Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (10): 2055-2061.doi: 10.12122/j.issn.1673-4254.2025.10.01
Yihan WANG1( ), Weiqing ZHANG2, Ting FANG2, Zhimin XIE1, Yongsheng FAN1, Xinchang WANG1(
), Weiqing ZHANG2, Ting FANG2, Zhimin XIE1, Yongsheng FAN1, Xinchang WANG1( )
)
Received:2025-04-28
Online:2025-10-20
Published:2025-10-24
Contact:
Xinchang WANG
E-mail:568398437@qq.com;ossani@126.com
Supported by:Yihan WANG, Weiqing ZHANG, Ting FANG, Zhimin XIE, Yongsheng FAN, Xinchang WANG. Elevated expressions of GRP78/CHOP in lupus nephritis: their diagnostic value and association with PERK/IRE1α pathway-mediated renal cell apoptosis[J]. Journal of Southern Medical University, 2025, 45(10): 2055-2061.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.10.01
| Item | LN (n=60) | SLE (n=35) | P |
|---|---|---|---|
| Age (year, Mean±SD) | 41.31±2.08 | 38.24±2.73 | 0.28 |
| Female [n (%)] | 56 (93.33%) | 33 (94.29%) | 0.85 |
| BMI (kg/m2) | 21.84±0.60 | 22.99±0.98 | 0.35 |
| SLEDAI | 8.14±0.74 | 5.25±0.58 | 0.05 |
| Illness duration (year) | 8.18±1.33 | 8.90±1.90 | 0.57 |
| 24 h UTP (g/d) | 1192.82±243.66 | 119.17±24.58 | 0.00 |
| Anti-dsDNA[+(%)] | 24 (40%) | 11 (31.43%) | 0.40 |
| Anti-Sm[+(%)] | 13 (21.67%) | 8 (22.86%) | 0.89 |
| Hb (g/L) | 115.25±3.04 | 123.04±5.66 | 0.10 |
| PLT (109/L) | 188.19±10.36 | 213.90±13.08 | 0.31 |
| WBC (1012/L) | 5.81±0.50 | 5.68±0.49 | 0.63 |
| C3 (g/L) | 0.70±0.04 | 0.74±0.04 | 0.80 |
| C4 (g/L) | 0.14±0.02 | 0.12±0.02 | 0.20 |
Tab.1 Comparison of general clinical data between the patients in LN group and SLE group
| Item | LN (n=60) | SLE (n=35) | P |
|---|---|---|---|
| Age (year, Mean±SD) | 41.31±2.08 | 38.24±2.73 | 0.28 |
| Female [n (%)] | 56 (93.33%) | 33 (94.29%) | 0.85 |
| BMI (kg/m2) | 21.84±0.60 | 22.99±0.98 | 0.35 |
| SLEDAI | 8.14±0.74 | 5.25±0.58 | 0.05 |
| Illness duration (year) | 8.18±1.33 | 8.90±1.90 | 0.57 |
| 24 h UTP (g/d) | 1192.82±243.66 | 119.17±24.58 | 0.00 |
| Anti-dsDNA[+(%)] | 24 (40%) | 11 (31.43%) | 0.40 |
| Anti-Sm[+(%)] | 13 (21.67%) | 8 (22.86%) | 0.89 |
| Hb (g/L) | 115.25±3.04 | 123.04±5.66 | 0.10 |
| PLT (109/L) | 188.19±10.36 | 213.90±13.08 | 0.31 |
| WBC (1012/L) | 5.81±0.50 | 5.68±0.49 | 0.63 |
| C3 (g/L) | 0.70±0.04 | 0.74±0.04 | 0.80 |
| C4 (g/L) | 0.14±0.02 | 0.12±0.02 | 0.20 |
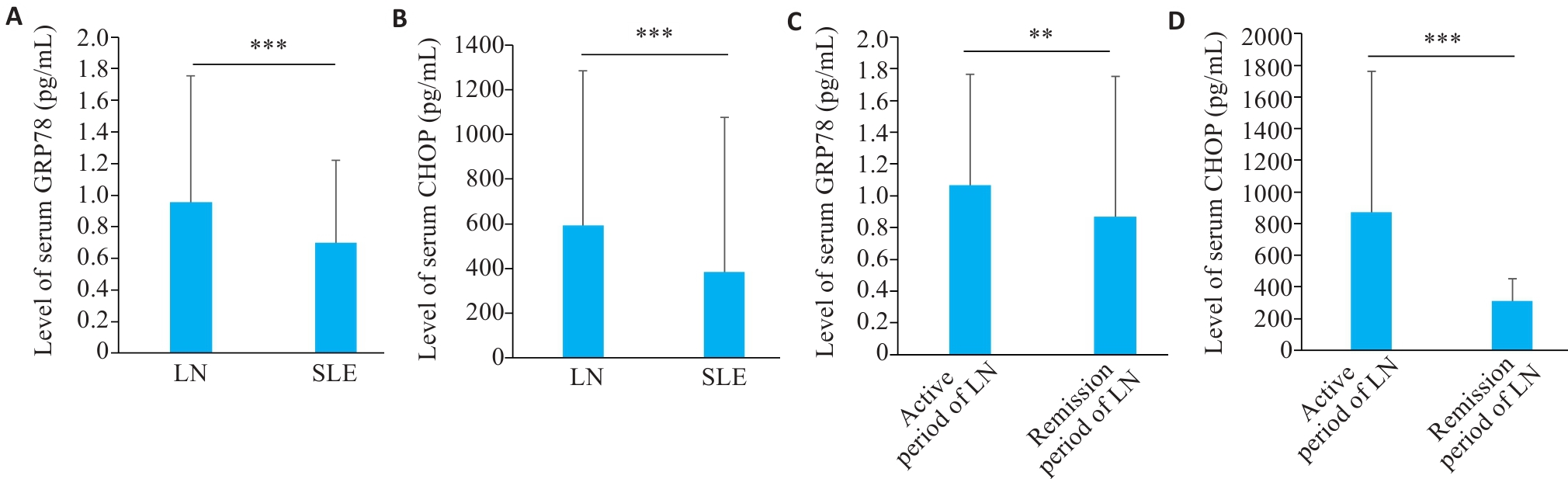
Fig.1 Level of serum GRP78 and CHOP in LN and SLE patients. A: Serum level of GRP78 in LN and SLE patients. B: Serum level of CHOP in LN and SLE patients. C: Serum level of GRP78 in LN patients in active and remission phase. D: Serum level of CHOP in LN patients in active and remission phase. **P<0.01, ***P<0.001.
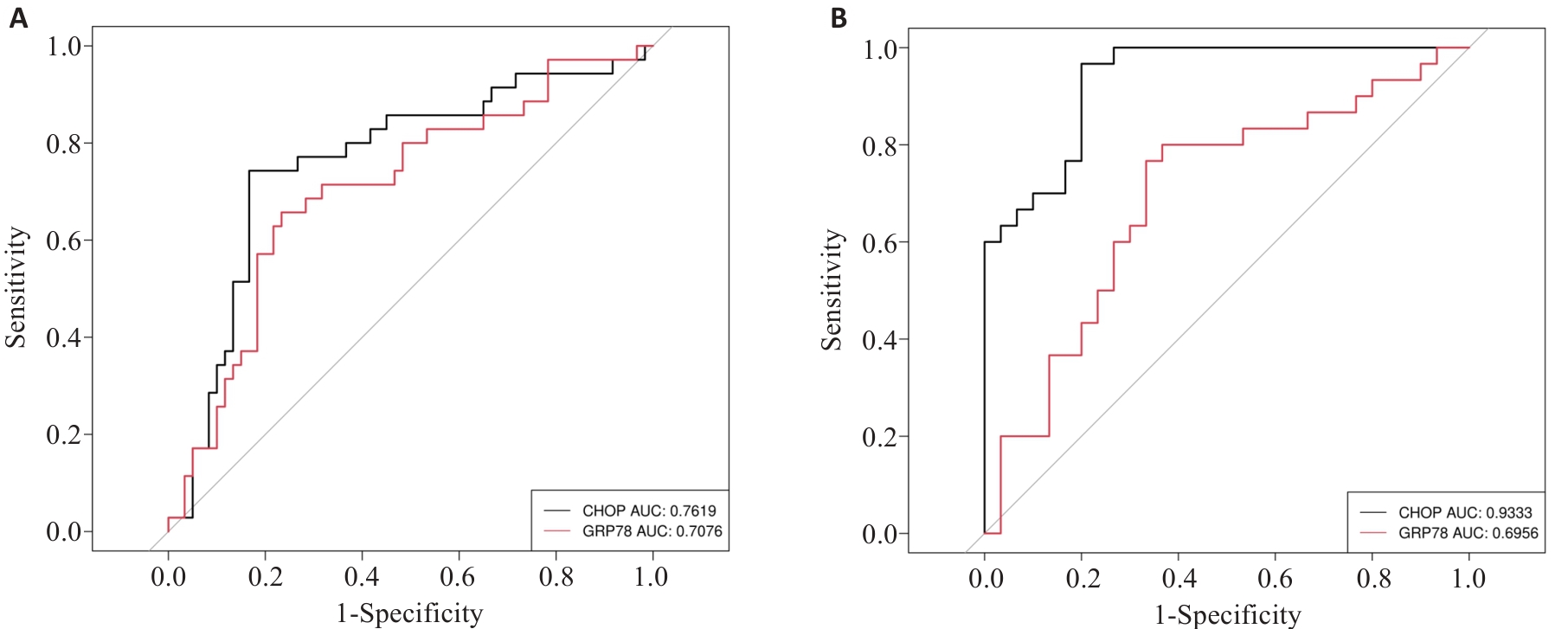
Fig.3 Diagnostic capabilities of GRP78 and CHOP for LN. A: ROC curves of peripheral blood GRP78 and CHOP for diagnosing LN. B: ROC curves of GRP78 and CHOP for diagnosis of LN in the active phase.
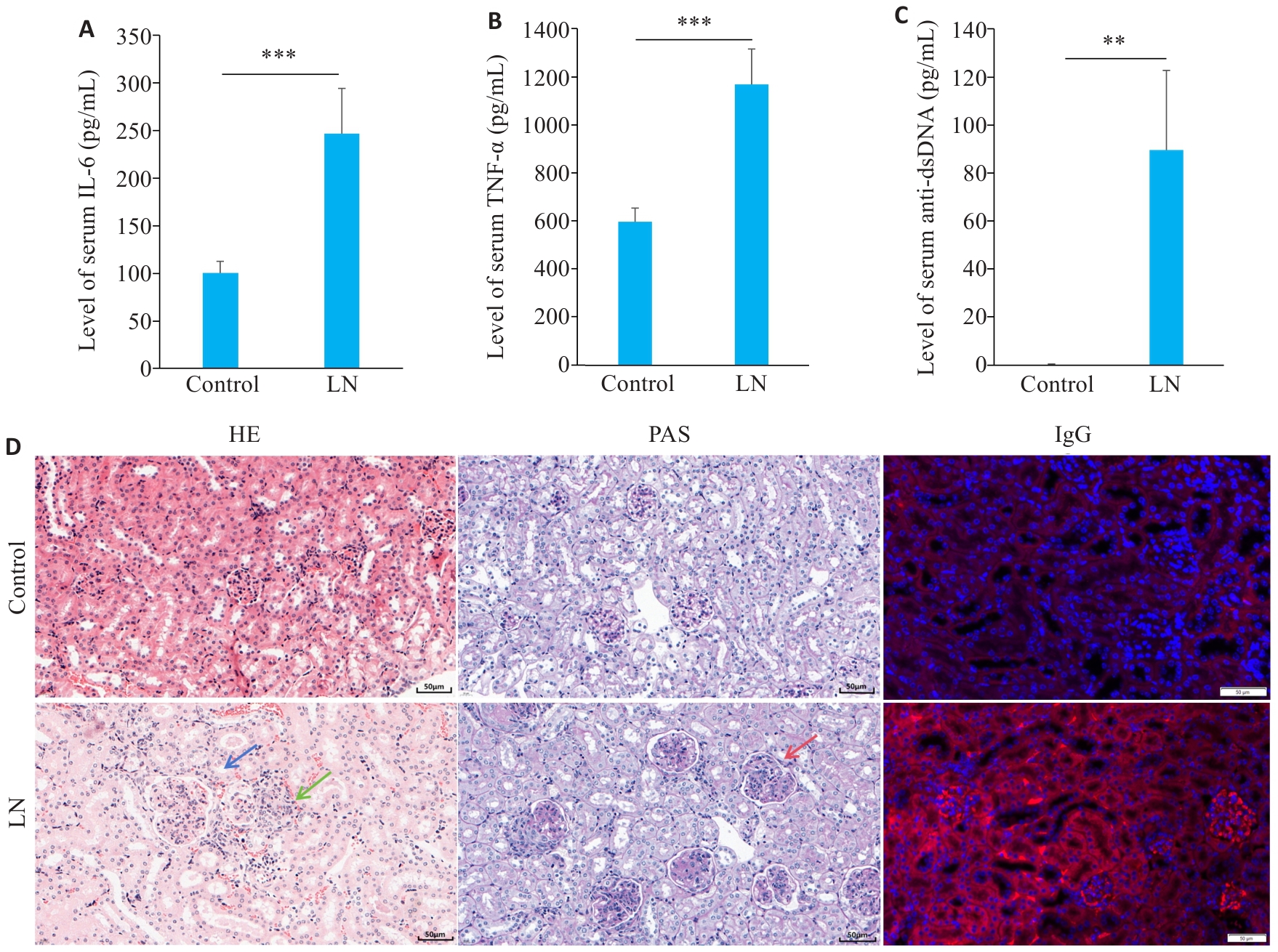
Fig.4 Disease characteristics in the mouse models of LN. A: Serum level of IL-6 in LN and control groups. B: Serum level of TNF-α in LN and control groups. C: Serum level of anti-dsDNA in LN and control groups. D: Renal HE staining, PAS staining and IgG staining showing mesangial cell proliferation (blue arrow), lymphocytes infiltration (green arrow), and basement membrane thickening (red arrow) (scale bar=50 μm). **P<0.01, ***P<0.001.
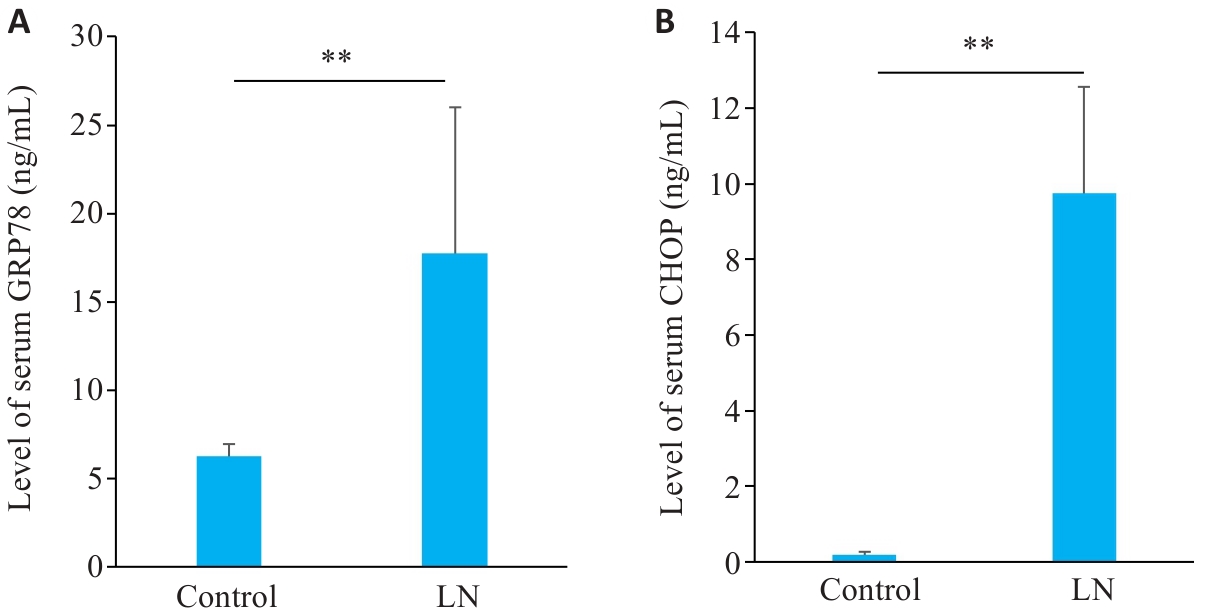
Fig.5 Serum levels of GRP78 and CHOP in mouse models of LN. A: Serum levels of GRP78 in LN and control groups. B: Serum levels of CHOP in LN and control groups. **P<0.01.
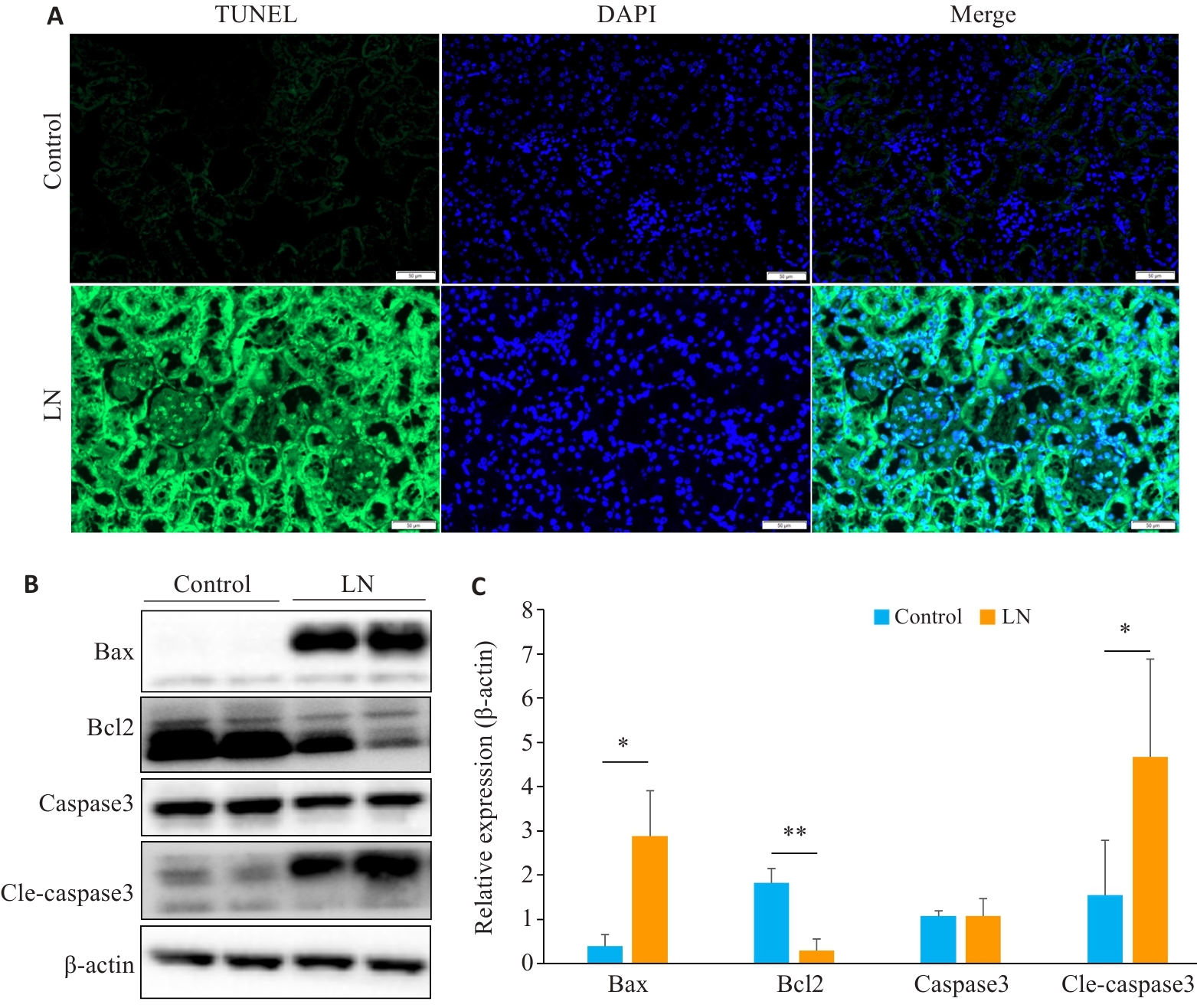
Fig.6 Renal apoptosis is increased in mouse models of LN. A: Kidney TUNEL staining in LN and control groups (scale bar=50 μm). B: Expression of apoptosis-related proteins in LN and control groups. C: Relative expression levels of apoptosis-related proteins in the two groups. *P<0.05, **P<0.01.
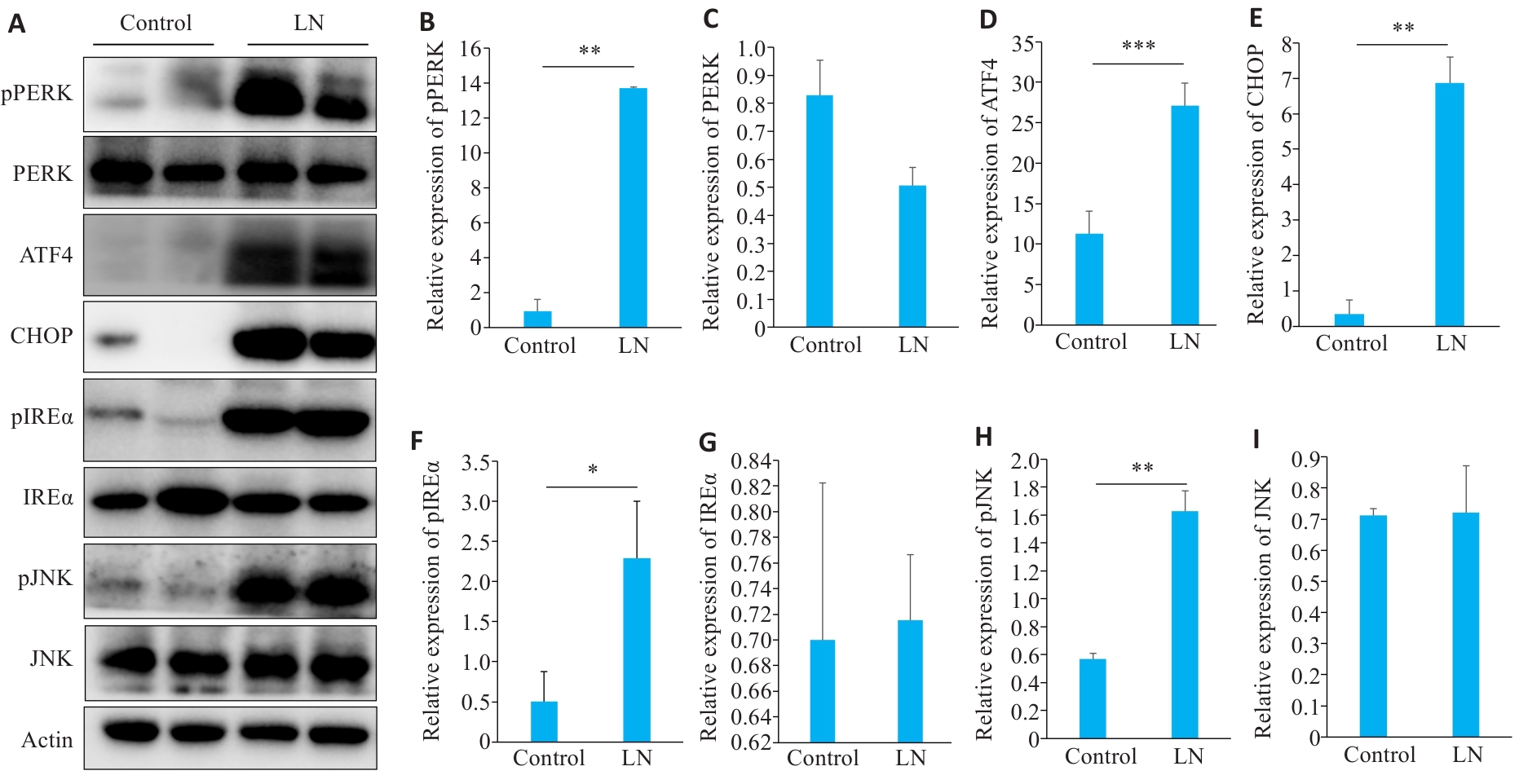
Fig.7 Expressions of proteins related to endoplasmic reticulum stress apoptosis in the kidney of LN mice. A: Protein bands in Western blotting of endoplasmic reticulum stress-related proteins. B-I: Relative PERK phosphorylation level and relative expression levels of PERK, ATF4, CHOP, IREα, IREα, phosphorylated JNK, and JNK, respectively. *P<0.05, **P<0.01, ***P<0.001.
| [1] | Siegel CH, Sammaritano LR. Systemic lupus erythematosus: a review[J]. JAMA, 2024, 331(17): 1480-91. doi:10.1001/jama.2024.2315 |
| [2] | Gasparotto M, Gatto M, Binda V, et al. Lupus nephritis: clinical presentations and outcomes in the 21st century[J]. Rheumatology (Oxford), 2020, 59(): v39-51. doi:10.1093/rheumatology/keaa381 |
| [3] | Anders HJ, Saxena R, Zhao M-H, et al. Lupus nephritis[J]. Nat Rev Dis Primers, 2020, 6: 7. doi:10.1038/s41572-019-0141-9 |
| [4] | Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease[J]. Nat Rev Drug Discov, 2022, 21(2): 115-40. doi:10.1038/s41573-021-00320-3 |
| [5] | Celik C, Lee SYT, Yap WS, et al. Endoplasmic reticulum stress and lipids in health and diseases[J]. Prog Lipid Res, 2023, 89: 101198. doi:10.1016/j.plipres.2022.101198 |
| [6] | Ke H, Su XZ, Dong CT, et al. Sigma-1 receptor exerts protective effects on ameliorating nephrolithiasis by modulating endoplasmic reticulum-mitochondrion association and inhibiting endoplasmic reticulum stress-induced apoptosis in renal tubular epithelial cells[J]. Redox Rep, 2024, 29(1): 2391139. doi:10.1080/13510002.2024.2391139 |
| [7] | Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases[J]. Nat Rev Nephrol, 2017, 13(11): 681-96. doi:10.1038/nrneph.2017.129 |
| [8] | Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: a cell’s response to stress[J]. Life Sci, 2019, 226: 156-63. doi:10.1016/j.lfs.2019.04.022 |
| [9] | Akinyemi AO, Simpson KE, Oyelere SF, et al. Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: a systematic review[J]. Mol Med, 2023, 29(1): 112. doi:10.1186/s10020-023-00706-6 |
| [10] | Xu ZH, Bu YW, Chitnis N, et al. miR-216b regulation of c-Jun mediates GADD153/CHOP-dependent apoptosis[J]. Nat Commun, 2016, 7: 11422. doi:10.1038/ncomms11422 |
| [11] | Gong QM, Lai TF, Liang LD, et al. Targeted inhibition of CX3CL1 limits podocytes ferroptosis to ameliorate cisplatin-induced acute kidney injury[J]. Mol Med, 2023, 29(1): 140. doi:10.1186/s10020-023-00733-3 |
| [12] | Sun MM, Wang FQ, Li HP, et al. Maresin-1 attenuates sepsis-associated acute kidney injury via suppressing inflammation, endoplasmic reticulum stress and pyroptosis by activating the AMPK/SIRT3 pathway[J]. J Inflamm Res, 2024, 17: 1349-64. doi:10.2147/jir.s442729 |
| [13] | Andrade-Silva M, Dhillon P, Sanchez-Navarro A, et al. The critical role of endoplasmic reticulum stress and the stimulator of interferon genes (STING) pathway in kidney fibrosis[J]. Kidney Int, 2025, 107(2): 302-16. doi:10.1016/j.kint.2024.10.021 |
| [14] | Li HY, Huang LF, Huang XR, et al. Endoplasmic reticulum stress in systemic lupus erythematosus and lupus nephritis: potential therapeutic target[J]. J Immunol Res, 2023, 2023: 7625817. doi:10.1155/2023/7625817 |
| [15] | Yu F, Haas M, Glassock R, et al. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes[J]. Nat Rev Nephrol, 2017, 13(8): 483-95. doi:10.1038/nrneph.2017.85 |
| [16] | Mejia-Vilet JM, Malvar A, Arazi A, et al. The lupus nephritis management renaissance[J]. Kidney Int, 2022, 101(2): 242-55. doi:10.1016/j.kint.2021.09.012 |
| [17] | Porter AW, Brodsky JL, Buck TM. Emerging links between endoplasmic reticulum stress responses and acute kidney injury[J]. Am J Physiol Cell Physiol, 2022, 323(6): C1697-703. doi:10.1152/ajpcell.00370.2022 |
| [18] | Chen XY, Shi CR, He MH, et al. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets[J]. Signal Transduct Target Ther, 2023, 8(1): 352. doi:10.1038/s41392-023-01570-w |
| [19] | Gallazzini M, Pallet N. Endoplasmic reticulum stress and kidney dysfunction[J]. Biol Cell, 2018, 110(9): 205-16. doi:10.1111/boc.201800019 |
| [20] | Hetz C, Zhang KZ, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response[J]. Nat Rev Mol Cell Biol, 2020, 21(8): 421-38. doi:10.1038/s41580-020-0250-z |
| [21] | Zhang RJ, Bian C, Gao J, et al. Endoplasmic reticulum stress in diabetic kidney disease: adaptation and apoptosis after three UPR pathways[J]. Apoptosis, 2023, 28(7/8): 977-96. doi:10.1007/s10495-023-01858-w |
| [22] | Kapuy O. Mechanism of decision making between autophagy and apoptosis induction upon endoplasmic reticulum stress[J]. Int J Mol Sci, 2024, 25(8): 4368. doi:10.3390/ijms25084368 |
| [23] | Verfaillie T, Rubio N, Garg AD, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress[J]. Cell Death Differ, 2012, 19(11): 1880-91. doi:10.1038/cdd.2012.74 |
| [24] | Chen S, Li X, Zhang XW, et al. PCV2 and PRV coinfection induces endoplasmic reticulum stress via PERK-eIF2α-ATF4-CHOP and IRE1-XBP1-EDEM pathways[J]. Int J Mol Sci, 2022, 23(9): 4479. doi:10.3390/ijms23094479 |
| [25] | Cao Y, Hu LT, Chen RK, et al. Unfolded protein response-activated NLRP3 inflammasome contributes to pyroptotic and apoptotic podocyte injury in diabetic kidney disease via the CHOP-TXNIP axis[J]. Cell Signal, 2025, 130: 111702. doi:10.1016/j.cellsig.2025.111702 |
| [26] | Nakatsuka A, Yamaguchi S, Jun WD. GRP78 contributes to the beneficial effects of SGLT2 inhibitor on proximal tubular cells in DKD[J]. Diabetes, 2024, 73(5): 763-79. doi:10.2337/db23-0581 |
| [27] | Trink J, Ahmed U, O’Neil K, et al. Cell surface GRP78 regulates TGFβ1-mediated profibrotic responses via TSP1 in diabetic kidney disease[J]. Front Pharmacol, 2023, 14: 1098321. doi:10.3389/fphar.2023.1098321 |
| [28] | Jin RB, Zhao AR, Han SY, et al. The interaction of S100A16 and GRP78 actives endoplasmic reticulum stress-mediated through the IRE1α/XBP1 pathway in renal tubulointerstitial fibrosis[J]. Cell Death Dis, 2021, 12(10): 942. doi:10.1038/s41419-021-04249-8 |
| [29] | Deng F, Zhang HP, Zhou W, et al. TRPA1 promotes cisplatin-induced acute kidney injury via regulating the endoplasmic reticulum stress-mitochondrial damage[J]. J Transl Med, 2023, 21(1): 695. doi:10.1186/s12967-023-04351-9 |
| [30] | Park SJ, Kim Y, Li C, et al. Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome[J]. Proc Natl Acad Sci USA, 2022, 119(35): e2116505119. doi:10.1073/pnas.2116505119 |
| [31] | Lin BB, Zhang XB, Xu XG. Nerve growth factor protects retinal ganglion cells related to inhibiting endoplasmic reticulum stress by inhibiting IRE1-JNK-CHOP signaling pathway[J]. Ocul Immunol Inflamm, 2022, 30(6): 1341-6. doi:10.1080/09273948.2021.1872651 |
| [1] | Xiaoyu CHANG, Hanwen ZHANG, Hongting CAO, Ling HOU, Xin MENG, Hong TAO, Yan LUO, Guanghua LI. Heat stress affects expression levels of circadian clock gene Bmal1 and cyclins in rat thoracic aortic endothelial cells [J]. Journal of Southern Medical University, 2025, 45(7): 1353-1362. |
| [2] | Yujia YANG, Lifang YANG, Yaling WU, Zhaoda DUAN, Chunze YU, Chunyun WU, Jianyun YU, Li YANG. Cannabidiol inhibits neuronal endoplasmic reticulum stress and apoptosis in rats with multiple concussions by regulating the PERK-eIF2α-ATF4-CHOP pathway [J]. Journal of Southern Medical University, 2025, 45(6): 1240-1250. |
| [3] | Yue CHEN, Linyu XIAO, Lü REN, Xue SONG, Jing LI, Jianguo HU. Monotropein improves motor function of mice with spinal cord injury by inhibiting the PI3K/AKT signaling pathway to suppress neuronal apoptosis [J]. Journal of Southern Medical University, 2025, 45(4): 774-784. |
| [4] | Fei CHU, Xiaohua CHEN, Bowen SONG, Jingjing YANG, Lugen ZUO. Moslosooflavone ameliorates dextran sulfate sodium-induced colitis in mice by suppressing intestinal epithelium apoptosis via inhibiting the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2025, 45(4): 819-828. |
| [5] | Yi ZHANG, Yu SHEN, Zhiqiang WAN, Song TAO, Yakui LIU, Shuanhu WANG. High expression of CDKN3 promotes migration and invasion of gastric cancer cells by regulating the p53/NF-κB signaling pathway and inhibiting cell apoptosis [J]. Journal of Southern Medical University, 2025, 45(4): 853-861. |
| [6] | Di CHEN, Ying LÜ, Yixin GUO, Yirong ZHANG, Ruixuan WANG, Xiaoruo ZHOU, Yuxin CHEN, Xiaohui WU. Dihydroartemisinin enhances doxorubicin-induced apoptosis of triple negative breast cancer cells by negatively regulating the STAT3/HIF-1α pathway [J]. Journal of Southern Medical University, 2025, 45(2): 254-260. |
| [7] | Yu BIN, Ziwen LI, Suwei ZUO, Sinuo SUN, Min LI, Jiayin SONG, Xu LIN, Gang XUE, Jingfang WU. High expression of apolipoprotein C1 promotes proliferation and inhibits apoptosis of papillary thyroid carcinoma cells by activating the JAK2/STAT3 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(2): 359-370. |
| [8] | Shuo LIU, Jing LI, Xingwang WU. Swertiamarin ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by inhibiting intestinal epithelial cell apoptosis [J]. Journal of Southern Medical University, 2024, 44(8): 1545-1552. |
| [9] | Xiaofan CONG, Teng CHEN, Shuo LI, Yuanyuan WANG, Longyun ZHOU, Xiaolong LI, Pei ZHANG, Xiaojin SUN, Surong ZHAO. Dihydroartemisinin enhances sensitivity of nasopharyngeal carcinoma HNE1/DDP cells to cisplatin-induced apoptosis by promoting ROS production [J]. Journal of Southern Medical University, 2024, 44(8): 1553-1560. |
| [10] | Linyu XIAO, Ting DUAN, Yongsheng XIA, Yue CHEN, Yang SUN, Yibo XU, Lei XU, Xingzhou YAN, Jianguo HU. Linarin inhibits microglia activation-mediated neuroinflammation and neuronal apoptosis in mouse spinal cord injury by inhibiting the TLR4/NF-κB pathway [J]. Journal of Southern Medical University, 2024, 44(8): 1589-1598. |
| [11] | Mengdong ZHENG, Yan LIU, Jiaojiao LIU, Qiaozhen KANG, Ting WANG. Effect of deletion of protein 4.1R on proliferation, apoptosis and glycolysis of hepatocyte HL-7702 cells [J]. Journal of Southern Medical University, 2024, 44(7): 1355-1360. |
| [12] | Yuanguo WANG, Peng ZHANG. Ferroptosis suppressor genes are highly expressed in esophageal cancer to inhibit tumor cell ferroptosis [J]. Journal of Southern Medical University, 2024, 44(7): 1389-1396. |
| [13] | Zhijun REN, Jianxin DIAO, Yiting WANG. Xionggui Decoction alleviates heart failure in mice with myocardial infarction by inhibiting oxidative stress-induced cardiomyocyte apoptosis [J]. Journal of Southern Medical University, 2024, 44(7): 1416-1424. |
| [14] | Guiling CHEN, Xiaofeng LIAO, Pengtao SUN, Huan CEN, Shengchun SHU, Bijing LI, Jinhua LI. Solasonine promotes apoptosis of non-small cell lung cancer cells by regulating the Bcl-2/Bax/caspase-3 pathway [J]. Journal of Southern Medical University, 2024, 44(6): 1109-1116. |
| [15] | Lingjun LU, Xiaodi YANG, Huaping ZHANG, Yuan LIANG, Xiulan SHI, Xin ZHOU. Recombinant Schistosoma japonicum cystatin alleviates acute liver injury in mice by inhibiting endoplasmic reticulum stress, inflammation and hepatocyte apoptosis [J]. Journal of Southern Medical University, 2024, 44(6): 1126-1134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||