Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (11): 2209-2219.doi: 10.12122/j.issn.1673-4254.2024.11.18
Previous Articles Next Articles
Ruoli DU1,2( ), Qi YUN2,3, Yiren WANG1,2, Xinyu DOU4, Hongwei YE1,2, Jiahui WANG2, Qin GAO1,2(
), Qi YUN2,3, Yiren WANG1,2, Xinyu DOU4, Hongwei YE1,2, Jiahui WANG2, Qin GAO1,2( )
)
Received:2024-08-10
Online:2024-11-20
Published:2024-11-29
Contact:
Qin GAO
E-mail:hello1112drl@126.com;bbmcgq@126.com
Ruoli DU, Qi YUN, Yiren WANG, Xinyu DOU, Hongwei YE, Jiahui WANG, Qin GAO. Plumbagin protect against sepsis-induced myocardial injury in mice by inhibiting the JAK2/STAT3 signaling pathway to reduce cardiomyocyte pyroptosis[J]. Journal of Southern Medical University, 2024, 44(11): 2209-2219.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.11.18
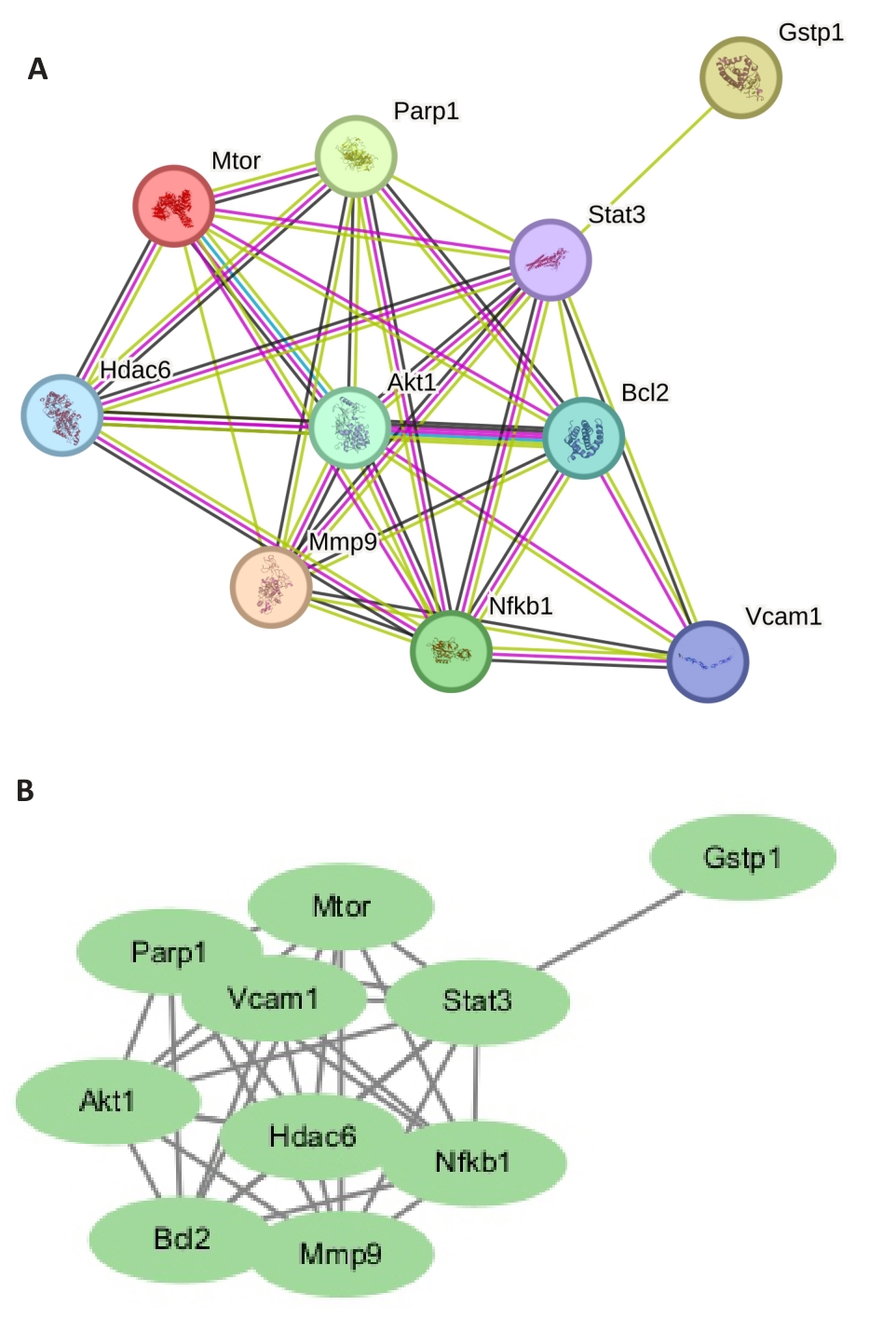
Fig.2 Core protein-protein interaction (PPI) network. A: Visual regulatory network of plumbagin-sepsis myocardial injury-pyroptosis. B: PPI network of plumbagin-sepsis myocardial injury-pyroptosis related targets.
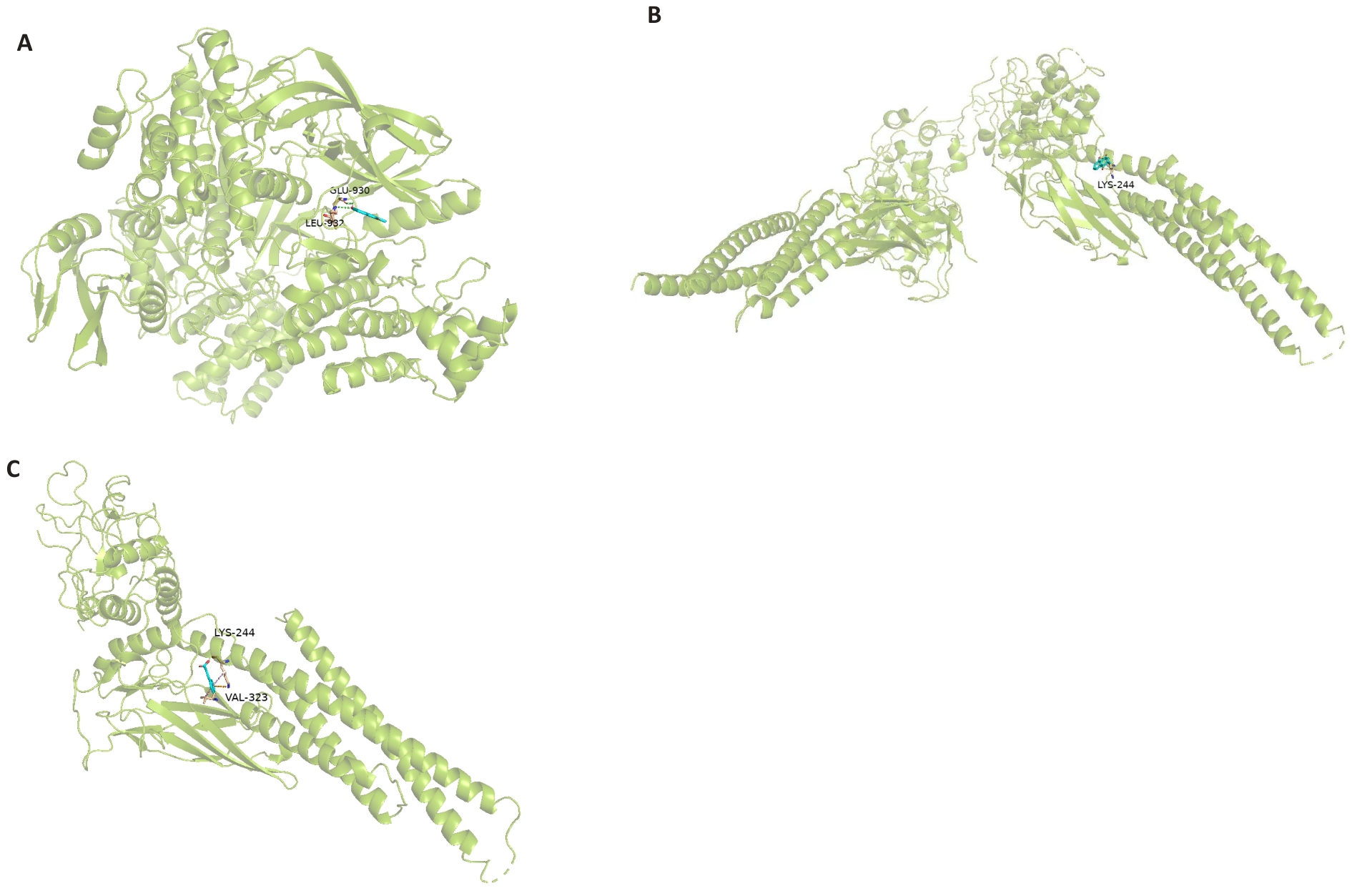
Fig.5 Molecular docking results. A: Molecular docking diagram of plumbagin and STAT3. B: Molecular docking diagram of plumbagin and JAK2. C: Molecular docking diagram of plumbagin and p-STAT3.
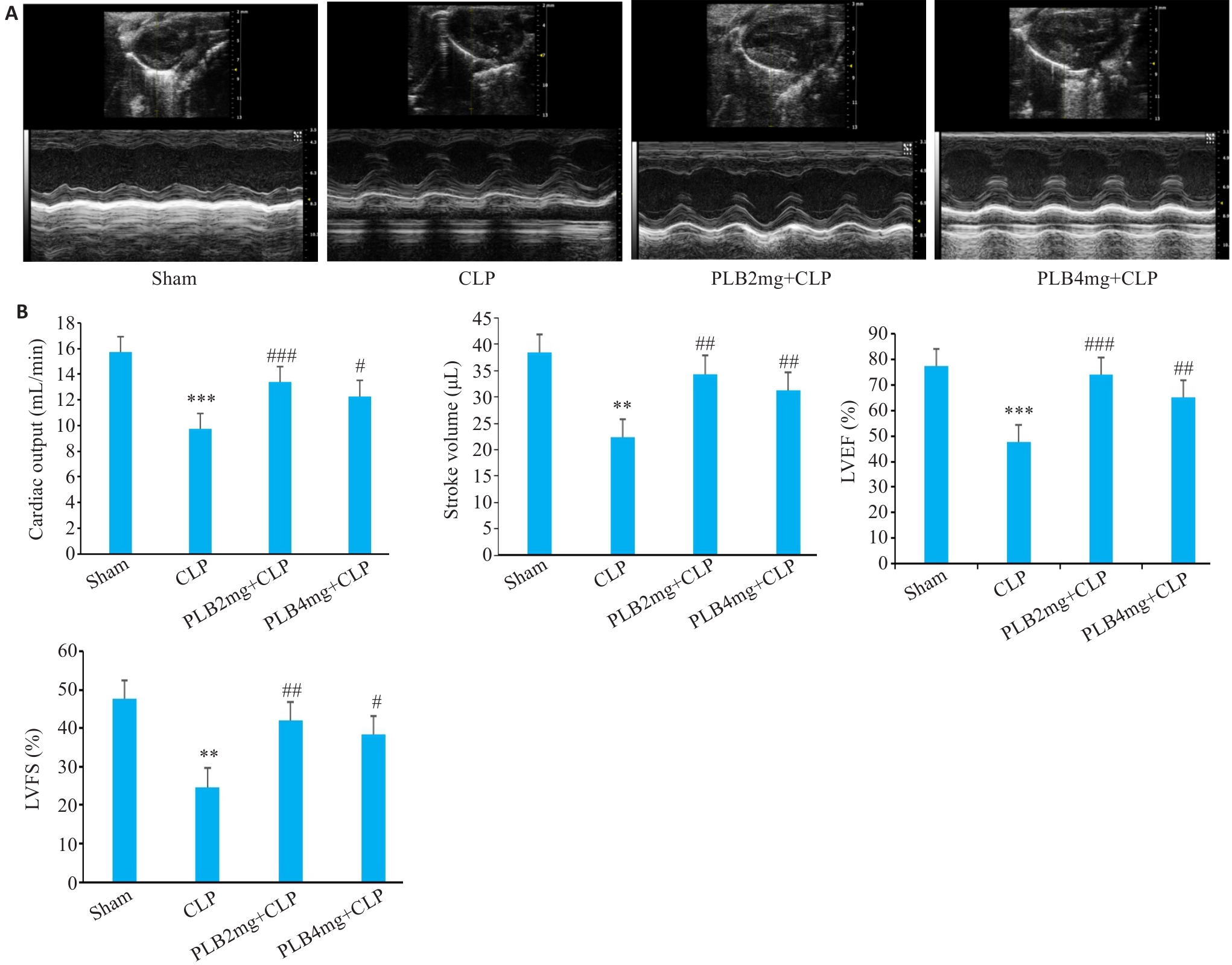
Fig.6 Cardiac ultrasound images and cardiac function indexes of the mice in different groups (Mean±SD, n=6). A: Evaluation of cardiac function by M-mode echocardiography. B: Comparison of the echocardiographic parameters (LVEF: Left ventricle ejection fraction; LVFS: Left ventricular fractional shortening). **P<0.01, ***P<0.001 vs Sham group, #P<0.05, ##P<0.01, ###P<0.001 vs CLP group.
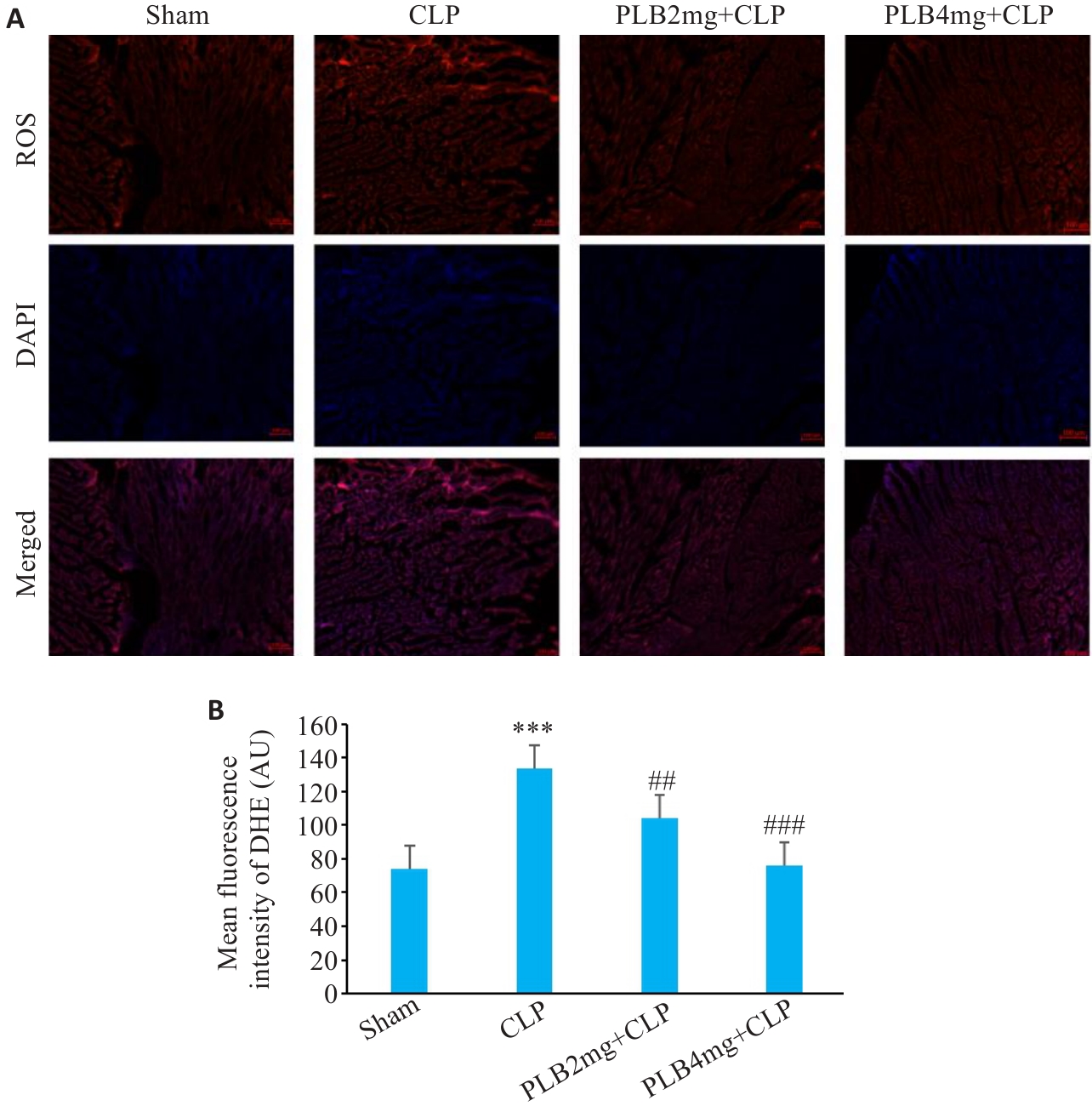
Fig.8 DHE fluorescence staining of myocardial fibers in different groups. A: Fluorescence probe for ROS in the cardiac tissues (Original magnification: ×200). B: ROS fluorescence intensity in each group (Mean±SD, n=6). ***P<0.001 vs sham group; ##P<0.01, ###P<0.001 vs the CLP group.

Fig.9 Serum IL-18 (A) and IL-1β (B) levels in the myocardial tissue in different groups (Mean±SD, n=6). ***P<0.001 vs sham group; #P<0.05 vs CLP group.

Fig.10 Serum MDA (A), CK-MB (B) and LDH (C) levels in the myocardial tissue in different groups (Mean±SD, n=6). ***P<0.001 vs sham group; #P<0.05, ##P<0.01 ###P<0.001 vs CLP group.
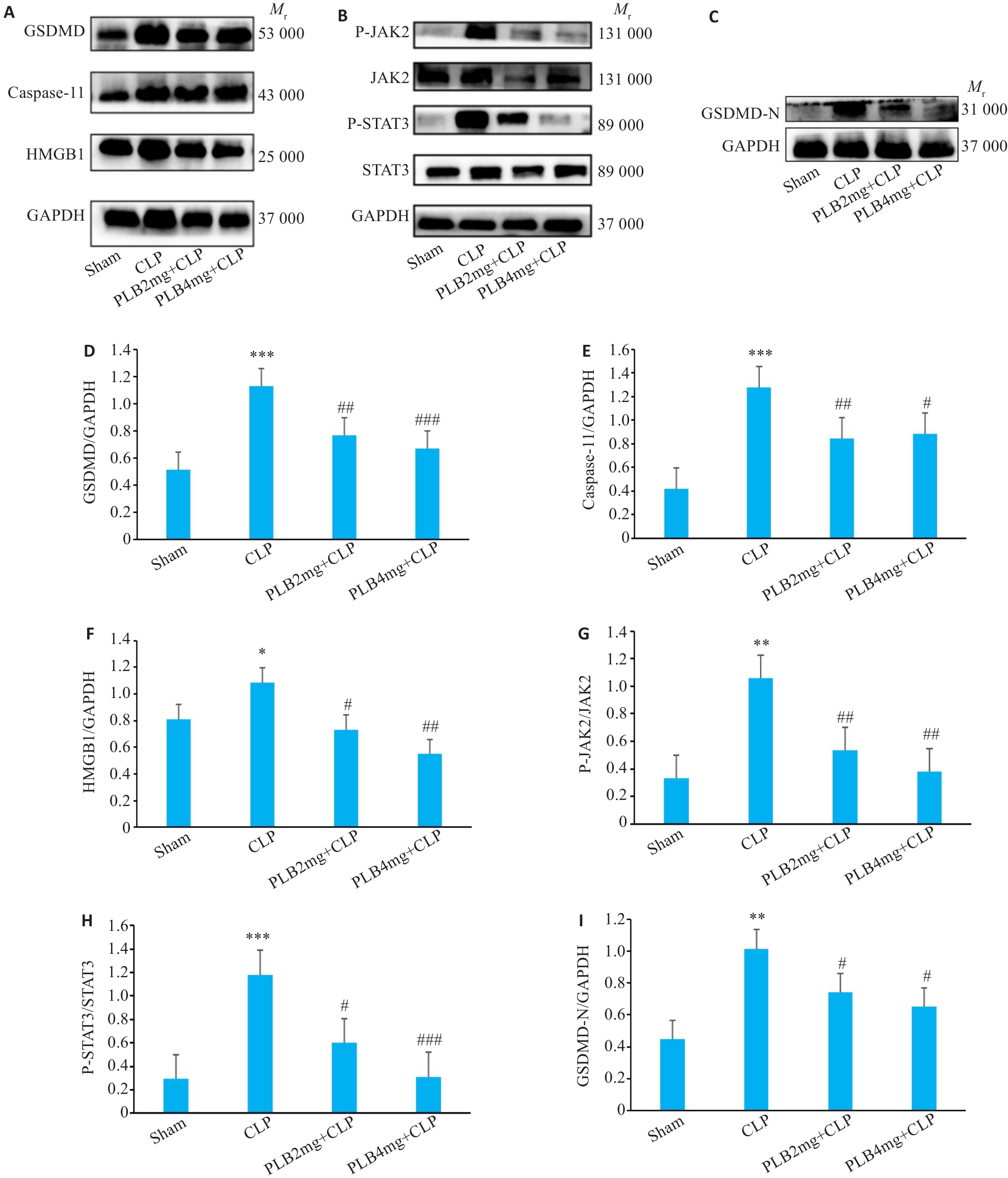
Fig.11 Comparison of GSDMD、Caspase-11、HMGB1、P-JAK2、JAK2、P-STAT3、STAT3 and GSDMD-N protein levels in the myocardial tissues among the 4 groups. A-C: Protein bands in Western blotting. D-I: Relative protein levels of GSDMD、Caspase-11、HMGB1、P-JAK2、JAK2、P-STAT3、STAT3 and GSDMD-N proteins (Mean±SD, n=3). *P<0.05, **P<0.01, ***P<0.001 vs sham group; #P<0.05, ##P<0.01, ###P<0.001 vs CLP group.
| 1 | Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 775-87. |
| 2 | Li Z, Wu BQ, Chen J, et al. WWP2 protects against sepsis-induced cardiac injury through inhibiting cardiomyocyte ferroptosis[J]. J Transl Int Med, 2024, 12(1): 35-50. |
| 3 | Zhao XJ, Xie JG, Duan CJ, et al. ADAR1 protects pulmonary macrophages from sepsis-induced pyroptosis and lung injury through miR-21/A20 signaling[J]. Int J Biol Sci, 2024, 20(2): 464-85. |
| 4 | Liu ZJ, Wei JH, Sun HB, et al. Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways[J]. J Cell Mol Med, 2024, 28(13): e18386. |
| 5 | Zhang QR, Fu HT, Gong WJ, et al. Plumbagin protects H9c2 cardiomyocytes against TBHP-induced cytotoxicity by alleviating ROS-induced apoptosis and modulating autophagy[J]. Exp Ther Med, 2022, 24(2): 501. |
| 6 | Li Z, Chinnathambi A, Ali Alharbi S, et al. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats[J]. Environ Toxicol, 2020, 35(12): 1374-85. |
| 7 | Petrocelli G, Marrazzo P, Bonsi L, et al. Plumbagin, a natural compound with several biological effects and anti-inflammatory properties[J]. Life, 2023, 13(6): 1303. |
| 8 | Wang SX, Wang J, Shao JB, et al. Plumbagin mediates cardioprotection against myocardial ischemia/reperfusion injury through nrf-2 signaling[J]. Med Sci Monit, 2016, 22: 1250-7. |
| 9 | Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula[J]. J Ethnopharmacol, 2023, 309: 116306. |
| 10 | Zhang ZX, Deng WJ, Kang R, et al. Plumbagin protects mice from lethal sepsis by modulating immunometabolism upstream of PKM2[J]. Mol Med, 2016, 22: 162-72. |
| 11 | Huang YH, Li L, Li YP, et al. Knockdown of LncRNA Lcn2-204 alleviates sepsis-induced myocardial injury by regulation of iron overload and ferroptosis[J]. J Mol Cell Cardiol, 2024, 192: 79-93. |
| 12 | Wang J, Wang XT, Liu DW, et al. Induction and deduction in sepsis-induced cardiomyopathy: five typical categories[J]. Chin Med J, 2020, 133(18): 2205-11. |
| 13 | Kumar Arora M, Ratra A, Asdaq SMB, et al. Plumbagin alleviates intracerebroventricular-quinolinic acid induced depression-like behavior and memory deficits in wistar rats[J]. Molecules, 2022, 27(6): 1834. |
| 14 | Guo YX, Liu L, Yan DZ, et al. Plumbagin prevents osteoarthritis in human chondrocytes through Nrf-2 activation[J]. Mol Med Rep, 2017, 15(4): 2333-8. |
| 15 | Monnet X, Lai C, Teboul JL. How I personalize fluid therapy in septic shock[J]? Crit Care,2023,27(1):123. |
| 16 | Vigneron C, Py BF, Monneret G, et al. The double sides of NLRP3 inflammasome activation in sepsis[J]. Clin Sci, 2023, 137(5): 333-51. |
| 17 | Yang R, Zhang XJ. A potential new pathway for heparin treatment of sepsis-induced lung injury: inhibition of pulmonary endothelial cell pyroptosis by blocking hMGB1-LPS-induced caspase-11 activation[J]. Front Cell Infect Microbiol, 2022, 12: 984835. |
| 18 | Sheng SY, Li JM, Hu XY, et al. Regulated cell death pathways in cardiomyopathy[J]. Acta Pharmacol Sin, 2023, 44(8): 1521-35. |
| 19 | Wu ZF, Deng JH, Zhou HW, et al. Programmed cell death in sepsis associated acute kidney injury[J]. Front Med, 2022, 9: 883028. |
| 20 | Cao ZZ, Qin HQ, Huang YH, et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model[J]. Bioengineered, 2022, 13(3): 4810-20. |
| 21 | Zangiabadi S, Abdul-Sater AA. Regulation of the NLRP3 inflammasome by posttranslational modifications[J]. J Immunol, 2022, 208(2): 286-92. |
| 22 | Jia YJ, Xiong S, Yao M, et al. HMGB1 inhibition blocks ferroptosis and oxidative stress to ameliorate sepsis-induced acute lung injury by activating the Nrf2 pathway[J]. Kaohsiung J Med Sci, 2024, 40(8): 710-21. |
| 23 | 梁 欢, 黄毓慧, 高 琴.非经典途径细胞焦亡在脓毒症等炎症性疾病中的作用[J].中南大学学报(医学版), 2021, 46(11): 1276-84. |
| 24 | Li X, Wei SZ, Niu SQ, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis[J]. Comput Biol Med, 2022, 144: 105389. |
| 25 | Yuan ZZ, Pan YY, Leng T, et al. Progress and prospects of research ideas and methods in the network pharmacology of traditional Chinese medicine[J]. J Pharm Pharm Sci, 2022, 25: 218-26. |
| 26 | Li YR, Jia YJ, Cui TF, et al. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac[J]. Exp Ther Med, 2021, 22(2): 890. |
| 27 | Foers AD, Garnham AL, Chatfield S, et al. Extracellular vesicles in synovial fluid from rheumatoid arthritis patients contain miRNAs with capacity to modulate inflammation[J]. Int J Mol Sci, 2021, 22(9): 4910. |
| 28 | Wang C, Liu N, Yang HT. Desflurane pretreatment can reduce sepsis-evoked lung injury in rats via inhibiting STAT3 pathway[J]. J Biol Regul Homeost Agents, 2020, 34(3): 935-42. |
| 29 | Jiang T, Peng DW, Shi W, et al. IL-6/STAT3 signaling promotes cardiac dysfunction by upregulating FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes formation in sepsis mice[J]. Front Cardiovasc Med, 2022, 8: 790612. |
| 30 | Li RQ, Li XQ, Zhao J, et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation[J]. Theranostics, 2022, 12(2): 976-98. |
| 31 | Shen YN, Zhang Y, Du JY, et al. CXCR5 down-regulation alleviates cognitive dysfunction in a mouse model of sepsis-associated encephalopathy: potential role of microglial autophagy and the p38MAPK/NF‑κB/STAT3 signaling pathway[J]. J Neuroinfl-ammation, 2021, 18(1): 246. |
| 32 | Xie LP, Wu YT, Zhou CY, et al. Piceatannol protects against sepsis-induced myocardial dysfunction via direct inhibition of JAK2[J]. Int Immunopharmacol, 2021, 96: 107639. |
| 33 | Zhen GS, Liang W, Jia HM, et al. Melatonin relieves sepsis-induced myocardial injury via regulating JAK2/STAT3 signaling pathway[J]. Minerva Med, 2022, 113(6): 983-9. |
| 34 | 孙 永, 史兆博, 刘美香, 等. 山药多糖对脓毒症大鼠心肌损伤及JAK2/STAT3信号通路的影响[J]. 中国动脉硬化杂志, 2022, 30(8): 669-75. |
| 35 | Jiang JX, Zhang D, Liu W, et al. Overexpression of NLRP12 enhances macrophage immune response and alleviates herpes simplex keratitis[J]. Front Cell Infect Microbiol, 2024, 14: 1416105. |
| [1] | Xinyuan CHEN, Chengting WU, Ruidi LI, Xueqin PAN, Yaodan ZHANG, Junyu TAO, Caizhi LIN. Shuangshu Decoction inhibits growth of gastric cancer cell xenografts by promoting cell ferroptosis via the P53/SLC7A11/GPX4 axis [J]. Journal of Southern Medical University, 2025, 45(7): 1363-1371. |
| [2] | Haiyi ZHOU, Siyi HE, Ruifang HAN, Yongge GUAN, Lijuan DONG, Yang SONG. Moxibustion promotes endometrial repair in rats with thin endometrium by inhibiting the NLRP3/pyroptosis axis via upregulating miR-223-3p [J]. Journal of Southern Medical University, 2025, 45(7): 1380-1388. |
| [3] | Liming WANG, Hongrui CHEN, Yan DU, Peng ZHAO, Yujie WANG, Yange TIAN, Xinguang LIU, Jiansheng LI. Yiqi Zishen Formula ameliorates inflammation in mice with chronic obstructive pulmonary disease by inhibiting the PI3K/Akt/NF-κB signaling pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1409-1422. |
| [4] | Yinfu ZHU, Yiran LI, Yi WANG, Yinger HUANG, Kunxiang GONG, Wenbo HAO, Lingling SUN. Therapeutic mechanism of hederagenin, an active component in Guizhi Fuling Pellets, against cervical cancer in nude mice [J]. Journal of Southern Medical University, 2025, 45(7): 1423-1433. |
| [5] | Lijun HE, Xiaofei CHEN, Chenxin YAN, Lin SHI. Inhibitory effect of Fuzheng Huaji Decoction against non-small cell lung cancer cells in vitro and the possible molecular mechanism [J]. Journal of Southern Medical University, 2025, 45(6): 1143-1152. |
| [6] | Guoyong LI, Renling LI, Yiting LIU, Hongxia KE, Jing LI, Xinhua WANG. Therapeutic mechanism of Arctium lappa extract for post-viral pneumonia pulmonary fibrosis: a metabolomics, network pharmacology analysis and experimental verification [J]. Journal of Southern Medical University, 2025, 45(6): 1185-1199. |
| [7] | Liping GUAN, Yan YAN, Xinyi LU, Zhifeng LI, Hui GAO, Dong CAO, Chenxi HOU, Jingyu ZENG, Xinyi LI, Yang ZHAO, Junjie WANG, Huilong FANG. Compound Centella asiatica formula alleviates Schistosoma japonicum-induced liver fibrosis in mice by inhibiting the inflammation-fibrosis cascade via regulating the TLR4/MyD88 pathway [J]. Journal of Southern Medical University, 2025, 45(6): 1307-1316. |
| [8] | Peipei TANG, Yong TAN, Yanyun YIN, Xiaowei NIE, Jingyu HUANG, Wenting ZUO, Yuling LI. Tiaozhou Ziyin recipe for treatment of premature ovarian insufficiency: efficacy, safety and mechanism [J]. Journal of Southern Medical University, 2025, 45(5): 929-941. |
| [9] | Fenlan BIAN, Shiyao NI, Peng ZHAO, Maonanxing QI, Bi TANG, Hongju WANG, Pinfang KANG, Jinjun LIU. Asiaticoside alleviates myocardial ischemia-reperfusion injury in rats by inhibiting NLRP3 inflammasome-mediated pyroptosis [J]. Journal of Southern Medical University, 2025, 45(5): 977-985. |
| [10] | Xiaotao LIANG, Yifan XIONG, Xueqi LIU, Xiaoshan LIANG, Xiaoyu ZHU, Wei XIE. Huoxue Shufeng Granule alleviates central sensitization in chronic migraine mice via TLR4/NF-κB inflammatory pathway [J]. Journal of Southern Medical University, 2025, 45(5): 986-994. |
| [11] | Yalei SUN, Meng LUO, Changsheng GUO, Jing GAO, Kaiqi SU, Lidian CHEN, Xiaodong FENG. Amentoflavone alleviates acute lung injury in mice by inhibiting cell pyroptosis [J]. Journal of Southern Medical University, 2025, 45(4): 692-701. |
| [12] | Zhengwang ZHU, Linlin WANG, Jinghan ZHAO, Ruixue MA, Yuchun YU, Qingchun CAI, Bing WANG, Pingsheng ZHU, Mingsan MIAO. Tuihuang Mixture improves α‑naphthylisothiocyanate-induced cholestasis in rats by inhibiting NLRP3 inflammasomes via regulating farnesoid X receptor [J]. Journal of Southern Medical University, 2025, 45(4): 718-724. |
| [13] | Niandong RAN, Jie LIU, Jian XU, Yongping ZHANG, Jiangtao GUO. n-butanol fraction of ethanol extract of Periploca forrestii Schltr.: its active components, targets and pathways for treating Alcheimer's disease in rats [J]. Journal of Southern Medical University, 2025, 45(4): 785-798. |
| [14] | Ju HUANG, Lixia YIN, Minzhu NIU, Zhijun GENG, Lugen ZUO, Jing LI, Jianguo HU. Nodakenin ameliorates TNBS-induced experimental colitis in mice by inhibiting pyroptosis of intestinal epithelial cells [J]. Journal of Southern Medical University, 2025, 45(2): 261-268. |
| [15] | Haonan¹ XU, Fang³ ZHANG, Yuying² HUANG, Qisheng⁴ YAO, Yueqin⁴ GUAN, Hao CHEN. Thesium chinense Turcz. alleviates antibiotic-associated diarrhea in mice by modulating gut microbiota structure and regulating the EGFR/PI3K/Akt signaling pathway [J]. Journal of Southern Medical University, 2025, 45(2): 285-295. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||