总结及肾衰方治疗慢性肾脏病的临床与实验研究[D]. 辽宁中医药大学, 2015. |
| [13] | ConsortiumUniProt. UniProt: the universal protein knowledgebase in 2023[J]. Nucleic Acids Res, 2023, 51(d1): D523-31. |
| [14] | Amberger JS, Hamosh A. Searching online mendelian inheritance in man (OMIM): a knowledgebase of human genes and genetic phenotypes[J]. Curr Protoc Bioinformatics, 2017, 58: 1.2.1-1.2.12. doi:10.1002/cpbi.27 |
| [15] | Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses[J]. Curr Protoc Bioinformatics, 2016, 54: 1.30.1-1.30.33. doi:10.1002/cpbi.5 |
| [16] | Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update[J]. Nucleic Acids Res, 2020, 48(d1): D845-55. |
| [17] | Szklarczyk D, Kirsch R, Koutrouli M, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest[J]. Nucleic Acids Res, 2023, 51(d1): D638-46. doi:10.1093/nar/gkac1000 |
| [18] | Sherman BT, Hao M, Qiu J, et al. DAVID a web server for functional enrichment analysis and functional annotation of gene lists (2021 update)[J]. Nucleic Acids Res, 2022, 50(w1): W216-21. doi:10.1093/nar/gkac194 |
| [19] | Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules[J]. Sci Rep, 2017, 7: 42717. doi:10.1038/srep42717 |
| [20] | Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules[J]. Nucleic Acids Res, 2019, 47(w1): W357-64. doi:10.1093/nar/gkz382 |
| [21] | 马园园, 刘成海, 陶艳艳. 肾纤维化动物模型特点与研究进展[J]. 中国实验动物学报, 2018, 26(3): 398-403. |
| [22] | 李建省, 王英明, 闫燕顺, 等. “肾虚络瘀,肾络癥瘕”病机观与肾间质纤维化中自噬不足的相关性[J]. 中国实验方剂学杂志, 2023, 29(2):186-94. |
| [23] | Li W, Zhang Y, Wang Q, et al. 6-Gingerol ameliorates ulcerative colitis by inhibiting ferroptosis based on the integrative analysis of plasma metabolomics and network pharmacology[J]. Food Funct, 2024, 15(11): 6054-67. doi:10.1039/d4fo00952e |
| [24] | 邓 婷, 傅 强, 李志樑, 等. 6-姜酚对心肾综合征大鼠心肾功能的影响[J]. 实用医学杂志, 2025, 41(11): 1627-36. |
| [25] | Wadekar PP, Salunkhe VR. Formulation and evaluation of nanobiotherapeutics of Terminalia arjuna through plant tissue culture for atherosclerosis[J]. Future J Pharm Sci, 2024, 10(1): 42. doi:10.1186/s43094-024-00613-5 |
| [26] | Nie R, Yuan Z, Wu Y, et al. Time-dose response and mechanistic specificity of berberine in renal fibrosis from a multi-model integration perspective: a systematic review and meta-analysis on animal models[J]. Front Pharmacol, 2025, 16: 1600408. doi:10.3389/fphar.2025.1600408 |
| [27] | 陈章平, 李信晓, 郭胜楠, 等. GABRG2基因敲除小鼠和HT22细胞通过激活PKA/NF-κB信号通路影响MMP3的表达[J].宁夏医科大学学报, 2022, 44(7): 656-62. |
| [28] | 刘星辰, 王 璟, 刘博阳, 等. PTH缺失通过诱导肾脏细胞衰老和衰老相关分泌表型分子表达而加速肾脏纤维化[J]. 现代生物医学进展,2024, 24(16): 3006-12. |
| [29] | Agrud A, Subburaju S, Goel P, et al. Gabrb3 endothelial cell-specific knockout mice display abnormal blood flow, hypertension, and behavioral dysfunction[J]. Sci Rep, 2022, 12(1): 4922. doi:10.1038/s41598-022-08806-9 |
| [30] | 秦一冰, 吕 静, 战丽彬, 等. 基于“脉络-血管系统”理论探讨肾纤维化的发病机制[J]. 世界中西医结合杂志, 2024, 19(6): 1256-9, 1264. |
| [31] | 张晓珣, 王 俊, 赵 宇, 等. 牛蒡子苷元对四氯化碳致大鼠肝纤维化的治疗作用[J]. 中国药理学与毒理学杂志, 2016, 30(1): 53-60. |
| [32] | García-Llorca A, Carta F, Supuran CT, et al. Carbonic anhydrase, its inhibitors and vascular function[J]. Front Mol Biosci, 2024, 11: 1338528. doi:10.3389/fmolb.2024.1338528 |
| [33] | Krishnan D, Liu L, Wiebe SA, et al. Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3[J]. Am J Physiol Renal Physiol, 2015, 309(4): F383-92. doi:10.1152/ajprenal.00464.2014 |
| [34] | Fang Z, Wang Q, Duan H, et al. 17β-Estradiol mediates TGFBR3/Smad2/3 signaling to attenuate the fibrosis of TGF‑β1-induced bovine endometrial epithelial cells via GPER[J]. J Cell Physiol, 2024, 239(1): 166-79. doi:10.1002/jcp.31153 |
| [35] | Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways[J]. Curr Opin Cell Biol, 1999, 11(2): 211-8. doi:10.1016/s0955-0674(99)80028-3 |
| [36] | Akhtar S, Al-Zaid B, El-Hashim AZ, et al. Impact of PAMAM delivery systems on signal transduction pathways in vivo: Modulation of ERK1/2 and p38 MAP kinase signaling in the normal and diabetic kidney[J]. Int J Pharm, 2016, 514(2): 353-63. doi:10.1016/j.ijpharm.2016.03.039 |
| [37] | Cheng X, Gao W, Dang Y, et al. Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation[J]. J Diabetes Res, 2013, 2013: 463740. doi:10.1155/2013/463740 |
| [38] | Wei X, Wang J, Deng YY, et al. Tubiechong patching promotes Tibia fracture healing in rats by regulating angiogenesis through the VEGF/ERK1/2 signaling pathway[J]. J Ethnopharmacol, 2023, 301: 115851. doi:10.1016/j.jep.2022.115851 |
| [39] | Wu Y, Wang L, Deng D, et al. Renalase protects against renal fibrosis by inhibiting the activation of the ERK signaling pathways[J]. Int J Mol Sci, 2017, 18(5): E855. doi:10.3390/ijms18050855 |
| [40] | Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6[J]. J Biol Chem, 1998, 273(3): 1741-8. doi:10.1074/jbc.273.3.1741 |
| [41] | Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development[J]. Nat Rev Cancer, 2009, 9(8): 537-49. doi:10.1038/nrc2694 |
| [42] | 阮颖新, 刘素雁, 李春媚, 等. p38MAPK通路参与肾间质纤维化的实验研究[J]. 临床泌尿外科杂志, 2007, (1): 63-6. |
| [43] | 霍振霞, 王保兴. p38MAPK在氧化应激诱导肾间质纤维化中的研究进展[J]. 中华临床医师杂志(电子版), 2016, 10(7): 1029-32. |
| [44] | Sari-Ak D, Torres-Gomez A, Yazicioglu YF, et al. Structural, biochemical, and functional properties of the Rap1-interacting adaptor molecule (RIAM)[J]. Biomed J, 2022, 45(2): 289-98. doi:10.1016/j.bj.2021.09.005 |
| [45] | Mochizuki N, Yamashita S, Kurokawa K, et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1[J]. Nature, 2001, 411(6841): 1065-8. doi:10.1038/35082594 |
| [46] | 朱雪婧. Rap1b对糖尿病肾病肾小管上皮细胞氧化损伤及凋亡的影响与机制研究[D]. 中南大学, 2011. |
| [47] | Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress[J]. Nature, 2002, 419(6904): 316-21. doi:10.1038/nature01036 |
| [48] | 李安琪, 祝晓梅, 黄九九, 等. 乳源六肽通过FoxO3a-MnSOD通路减少酒精诱导的氧化应激缓解肝损伤[J]. 安徽医科大学学报, 2022, 57(12): 1864-9. |
| [49] | 龚武清, 彭书生, 杜海林. 基于TNF-α/PI3K-Akt/NF-κB信号通路研究瑞舒伐他汀介导MCP-1对深静脉血栓大鼠模型凝血功能、炎症因子及纤溶指标的影响[J]. 中国老年学杂志, 2025, 45(13): 3272-6. |
| [50] | 李玮怡, 江 露, 张宗星, 等. 强骨康疏方通过抑制HIF-1α/BNIP3自噬信号通路减少类风湿性关节炎大鼠的破骨细胞分化[J]. 南方医科大学学报, 2025, 45(7): 1389-96. |
| [51] | Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure[J]. Adv Drug Deliv Rev, 2016, 97: 4-27. doi:10.1016/j.addr.2015.11.001 |
| [52] | Lv W, Booz GW, Fan F, et al. Oxidative stress and renal fibrosis: recent insights for the development of novel therapeutic strategies[J]. Front Physiol, 2018, 9: 105. doi:10.3389/fphys.2018.00105 |
| [53] | Kuppe C, Ibrahim MM, Kranz J, et al. Decoding myofibroblast origins in human kidney fibrosis[J]. Nature, 2021, 589(7841): 281-6. doi:10.1038/s41586-020-2941-1 |
| [54] | Genovese F, Manresa AA, Leeming DJ, et al. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis[J]? Fibrogenesis Tissue Repair, 2014, 7(1): 4. doi:10.1186/1755-1536-7-4 |
| [55] | Sharma AK, Mauer SM, Kim Y, et al. Interstitial fibrosis in obstructive nephropathy[J]. Kidney Int, 1993, 44(4): 774-88. doi:10.1038/ki.1993.312 |
| [56] | Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome[J]. Nephrol Dial Transplant, 2003, 18(7): 1272-80. doi:10.1093/ndt/gfg074 |
| [57] | Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on oxidative stress in chronic kidney disease[J]. Nephrology: Carlton, 2015, 20(10): 697-705. doi:10.1111/nep.12502 |
| [58] | Zachara BA. Chapter five selenium and selenium-dependent antioxidants in chronic kidney disease[J]. Adv Clin Chem, 2015, 68: 131-51. doi:10.1016/bs.acc.2014.11.006 |
| [59] | Tsikas D. Assessment of lipid peroxidation by measuring malon-dialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges[J]. Anal Biochem, 2017, 524: 13-30. doi:10.1016/j.ab.2016.10.021 |
| [60] | Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update[J]. Front Immunol, 2017, 8: 405. doi:10.3389/fimmu.2017.00405 |
| [61] | Meldrum KK, Misseri R, Metcalfe P, et al. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction[J]. Am J Physiol Regul Integr Comp Physiol, 2007, 292(4): R1456-64. doi:10.1152/ajpregu.00620.2005 |
| [62] | Wittchen ES, Worthylake RA, Kelly P, et al. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function[J]. J Biol Chem, 2005, 280(12): 11675-82. doi:10.1074/jbc.m412595200 |
| [63] | 吴华英, 邓 凯, 李 静, 等. 益气活血方调控cAMP/Epac1/Rap1信号改善冠心病气虚血瘀证大鼠的验证[J]. 中国实验方剂学杂志, 2024,30(18): 107-16. |
| [64] | 沈鑫蕾, 朱清如, 余文凯, 等. 盐炙车前子调控肾小管上皮-间充质转化改善肾纤维化的机制研究[J].中国中药杂志, 2025, 50(5):1195-208. |
| [65] | 郭锦晨. 健脾化湿清热通络法改善类风湿关节炎患者SOD的数据挖掘研究及对AMPK-FoxO3a-MnSOD信号通路的影响[D]. 安徽中医药大学, 2018. |
| [66] | 孙荣嵘, 冯小华, 王婷婷, 等. AKT/FoxO3a通路在白藜芦醇抑制大鼠肾脏间质纤维化中的作用[J]. 药物评价研究, 2019, 42(11):2165-8. |
| [67] | 杨伟源, 李 理, 郑林鑫, 等. EGFR/Foxo3a/Snail1通路在博来霉素肺纤维化小鼠中的作用机制探讨[J]. 中国呼吸与危重监护杂志, 2020,19(4): 371-8. |
| [1] | Jingxian WANG, Zijing REN, Peiyang ZHOU. S1PR5 activation or overexpression enhances barrier function of mouse brain microvascular endothelial cells against OGD/R injury by modulating oxidative stress [J]. Journal of Southern Medical University, 2025, 45(7): 1451-1459. |
| [2] | Anbang ZHANG, Xiuqi SUN, Bo PANG, Yuanhua WU, Jingyu SHI, Ning ZHANG, Tao YE. Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury in rats by inhibiting ferroptosis through the gut-brain axis and the Nrf2/HO-1 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(5): 911-920. |
| [3] | Zhi GAO, Ao WU, Zhongxiang HU, Peiyang SUN. Bioinformatics analysis of oxidative stress and immune infiltration in rheumatoid arthritis [J]. Journal of Southern Medical University, 2025, 45(4): 862-870. |
| [4] | Pengwei HUANG, Jie CHEN, Jinhu ZOU, Xuefeng GAO, Hong CAO. Quercetin mitigates HIV-1 gp120-induced rat astrocyte neurotoxicity via promoting G3BP1 disassembly in stress granules [J]. Journal of Southern Medical University, 2025, 45(2): 304-312. |
| [5] | Zhoufang CAO, Yuan WANG, Mengna WANG, Yue SUN, Feifei LIU. LINC00837/miR-671-5p/SERPINE2 functional axis promotes pathological processes of fibroblast-like synovial cells in rheumatoid arthritis [J]. Journal of Southern Medical University, 2025, 45(2): 371-378. |
| [6] | Shuxian LIN, Lina GUO, Yan MA, Yao XIONG, Yingxi HE, Xinzhu XU, Wen SHENG, Suhua XU, Feng QIU. Lactobacillus plantarum ZG03 alleviates oxidative stress via its metabolites short-chain fatty acids [J]. Journal of Southern Medical University, 2025, 45(10): 2223-2230. |
| [7] | Yuru ZHANG, Lei WAN, Haoxiang FANG, Fangze LI, Liwen WANG, Kefei LI, Peiwen YAN, Hui JIANG. Inhibiting miR-155-5p promotes proliferation of human submandibular gland epithelial cells in primary Sjogren's syndrome by negatively regulating the PI3K/AKT signaling pathway via PIK3R1 [J]. Journal of Southern Medical University, 2025, 45(1): 65-71. |
| [8] | Rong DAI, Zeping CAO, Chuanjiao LIU, Yong GE, Meng CHENG, Weili WANG, Yizhen CHEN, Lei ZHANG, Yiping WANG. Qingshen Granules alleviates renal fibrosis in mice by regulating exosomes, miR-330-3p, and CREBBP expression [J]. Journal of Southern Medical University, 2024, 44(8): 1431-1440. |
| [9] | Yuming ZHANG, Shicheng XIA, Linlin ZHANG, Mengxi CHEN, Xiaojing LIU, Qin GAO, Hongwei YE. Protective effect of Lonicerae japonicae flos extract against doxorubicin-induced liver injury in mice [J]. Journal of Southern Medical University, 2024, 44(8): 1571-1581. |
| [10] | Zhijun REN, Jianxin DIAO, Yiting WANG. Xionggui Decoction alleviates heart failure in mice with myocardial infarction by inhibiting oxidative stress-induced cardiomyocyte apoptosis [J]. Journal of Southern Medical University, 2024, 44(7): 1416-1424. |
| [11] | Guoxin LIANG, Hongyue TANG, Chang GUO, Mingming ZHANG. MiR-224-5p overexpression inhibits oxidative stress by regulating the PI3K/Akt/FoxO1 axis to attenuate hypoxia/reoxygenation-induced cardiomyocyte injury [J]. Journal of Southern Medical University, 2024, 44(6): 1173-1181. |
| [12] | CHEN Guodong, LUO Suxin. Colchicine alleviates myocardial ischemia-reperfusion injury in mice by activating AMPK [J]. Journal of Southern Medical University, 2024, 44(2): 226-235. |
| [13] | LING Xuguang, XU Wenwen, PANG Guanlai, HONG Xuxing, LIU Fengqin, LI Yang. Tea polyphenols ameliorates acute lung injury in septic mice by inhibiting NLRP3 inflammasomes [J]. Journal of Southern Medical University, 2024, 44(2): 381-386. |
| [14] | SUN Xiaopeng, SHI Hang, ZHANG Lei, LIU Zhong, LI Kewei, QIAN Lingling, ZHU Xingyu, YANG Kangjia, FU Qiang, DING Hua. Exosomes from ectoderm mesenchymal stem cells inhibits lipopolysaccharide-induced microglial M1 polarization and promotes survival of H2O2-exposed PC12 cells by suppressing inflammatory response and oxidative stress [J]. Journal of Southern Medical University, 2024, 44(1): 119-128. |
| [15] | YE Hongwei, ZHANG Yuming, YUN Qi, DU Ruoli, LI Lu, LI Yuping, GAO Qin. Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression [J]. Journal of Southern Medical University, 2024, 44(1): 45-51. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||