南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (12): 2421-2433.doi: 10.12122/j.issn.1673-4254.2024.12.19
张富星1( ), 刘国庆1, 董锐1, 高磊1, 陆伟晨1, 高连霞1, 赵忠扩2, 陆飞2(
), 刘国庆1, 董锐1, 高磊1, 陆伟晨1, 高连霞1, 赵忠扩2, 陆飞2( ), 刘牧林1(
), 刘牧林1( )
)
收稿日期:2024-07-31
出版日期:2024-12-20
发布日期:2024-12-26
通讯作者:
陆飞,刘牧林
E-mail:1430078329@qq.com;8022038@zju.edu.cn;liumulin66@aliyun.com
作者简介:张富星,在读硕士研究生,E-mail: 1430078329@qq.com
基金资助:
Fuxing ZHANG1( ), Guoqing LIU1, Rui DONG1, Lei GAO1, Weichen LU1, Lianxia GAO1, Zhongkuo ZHAO2, Fei LU2(
), Guoqing LIU1, Rui DONG1, Lei GAO1, Weichen LU1, Lianxia GAO1, Zhongkuo ZHAO2, Fei LU2( ), Mulin LIU1(
), Mulin LIU1( )
)
Received:2024-07-31
Online:2024-12-20
Published:2024-12-26
Contact:
Fei LU, Mulin LIU
E-mail:1430078329@qq.com;8022038@zju.edu.cn;liumulin66@aliyun.com
摘要:
目的 探究软骨酸性蛋白1(CRTAC1)参与影响胃癌生物学行为和免疫浸润的机制。 方法 采用转录组分析CRTAC1在胃癌肿瘤细胞中表达与预后的关系,GO和KEGG富集分析CRTAC1表达水平可能参与细胞的功能及信号通路。ESTIMATE算法分析CRTAC1表达对肿瘤微环境及肿瘤突变负荷的影响。CCK-8、EdU、克隆形成以及流式细胞周期实验检测CRTAC1参与胃癌细胞的增殖。流式细胞凋亡、Hoechst和Western blotting实验检测CRTAC1抑制胃癌细胞凋亡。划痕伤口愈合、Transwell以及Western blotting实验分析CRTAC1参与胃癌迁移的机制。 结果 生物信息学分析结果显示:CRTAC1在胃癌和癌旁组织中的表达量差异有统计学意义(P<0.05);单因素Cox和多因素Cox回归分析表明年龄和分期分别是CRTAC1高表达胃癌患者的预后风险因素(P<0.001)。采用ESTIMATE算法结果显示:CRTAC1的表达增加免疫细胞浸润丰度(P<0.001);同时CRTAC1高表达减少肿瘤突变负荷(P<0.001)。采用CCK-8、EdU、克隆形成实验检测敲低CRTAC1后明显抑制细胞增值(P<0.001);流式细胞周期实验检测敲低CRTAC1后细胞阻滞在G1期(P<0.001)。流式细胞凋亡、Hoechst实验结果显示敲低CRTAC1后细胞凋亡增加(P<0.001);生物信息学预测胃癌患者中CRTAC1与凋亡相关蛋白BAX和BCL2的关系(P<0.05),Western blotting实验验证敲低CRTAC1后CRTAC1、BAX和BCL2的表达水平变化(P<0.05)。划痕伤口愈合、Transwell实验显示,敲低CRTAC1可抑制细胞的增值迁移(P<0.001);生物信息学预测胃癌患者中CRTAC1与EMT相关蛋白E-cadherin和Vimentin与CRTAC1的关系(P<0.05),Western blotting实验验证敲低CRTAC1后E-cadherin和Vimentin的表达水平改变(P<0.01)。KEGG富集分析显示,CRTAC1生物功能可能与PI3K/AKT信号相关,Western blotting实验证实CRTAC1可促进p-PI3K、AKT2、p-AKT、p-mTOR的表达(P<0.05)。 结论 CRTAC1在胃癌组织中高表达影响患者的免疫治疗效果和预后,可能通过调控肿瘤突变负荷和肿瘤微环境,以及PI3K/AKT信号通路促进胃癌细胞EMT进程相关。
张富星, 刘国庆, 董锐, 高磊, 陆伟晨, 高连霞, 赵忠扩, 陆飞, 刘牧林. 高表达CRTAC1通过调控PI3K信号通路促进胃癌细胞增殖、迁移及免疫浸润[J]. 南方医科大学学报, 2024, 44(12): 2421-2433.
Fuxing ZHANG, Guoqing LIU, Rui DONG, Lei GAO, Weichen LU, Lianxia GAO, Zhongkuo ZHAO, Fei LU, Mulin LIU. High expression of CRTAC1 promotes proliferation, migration and immune cell infiltration of gastric cancer by regulating the PI3K/AKT signaling pathway[J]. Journal of Southern Medical University, 2024, 44(12): 2421-2433.
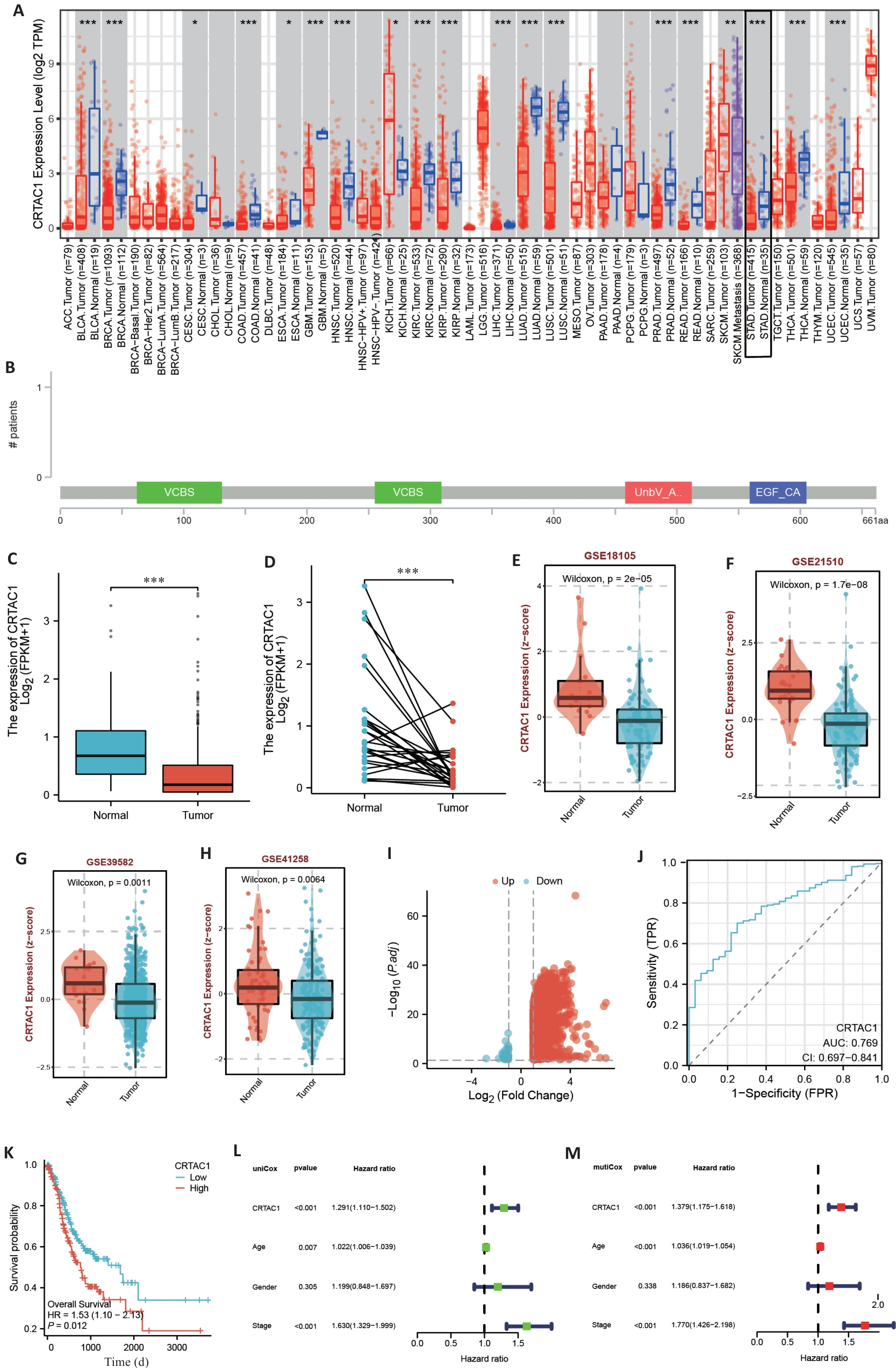
图1 CRTAC1在胃癌中表达水平与患者的预后相关
Fig.1 CRTAC1 expression level in gastric cancer is significantly correlated with the patients' prognosis. A: Pan-cancer analysis of TIMER2.0 database. B: Mutation location of CRTAC1 in gastric cancer transcriptome data of cBioPortal database. C, D: Differential analysis of CRTAC1 expression in unpaired and paired gastric cancer transcriptome data of TCGA database. E-H: Differential analysis of CRTAC1 expression in transcriptome data of BEST website of GSE18105, GSE21510, GSE39582 and GSE41258 transcriptome data in BEST website. I: Differentially expressed genes between high and low CRTAC1 expression groups in TCGA database gastric cancer transcriptome data and plotting of volcano plots. J: Expression level of CRTAC1 in TCGA database gastric cancer transcriptome data for ROC curves plotting. K: Relationship between CRTAC1 expression level and gastric cancer patients' overall survival (OS). L, M: One-way Cox and multifactorial Cox analysis of CRTAC1 in gastric cancer patients. *P<0.05,**P<0. 01, ***P<0. 001.
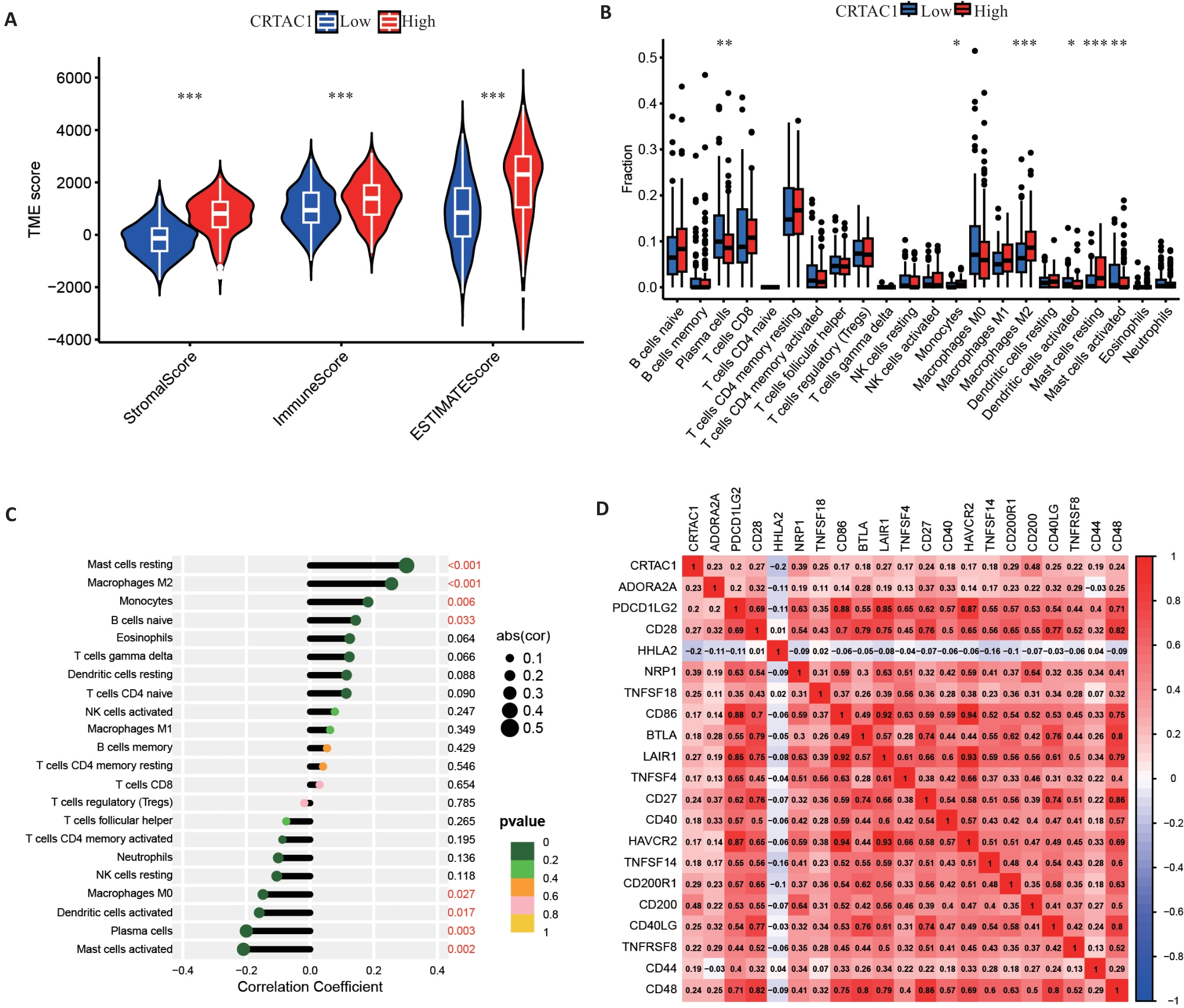
图2 CRTAC1表达影响胃癌肿瘤微环境
Fig.2 CRTAC1 expression affects tumor microenvironment in gastric cancer. A: Stromal cell scores, immune cell scores and tumor microenvironment scores of patients in the high and low CRTAC1 expression groups. B, C: Differential and correlation analyses of immune cells in patients in high and low CRTAC1 expression groups. D: Correlation analysis of immune checkpoints in patients in high and low CRTAC1 expression groups. *P<0.05, **P<0.01, ***P<0.001.
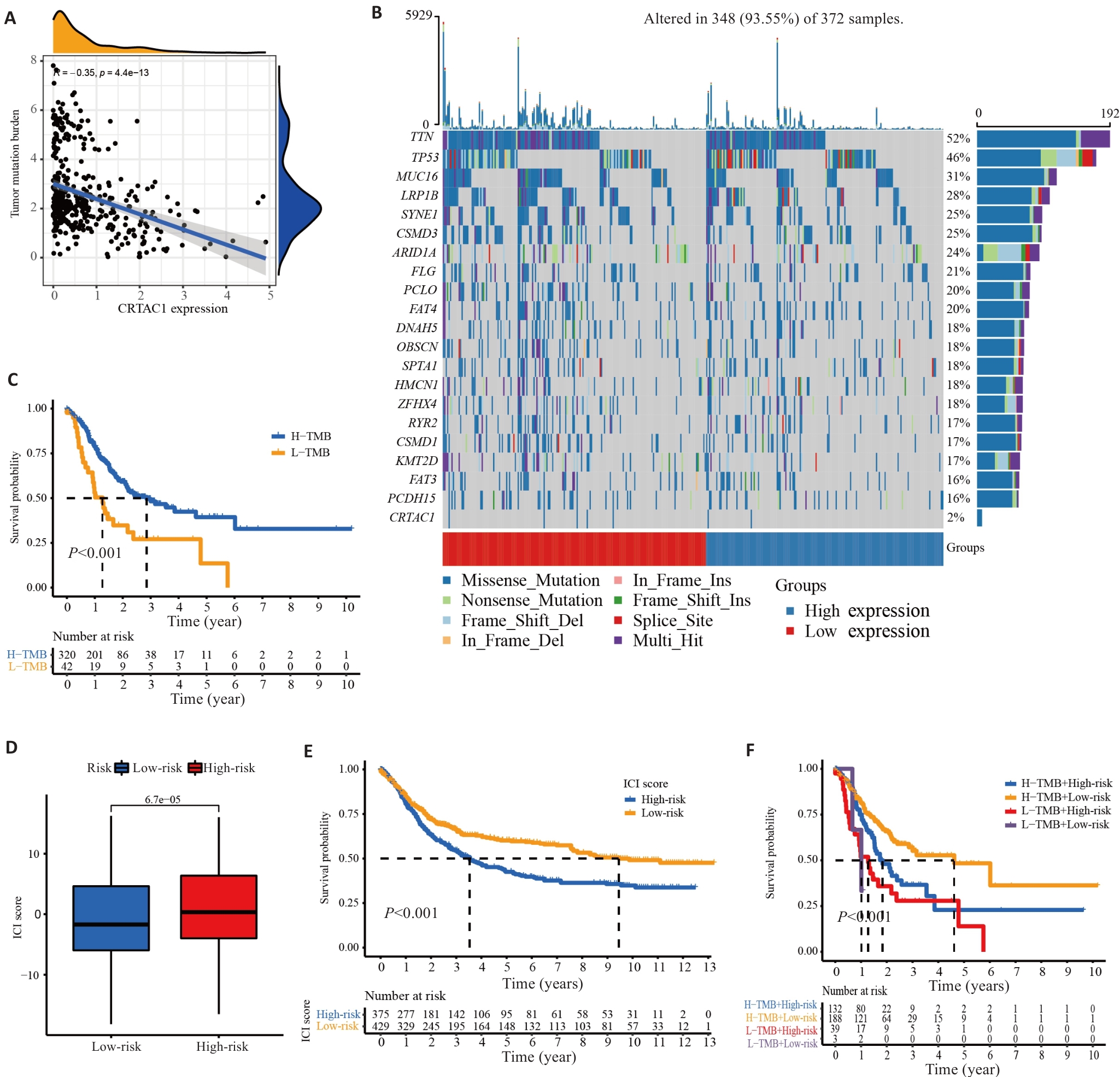
图3 CRTAC1在胃癌中的表达水平影响肿瘤突变负荷及预后
Fig.3 CRTAC1 expression level in gastric cancer affects tumor mutation load and prognosis. A: Scatter plot of correlation between CRTAC1 expression and TMB. B: Waterfall plot of the top 20 genes with mutation frequency in samples from high and low CRTAC1 expression groups. C: Kaplan-Meier curve of TMB affecting the overall survival of gastric cancer patients. D: Box line plot of TMB combined affecting the risk scores of gastric cancer patients. E: Kaplan-Meier curves of TMB affecting the risk scores of gastric cancer patients' overall survival. F: Kaplan-Meier curves of the effect of TMB combined with CRTAC1 expression on overall survival of gastric cancer patients. *P<0.05, **P<0. 01, ***P<0. 001.
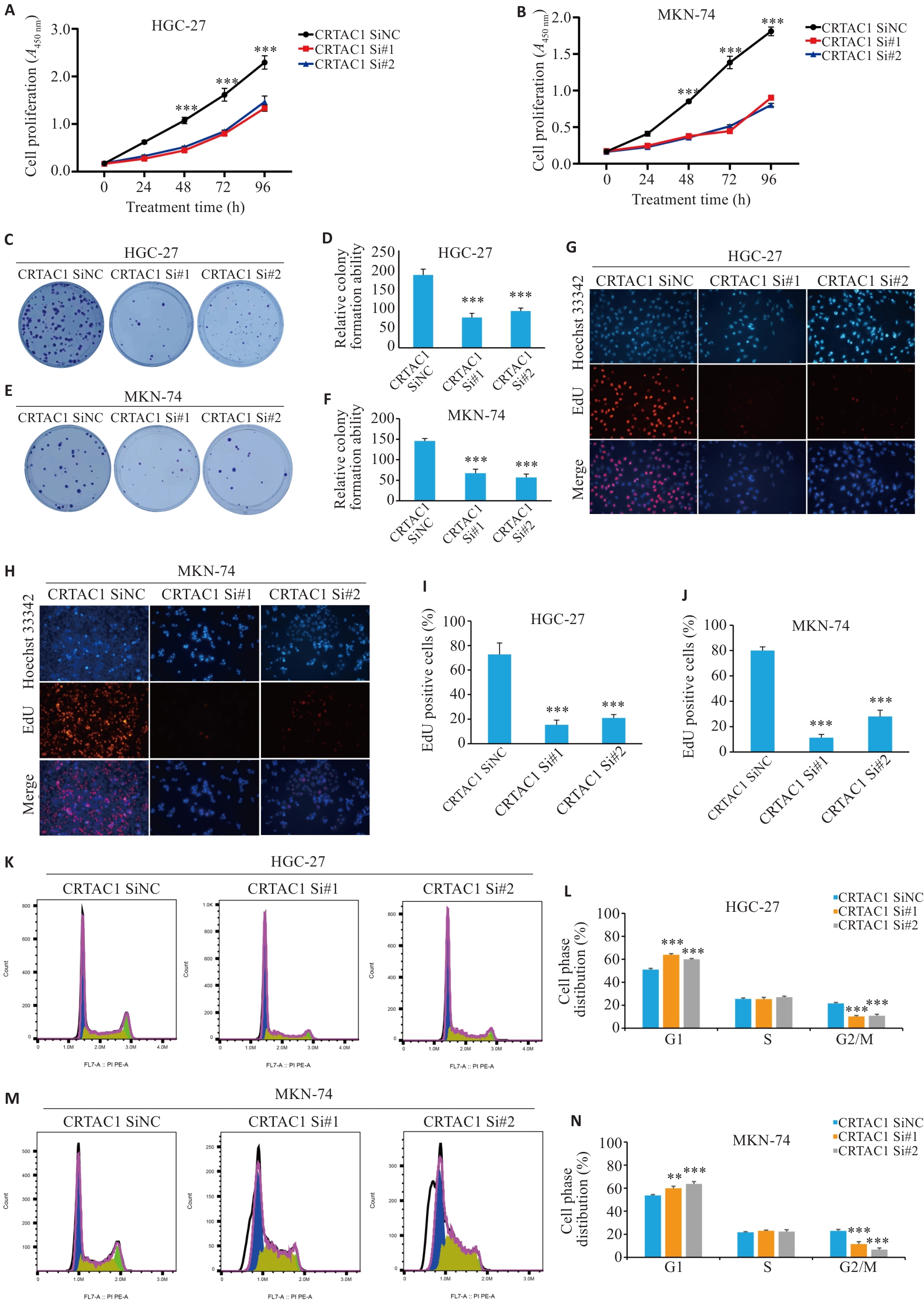
图4 CRTAC1高表达促进胃癌细胞的生长增殖
Fig. 4 High expression of CRTAC1 promotes proliferation of gastric cancer cells. A, B: Detection of proliferative capacity of the cells with CRTAC1 knockdown using CCK8 assay. C-F: Colony formation assay of the cells with CRTAC1 knockdown. G-J: EdU assay for assessing proliferation of the cells with CRTAC1 knockdown (Original magnification: ×20). K-N: Flow cytometry for assessing cell cycle changes in the cells with CRTAC1 knockdown. **P<0. 01, ***P<0. 001 vs CRTAC1 SiNC group.
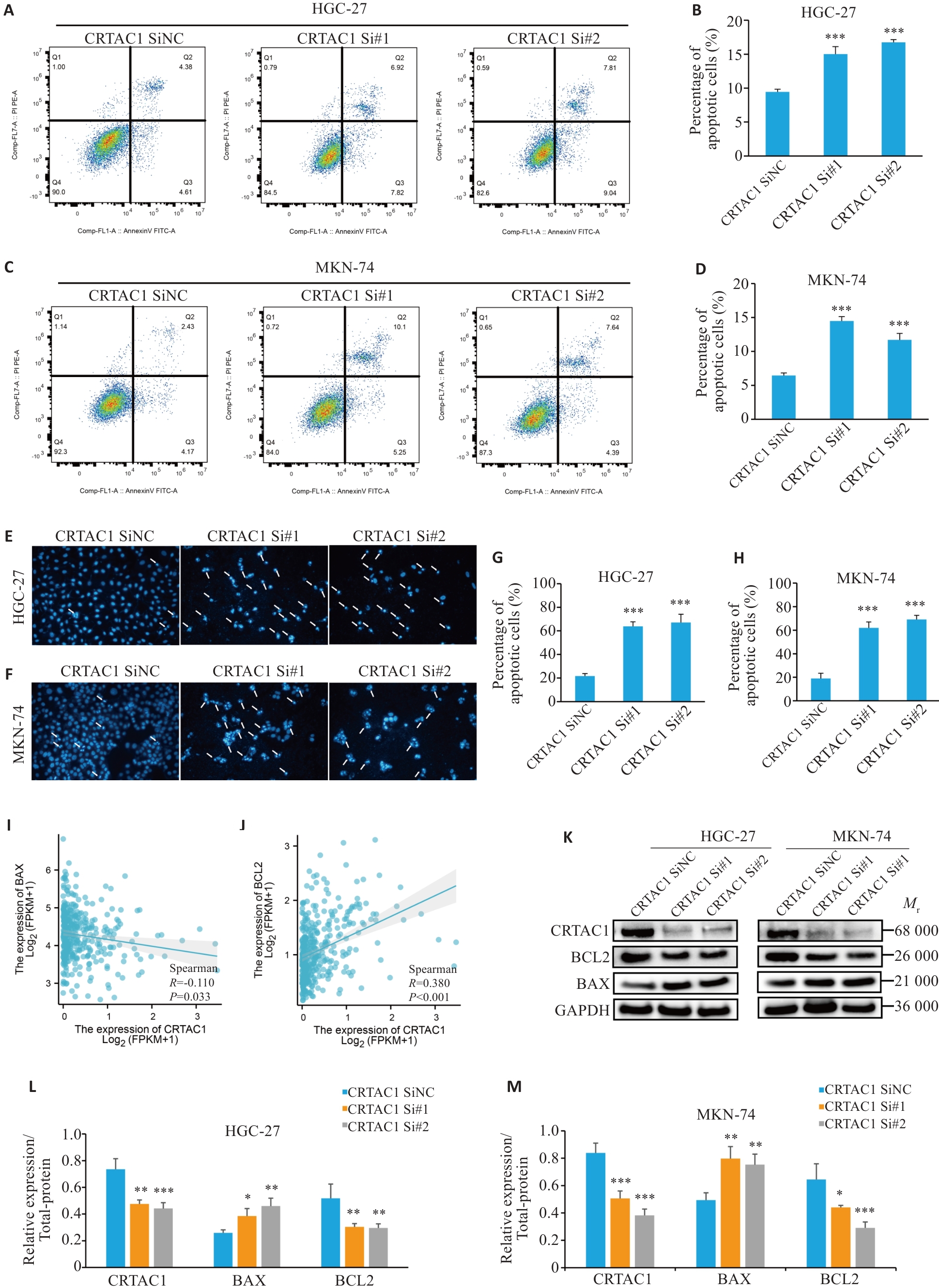
图5 CRTAC1的高表达抑制胃癌细胞凋亡
Fig.5 High expression of CRTAC1 significantly inhibits apoptosis in gastric cancer cells. A-H: Flow apoptosis assay and Hoechst assay showing that CRTAC1 knockdown promotes apoptosis of gastric cancer cells. I-J: Spearman's statistical analysis of the changes in BAX and BCL-2 expressions following CRTAC1 knockdown using the R language (×20). K-M: Changes in BAX and BCL-2 expressions in GC cells with CRTAC1 knockdown detected by Western blotting. *P<0.05, **P<0. 01, ***P<0. 001 vs CRTAC1 SiNC group.
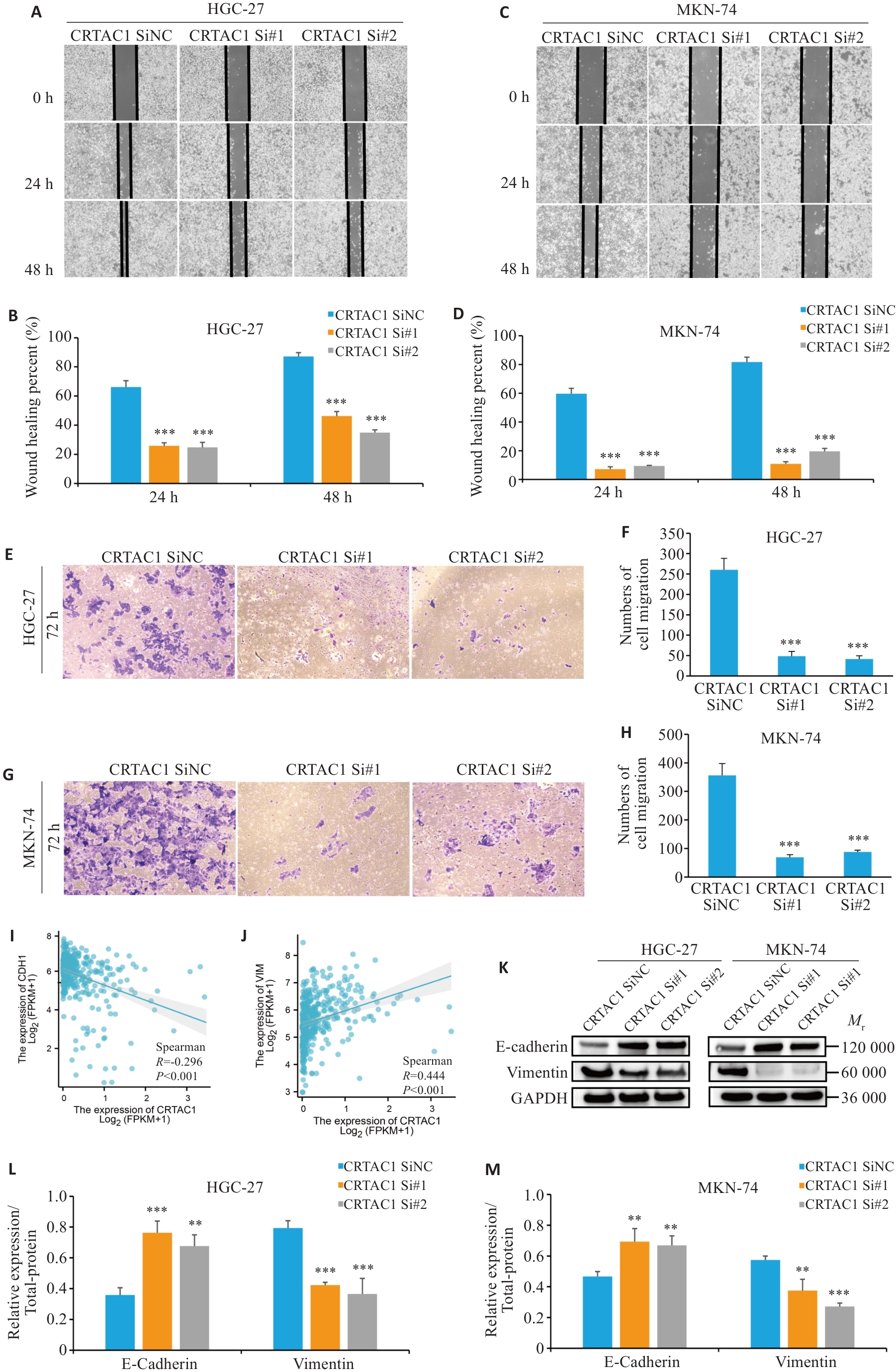
图6 CRTAC1高表达促进胃癌细胞的增殖迁移
Fig.6 High expression of CRTAC1 promotes proliferation and migration of gastric cancer cells. A-H: Scratch wound healing assay and Transwell assay for assessing migration ability of the cells with CRTAC1 knockdown (×20). I-J: Spearman's statistical analysis of the correlation between E-cadherin (CDH1) and Vimentin (VIM) expression using R language. K-M: Western blotting of E-cadherin and vimentin protein expressions in the cells with CRTAC1 knockdown. **P<0. 01, ***P<0. 001 vs CRTAC1 SiNC group.
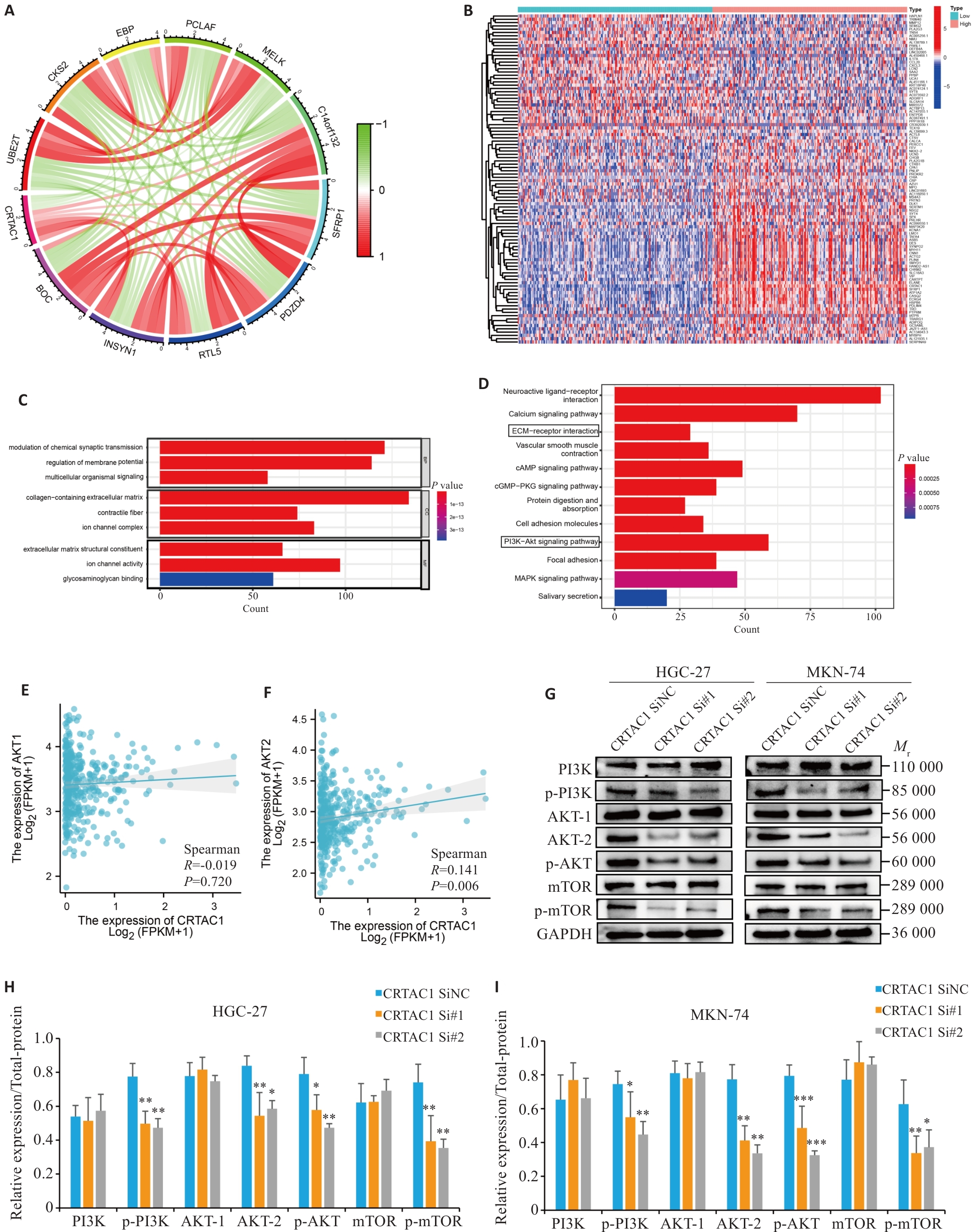
图7 CRTAC1通过PI3K信号通路促进胃癌细胞增殖及迁移
Fig. 7 High expression of CRTAC1 promotes proliferation and migration of gastric cancer cells through the PI3K signaling pathway. A: Circle plot of co-expression analysis of CRTAC1 performed in gastric cancer genome (red, white, and green scatters are positively, non-significantly, and negatively correlated genes, respectively, with CRTAC1 expression). B: Heat map of differential genes between high and low CRTAC1 expression groups in gastric cancer (red, gray, and blue scatters are positively, non-significantly, and negatively correlated genes, respectively, with CRTAC1 expression). C, D: Histograms of GO and KEGG enrichment analysis of CRTAC1 differential genes, FDR<0.05. E, F: Spearman's statistical analysis of the correlation between AKT2 expressions using R language. G-I: Western blotting of the PI3K signaling pathway protein expressions in cells with CRTAC1 knockdown. *P<0.05, **P<0. 01, ***P<0. 001 vs CRTAC1 SiNC group.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. |
| 2 | Thrift AP, El-Serag HB. Burden of gastric cancer[J]. Clin Gastroenterol Hepatol, 2020, 18(3): 534-42. |
| 3 | Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer[J]. Lancet, 2009, 374(9688): 477-90. |
| 4 | Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection[J]. Surg Today, 2020, 50(1): 3-11. |
| 5 | Zhang KC, Chen L. Chinese consensus on the diagnosis and treatment of gastric cancer with liver metastases[J]. Ther Adv Med Oncol, 2020, 12: 1758835920904803. |
| 6 | Lei YH, Lin L, Cheng SY, et al. Acute inflammatory reaction during anti-angiogenesis therapy combined with immunotherapy as a possible indicator of the therapeutic effect: three case reports and literature review[J]. Front Oncol, 2023, 13: 1072480. |
| 7 | Anjos L, Morgado I, Guerreiro M, et al. Cartilage acidic protein 1, a new member of the beta-propeller protein family with amyloid propensity[J]. Proteins, 2017, 85(2): 242-55. |
| 8 | Jin ZH, Zhao LL, Chang YX, et al. CRTAC1 enhances the chemosensitivity of non-small cell lung cancer to cisplatin by eliciting RyR-mediated calcium release and inhibiting Akt1 expression[J]. Cell Death Dis, 2023, 14(8): 563. |
| 9 | Chemaly ER, Troncone L, Lebeche D. SERCA control of cell death and survival[J]. Cell Calcium, 2018, 69: 46-61. |
| 10 | Yang JH, Fan L, Liao XX, et al. CRTAC1 (Cartilage acidic protein 1) inhibits cell proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) process in bladder cancer by downregulating Yin Yang 1 (YY1) to inactivate the TGF-β pathway[J]. Bioengineered, 2021, 12(2): 9377-89. |
| 11 | Li WM, Chan TC, Wei YC, et al. Downregulation of CRTAC1 in urothelial carcinoma promotes tumor aggressiveness and confers poor prognosis[J]. Front Biosci, 2023, 28(9): 217. |
| 12 | Shen YP, Chen K, Gu CJ. Identification of a chemotherapy-associated gene signature for a risk model of prognosis in gastric adenocarcinoma through bioinformatics analysis[J]. J Gastrointest Oncol, 2022, 13(5): 2219-33. |
| 13 | Zeng DQ, Li MY, Zhou R, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures[J]. Cancer Immunol Res, 2019, 7(5): 737-50. |
| 14 | Kushnir A, Wajsberg B, Marks AR. Ryanodine receptor dysfunction in human disorders[J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(11 Pt B): 1687-97. |
| 15 | Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors[J]. Cancer Treat Rev, 2018, 62: 50-60. |
| 16 | Genovese I, Carinci M, Modesti L, et al. Mitochondria: insights into crucial features to overcome cancer chemoresistance[J]. Int J Mol Sci, 2021, 22(9): 4770. |
| 17 | Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival[J]. Nat Rev Cancer, 2008, 8(5): 361-75. |
| 18 | Li J, Qi FZ, Su HS, et al. GRP75-faciliated Mitochondria-associated ER Membrane (MAM) Integrity controls Cisplatin-resistance in Ovarian Cancer Patients[J]. Int J Biol Sci, 2022, 18(7): 2914-31. |
| 19 | Bruce JIE, James AD. Targeting the calcium signalling machinery in cancer[J]. Cancers, 2020, 12(9): 2351. |
| 20 | Wei CL, Wang XH, Chen M, et al. Calcium flickers steer cell migration[J]. Nature, 2009, 457(7231): 901-5. |
| 21 | Neophytou CM, Kyriakou TC, Papageorgis P. Mechanisms of metastatic tumor dormancy and implications for cancer therapy[J]. Int J Mol Sci, 2019, 20(24): 6158. |
| 22 | Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells[J]. Nature, 2016, 529(7586): 298-306. |
| 23 | Wang C, Yang Z, Xu E, et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway[J]. Clin Transl Med, 2021, 11(8): e522. |
| 24 | Duan SY, Huang WQ, Liu XT, et al. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways[J]. J Exp Clin Cancer Res, 2018, 37(1): 304. |
| 25 | Tian LY, Smit DJ, Jücker M. The role of PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism[J]. Int J Mol Sci, 2023, 24(3): 2652. |
| 26 | Xu H, Wang J, Al-Nusaif M, et al. CCL2 promotes metastasis and epithelial-mesenchymal transition of non-small cell lung cancer via PI3K/Akt/mTOR and autophagy pathways[J]. Cell Prolif, 2024, 57(3): e13560. |
| 27 | Wang Q, Chen XY, Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice)[J]. Br J Cancer, 2017, 117(2): 159-63. |
| 28 | Degan SE, Gelman IH. Emerging roles for AKT isoform preference in cancer progression pathways[J]. Mol Cancer Res, 2021, 19(8): 1251-7. |
| 29 | Zhang HK, Cheng Y, Jia CW, et al. MicroRNA-29s could target AKT2 to inhibit gastric cancer cells invasion ability[J]. Med Oncol, 2015, 32(1): 342. |
| 30 | Cheng ZY, Liu GY, Huang CJ, et al. KLF5 activates lncRNA DANCR and inhibits cancer cell autophagy accelerating gastric cancer progression[J]. NPJ Genom Med, 2021, 6(1): 75. |
| 31 | Ganesh K, Massagué J. Targeting metastatic cancer[J]. Nat Med, 2021, 27(1): 34-44. |
| 32 | Lüönd F, Sugiyama N, Bill R, et al. Distinct contributions of partial and full EMT to breast cancer malignancy[J]. Dev Cell, 2021, 56(23): 3203-21.e11. |
| 33 | Aiello NM, Kang YB. Context-dependent EMT programs in cancer metastasis[J]. J Exp Med, 2019, 216(5): 1016-26. |
| 34 | Akhmetkaliyev A, Alibrahim N, Shafiee D, et al. EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: the two sides of the same coin[J]? Mol Cancer, 2023, 22(1): 90. |
| 35 | Wu N, Jiang MZ, Liu HM, et al. LINC00941 promotes CRC metastasis through preventing SMAD4 protein degradation and activating the TGF-β/SMAD2/3 signaling pathway[J]. Cell Death Differ, 2021, 28(1): 219-32. |
| 36 | Tan ZM, Sun WY, Li Y, et al. Current progress of EMT: a new direction of targeted therapy for colorectal cancer with invasion and metastasis[J]. Biomolecules, 2022, 12(12): 1723. |
| 37 | Li XC, Sun Z, Peng GX, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer[J]. Theranostics, 2022, 12(2): 620-38. |
| 38 | Matsueda S, Graham DY. Immunotherapy in gastric cancer[J]. World J Gastroenterol, 2014, 20(7): 1657-66. |
| 39 | Wang JQ, Du LY, Chen XJ. Adenosine signaling: optimal target for gastric cancer immunotherapy[J]. Front Immunol, 2022, 13: 1027838. |
| 40 | Li W, Zhang X, Wu FL, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer[J]. Cell Death Dis, 2019, 10(12): 918. |
| 41 | Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer[J]. Gastric Cancer, 2020, 23(4): 565-78. |
| 42 | Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition[J]. N Engl J Med, 2017, 377(25): 2500-1. |
| 43 | McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types[J]. Ann Oncol, 2021, 32(5): 661-72. |
| 44 | Zhao ZD, Mak TK, Shi YT, et al. The DNA damage repair-related lncRNAs signature predicts the prognosis and immunotherapy response in gastric cancer[J]. Front Immunol, 2023, 14: 1117255. |
| 45 | Li XC, Pasche B, Zhang W, et al. Association of MUC16 mutation with tumor mutation load and outcomes in patients with gastric cancer[J]. JAMA Oncol, 2018, 4(12): 1691-8. |
| 46 | Yang Y, Zhang JY, Chen YX, et al. MUC4, MUC16, and TTN genes mutation correlated with prognosis, and predicted tumor mutation burden and immunotherapy efficacy in gastric cancer and pan-cancer[J]. Clin Transl Med, 2020, 10(4): e155. |
| 47 | Cao JL, Yang X, Chen SY, et al. The predictive efficacy of tumor mutation burden in immunotherapy across multiple cancer types: a meta-analysis and bioinformatics analysis[J]. Transl Oncol, 2022, 20: 101375. |
| [1] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [2] | 庞金龙, 赵新丽, 张振, 王豪杰, 周星琦, 杨玉梅, 李姗姗, 常小强, 李锋, 李娴. 皮肤黑色素瘤中MMRN2高表达促进肿瘤细胞的侵袭和迁移并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1479-1489. |
| [3] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [4] | 侯鑫睿, 张振东, 曹明远, 杜予心, 王小平. 红景天苷靶向miR-1343-3p-OGDHL/PDHB糖代谢轴抑制胃癌细胞的体内外增殖[J]. 南方医科大学学报, 2025, 45(6): 1226-1239. |
| [5] | 郭晓娟, 杜瑞娟, 陈丽平, 郭克磊, 周彪, 卞华, 韩立. WW结构域E3泛素连接酶1调控卵巢癌肿瘤微环境中的免疫浸润[J]. 南方医科大学学报, 2025, 45(5): 1063-1073. |
| [6] | 马振南, 刘福全, 赵雪峰, 张晓微. DTX2促进奥沙利铂耐药的结直肠癌细胞增殖、侵袭和上皮间质转化[J]. 南方医科大学学报, 2025, 45(4): 829-836. |
| [7] | 张毅, 沈昱, 万志强, 陶嵩, 柳亚魁, 王栓虎. CDKN3高表达促进胃癌细胞的迁移和侵袭:基于调控p53/NF-κB信号通路和抑制胃癌细胞凋亡[J]. 南方医科大学学报, 2025, 45(4): 853-861. |
| [8] | 高志, 吴傲, 胡仲翔, 孙培养. 类风湿性关节炎中氧化应激与免疫浸润的生物信息学分析[J]. 南方医科大学学报, 2025, 45(4): 862-870. |
| [9] | 陶露, 韦卓利, 王月月, 项平. CEACAM6通过调控上皮间质转化抑制鼻咽癌细胞的增殖和迁移[J]. 南方医科大学学报, 2025, 45(3): 566-576. |
| [10] | 黄晴晴, 张文静, 张小凤, 王炼, 宋雪, 耿志军, 左芦根, 王月月, 李静, 胡建国. 高表达MYO1B促进胃癌细胞增殖、迁移和侵袭并与患者的不良预后有关[J]. 南方医科大学学报, 2025, 45(3): 622-631. |
| [11] | 宋雪, 陈悦, 张敏, 张诺, 左芦根, 李静, 耿志军, 张小凤, 王月月, 王炼, 胡建国. GPSM2在胃癌组织中高表达并通过促进肿瘤细胞的增殖影响患者预后[J]. 南方医科大学学报, 2025, 45(2): 229-238. |
| [12] | 王耀彬, 陈柳燕, 罗伊凌, 申继清, 周素芳. NUF2对泛癌的预后和免疫治疗效果的预测价值[J]. 南方医科大学学报, 2025, 45(1): 137-149. |
| [13] | 陈孝华, 鲁辉, 王子良, 王炼, 夏勇生, 耿志军, 张小凤, 宋雪, 王月月, 李静, 胡建国, 左芦根. ABI2在胃癌进展和预后中的作用及其调控机制[J]. 南方医科大学学报, 2024, 44(9): 1653-1661. |
| [14] | 叶梦楠, 武鸿美, 梅琰, 张庆玲. CREM在胃癌中高表达并与患者的不良预后相关[J]. 南方医科大学学报, 2024, 44(9): 1776-1782. |
| [15] | 朱梦云, 王剑锋. 康柏西普可逆转TGF-β2诱导的晶状体上皮细胞发生上皮间质转化:基于调节TGF-β/Smad信号通路[J]. 南方医科大学学报, 2024, 44(8): 1459-1466. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||