南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (11): 2209-2219.doi: 10.12122/j.issn.1673-4254.2024.11.18
杜若丽1,2( ), 云琦2,3, 王奕人1,2, 窦欣雨4, 叶红伟1,2, 王佳慧2, 高琴1,2(
), 云琦2,3, 王奕人1,2, 窦欣雨4, 叶红伟1,2, 王佳慧2, 高琴1,2( )
)
收稿日期:2024-08-10
出版日期:2024-11-20
发布日期:2024-11-29
通讯作者:
高琴
E-mail:hello1112drl@126.com;bbmcgq@126.com
作者简介:杜若丽,在读硕士研究生,E-mail: hello1112drl@126.com
基金资助:
Ruoli DU1,2( ), Qi YUN2,3, Yiren WANG1,2, Xinyu DOU4, Hongwei YE1,2, Jiahui WANG2, Qin GAO1,2(
), Qi YUN2,3, Yiren WANG1,2, Xinyu DOU4, Hongwei YE1,2, Jiahui WANG2, Qin GAO1,2( )
)
Received:2024-08-10
Online:2024-11-20
Published:2024-11-29
Contact:
Qin GAO
E-mail:hello1112drl@126.com;bbmcgq@126.com
摘要:
目的 基于网络药理学探讨白花丹素是否通过减轻焦亡来抑制脓毒症心肌损伤的机制。 方法 通过网络药理学方法获得白花丹素与疾病的关键靶点,进行GO、KEGG分析,通过分子对接验证结合能。将小鼠随机分为4组,8只/组:Sham组、盲肠结扎组(CLP)、白花丹素(PLB,2 mg/kg)+CLP组和PLB(4 mg/kg)+CLP组。采用CLP诱导脓毒症小鼠心脏损伤。超声心动图和HE染色检测心肌功能和形态的变化;检测小鼠血清CK-MB、LDH、MDA和心肌ROS水平,ELISA检测小鼠血清IL-1β和IL-18水平。Western blotting测定心肌STAT3、GSDMD、Caspase-11、JAK2、P-STAT3、P-JAK2、GSDMD-N和HMGB1的蛋白水平。 结果 从交集的10个基因中筛选出5个核心靶点,分子对接显示,白花丹素与STAT3、p-STAT3和JAK2结合较好。与Sham组相比,CLP组的CO、LVEF、LVFS和SV水平下降(P<0.01)。血清 CK-MB、LDH、MDA、心肌炎症因子和ROS水平升高(P<0.01),HE染色结果显示心脏损伤;相关蛋白水平升高(P<0.05)。与CLP组相比,白花丹素处理组的心超功能指标水平升高(P<0.05);血清 CK-MB、LDH、MDA、炎症因子和心肌ROS水平降低(P<0.01);相关蛋白水平降低(P<0.05)。 结论 白花丹素减轻小鼠脓毒症心肌损伤作用,其机制可能与抑制STAT3减轻焦亡有关。
杜若丽, 云琦, 王奕人, 窦欣雨, 叶红伟, 王佳慧, 高琴. 白花丹素通过抑制JAK2/STAT3通路减弱焦亡对抗脓毒症心肌损伤[J]. 南方医科大学学报, 2024, 44(11): 2209-2219.
Ruoli DU, Qi YUN, Yiren WANG, Xinyu DOU, Hongwei YE, Jiahui WANG, Qin GAO. Plumbagin protect against sepsis-induced myocardial injury in mice by inhibiting the JAK2/STAT3 signaling pathway to reduce cardiomyocyte pyroptosis[J]. Journal of Southern Medical University, 2024, 44(11): 2209-2219.
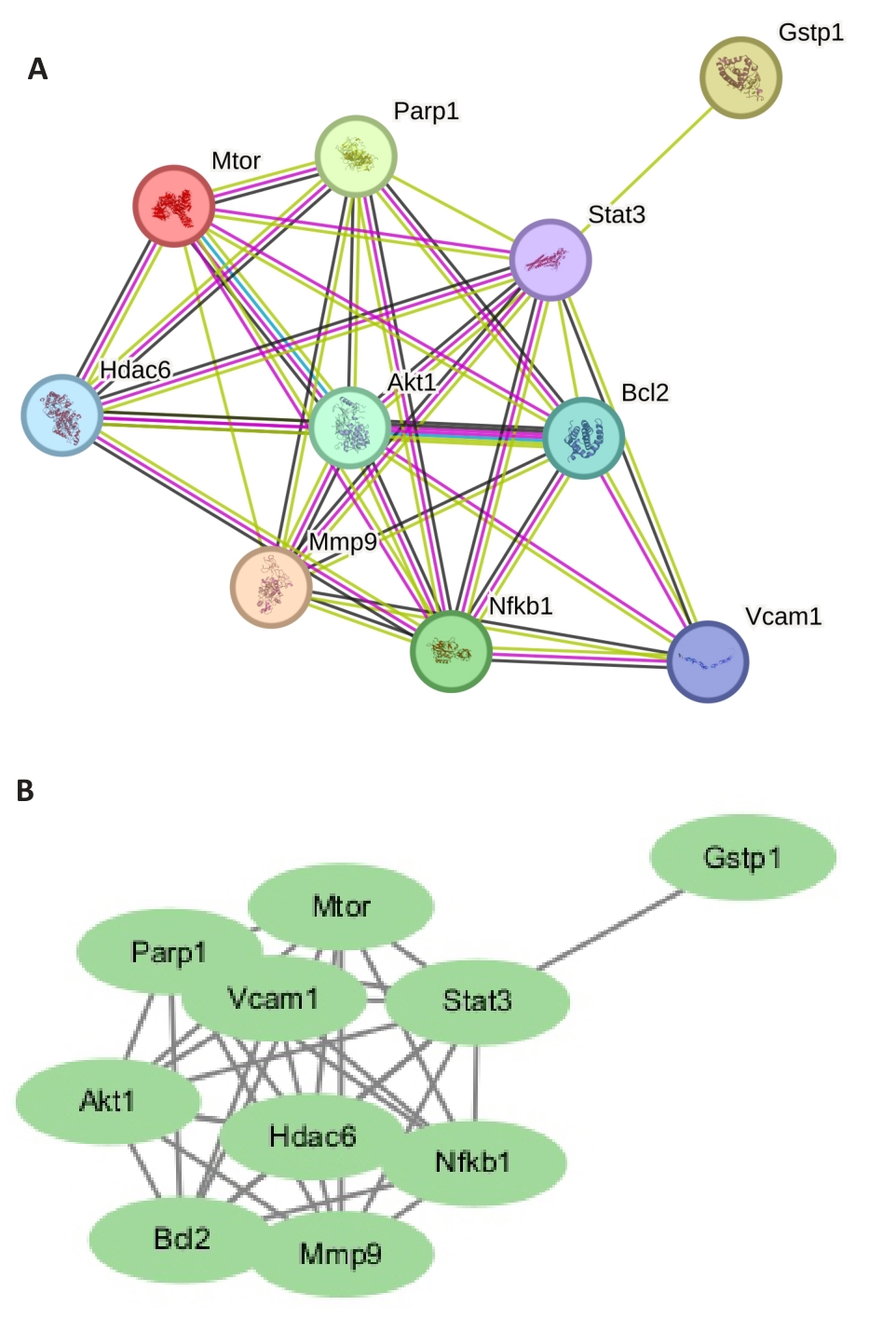
图2 核心PPI网络
Fig.2 Core protein-protein interaction (PPI) network. A: Visual regulatory network of plumbagin-sepsis myocardial injury-pyroptosis. B: PPI network of plumbagin-sepsis myocardial injury-pyroptosis related targets.
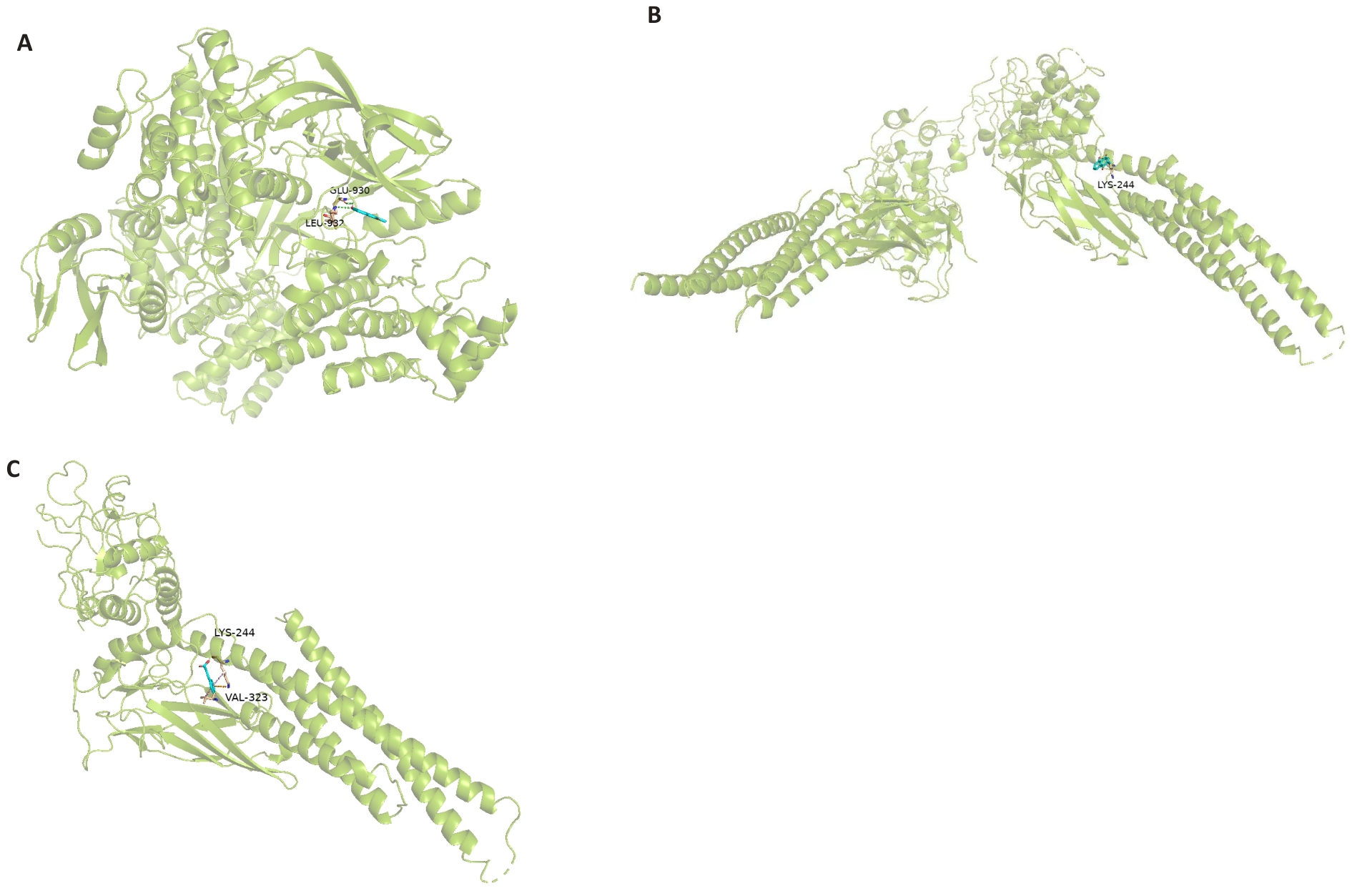
图5 分子对接结果
Fig.5 Molecular docking results. A: Molecular docking diagram of plumbagin and STAT3. B: Molecular docking diagram of plumbagin and JAK2. C: Molecular docking diagram of plumbagin and p-STAT3.
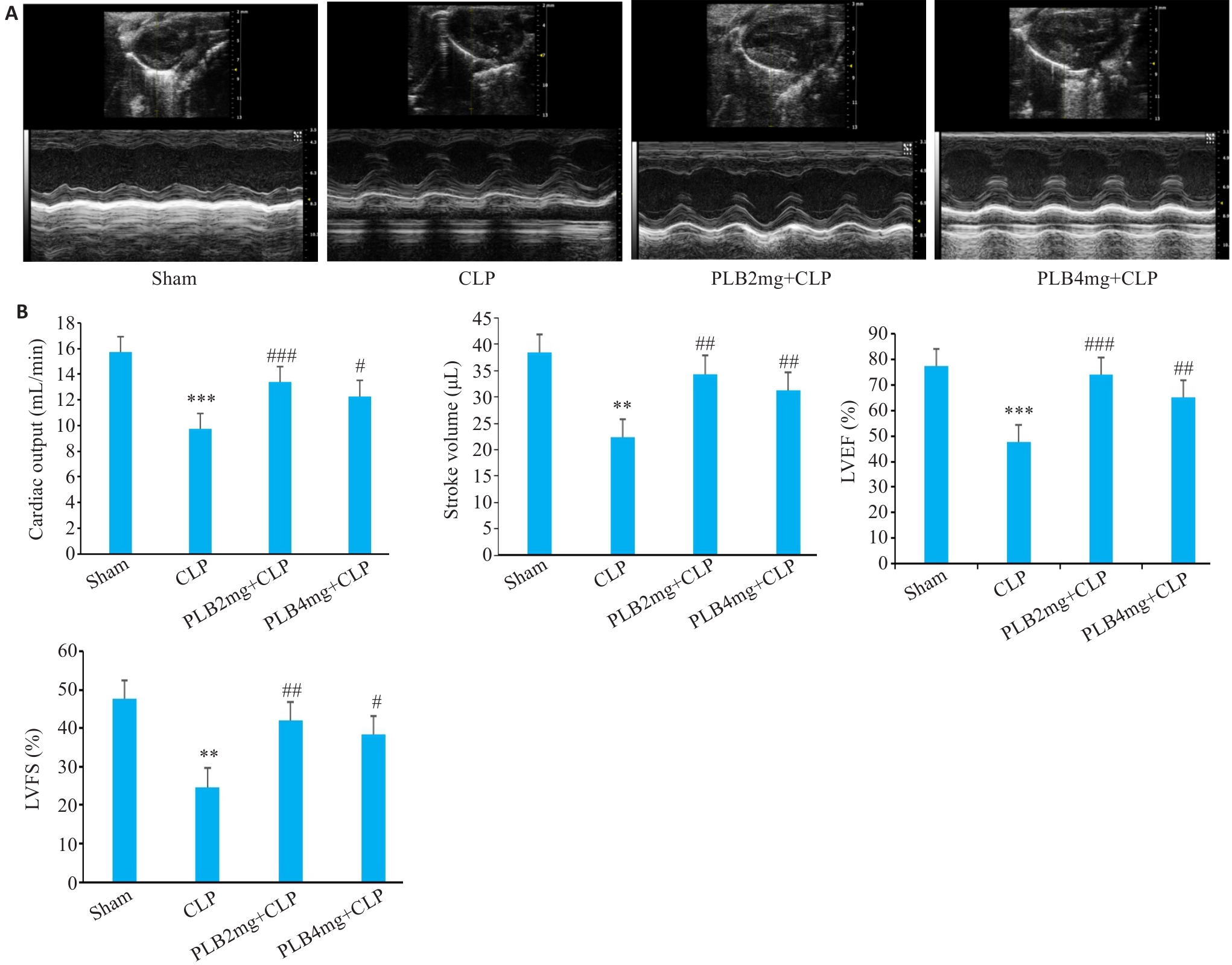
图6 各组小鼠心脏超声图像和心功能各指标变化
Fig.6 Cardiac ultrasound images and cardiac function indexes of the mice in different groups (Mean±SD, n=6). A: Evaluation of cardiac function by M-mode echocardiography. B: Comparison of the echocardiographic parameters (LVEF: Left ventricle ejection fraction; LVFS: Left ventricular fractional shortening). **P<0.01, ***P<0.001 vs Sham group, #P<0.05, ##P<0.01, ###P<0.001 vs CLP group.
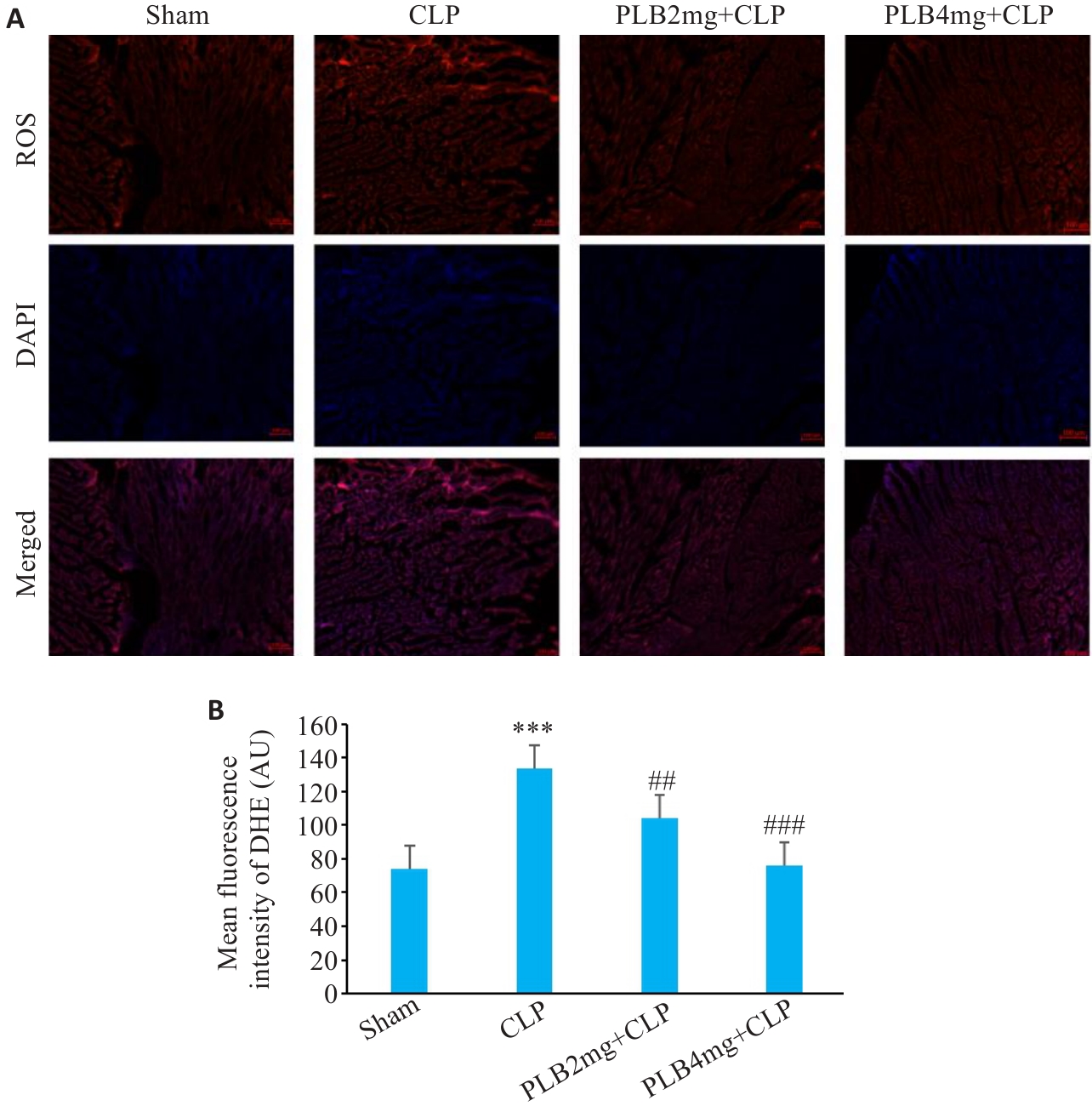
图8 各组小鼠心肌纤维DHE荧光染色代表性图片及分析
Fig.8 DHE fluorescence staining of myocardial fibers in different groups. A: Fluorescence probe for ROS in the cardiac tissues (Original magnification: ×200). B: ROS fluorescence intensity in each group (Mean±SD, n=6). ***P<0.001 vs sham group; ##P<0.01, ###P<0.001 vs the CLP group.

图9 各组小鼠血清IL-18和IL-1β的变化
Fig.9 Serum IL-18 (A) and IL-1β (B) levels in the myocardial tissue in different groups (Mean±SD, n=6). ***P<0.001 vs sham group; #P<0.05 vs CLP group.

图10 各组小鼠血清MDA、CK-MB和LDH的变化
Fig.10 Serum MDA (A), CK-MB (B) and LDH (C) levels in the myocardial tissue in different groups (Mean±SD, n=6). ***P<0.001 vs sham group; #P<0.05, ##P<0.01 ###P<0.001 vs CLP group.
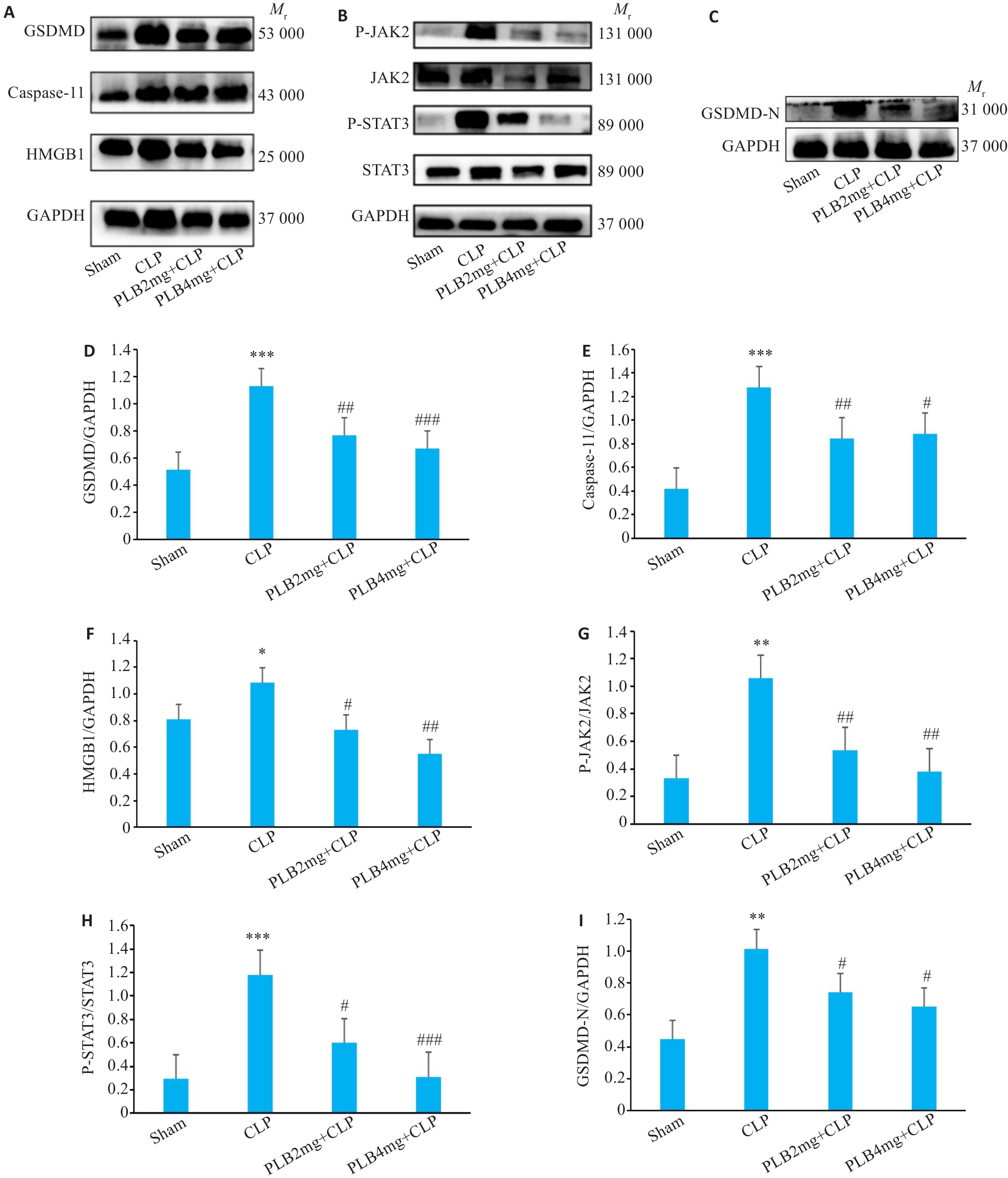
图11 各组小鼠心肌组织GSDMD、Caspase-11、HMGB1、P-JAK2、JAK2、P-STAT3、STAT3和GSDMD-N蛋白水平比较
Fig.11 Comparison of GSDMD、Caspase-11、HMGB1、P-JAK2、JAK2、P-STAT3、STAT3 and GSDMD-N protein levels in the myocardial tissues among the 4 groups. A-C: Protein bands in Western blotting. D-I: Relative protein levels of GSDMD、Caspase-11、HMGB1、P-JAK2、JAK2、P-STAT3、STAT3 and GSDMD-N proteins (Mean±SD, n=3). *P<0.05, **P<0.01, ***P<0.001 vs sham group; #P<0.05, ##P<0.01, ###P<0.001 vs CLP group.
| 1 | Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 775-87. |
| 2 | Li Z, Wu BQ, Chen J, et al. WWP2 protects against sepsis-induced cardiac injury through inhibiting cardiomyocyte ferroptosis[J]. J Transl Int Med, 2024, 12(1): 35-50. |
| 3 | Zhao XJ, Xie JG, Duan CJ, et al. ADAR1 protects pulmonary macrophages from sepsis-induced pyroptosis and lung injury through miR-21/A20 signaling[J]. Int J Biol Sci, 2024, 20(2): 464-85. |
| 4 | Liu ZJ, Wei JH, Sun HB, et al. Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways[J]. J Cell Mol Med, 2024, 28(13): e18386. |
| 5 | Zhang QR, Fu HT, Gong WJ, et al. Plumbagin protects H9c2 cardiomyocytes against TBHP-induced cytotoxicity by alleviating ROS-induced apoptosis and modulating autophagy[J]. Exp Ther Med, 2022, 24(2): 501. |
| 6 | Li Z, Chinnathambi A, Ali Alharbi S, et al. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats[J]. Environ Toxicol, 2020, 35(12): 1374-85. |
| 7 | Petrocelli G, Marrazzo P, Bonsi L, et al. Plumbagin, a natural compound with several biological effects and anti-inflammatory properties[J]. Life, 2023, 13(6): 1303. |
| 8 | Wang SX, Wang J, Shao JB, et al. Plumbagin mediates cardioprotection against myocardial ischemia/reperfusion injury through nrf-2 signaling[J]. Med Sci Monit, 2016, 22: 1250-7. |
| 9 | Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula[J]. J Ethnopharmacol, 2023, 309: 116306. |
| 10 | Zhang ZX, Deng WJ, Kang R, et al. Plumbagin protects mice from lethal sepsis by modulating immunometabolism upstream of PKM2[J]. Mol Med, 2016, 22: 162-72. |
| 11 | Huang YH, Li L, Li YP, et al. Knockdown of LncRNA Lcn2-204 alleviates sepsis-induced myocardial injury by regulation of iron overload and ferroptosis[J]. J Mol Cell Cardiol, 2024, 192: 79-93. |
| 12 | Wang J, Wang XT, Liu DW, et al. Induction and deduction in sepsis-induced cardiomyopathy: five typical categories[J]. Chin Med J, 2020, 133(18): 2205-11. |
| 13 | Kumar Arora M, Ratra A, Asdaq SMB, et al. Plumbagin alleviates intracerebroventricular-quinolinic acid induced depression-like behavior and memory deficits in wistar rats[J]. Molecules, 2022, 27(6): 1834. |
| 14 | Guo YX, Liu L, Yan DZ, et al. Plumbagin prevents osteoarthritis in human chondrocytes through Nrf-2 activation[J]. Mol Med Rep, 2017, 15(4): 2333-8. |
| 15 | Monnet X, Lai C, Teboul JL. How I personalize fluid therapy in septic shock[J]? Crit Care,2023,27(1):123. |
| 16 | Vigneron C, Py BF, Monneret G, et al. The double sides of NLRP3 inflammasome activation in sepsis[J]. Clin Sci, 2023, 137(5): 333-51. |
| 17 | Yang R, Zhang XJ. A potential new pathway for heparin treatment of sepsis-induced lung injury: inhibition of pulmonary endothelial cell pyroptosis by blocking hMGB1-LPS-induced caspase-11 activation[J]. Front Cell Infect Microbiol, 2022, 12: 984835. |
| 18 | Sheng SY, Li JM, Hu XY, et al. Regulated cell death pathways in cardiomyopathy[J]. Acta Pharmacol Sin, 2023, 44(8): 1521-35. |
| 19 | Wu ZF, Deng JH, Zhou HW, et al. Programmed cell death in sepsis associated acute kidney injury[J]. Front Med, 2022, 9: 883028. |
| 20 | Cao ZZ, Qin HQ, Huang YH, et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model[J]. Bioengineered, 2022, 13(3): 4810-20. |
| 21 | Zangiabadi S, Abdul-Sater AA. Regulation of the NLRP3 inflammasome by posttranslational modifications[J]. J Immunol, 2022, 208(2): 286-92. |
| 22 | Jia YJ, Xiong S, Yao M, et al. HMGB1 inhibition blocks ferroptosis and oxidative stress to ameliorate sepsis-induced acute lung injury by activating the Nrf2 pathway[J]. Kaohsiung J Med Sci, 2024, 40(8): 710-21. |
| 23 | 梁 欢, 黄毓慧, 高 琴.非经典途径细胞焦亡在脓毒症等炎症性疾病中的作用[J].中南大学学报(医学版), 2021, 46(11): 1276-84. |
| 24 | Li X, Wei SZ, Niu SQ, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis[J]. Comput Biol Med, 2022, 144: 105389. |
| 25 | Yuan ZZ, Pan YY, Leng T, et al. Progress and prospects of research ideas and methods in the network pharmacology of traditional Chinese medicine[J]. J Pharm Pharm Sci, 2022, 25: 218-26. |
| 26 | Li YR, Jia YJ, Cui TF, et al. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac[J]. Exp Ther Med, 2021, 22(2): 890. |
| 27 | Foers AD, Garnham AL, Chatfield S, et al. Extracellular vesicles in synovial fluid from rheumatoid arthritis patients contain miRNAs with capacity to modulate inflammation[J]. Int J Mol Sci, 2021, 22(9): 4910. |
| 28 | Wang C, Liu N, Yang HT. Desflurane pretreatment can reduce sepsis-evoked lung injury in rats via inhibiting STAT3 pathway[J]. J Biol Regul Homeost Agents, 2020, 34(3): 935-42. |
| 29 | Jiang T, Peng DW, Shi W, et al. IL-6/STAT3 signaling promotes cardiac dysfunction by upregulating FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes formation in sepsis mice[J]. Front Cardiovasc Med, 2022, 8: 790612. |
| 30 | Li RQ, Li XQ, Zhao J, et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation[J]. Theranostics, 2022, 12(2): 976-98. |
| 31 | Shen YN, Zhang Y, Du JY, et al. CXCR5 down-regulation alleviates cognitive dysfunction in a mouse model of sepsis-associated encephalopathy: potential role of microglial autophagy and the p38MAPK/NF‑κB/STAT3 signaling pathway[J]. J Neuroinfl-ammation, 2021, 18(1): 246. |
| 32 | Xie LP, Wu YT, Zhou CY, et al. Piceatannol protects against sepsis-induced myocardial dysfunction via direct inhibition of JAK2[J]. Int Immunopharmacol, 2021, 96: 107639. |
| 33 | Zhen GS, Liang W, Jia HM, et al. Melatonin relieves sepsis-induced myocardial injury via regulating JAK2/STAT3 signaling pathway[J]. Minerva Med, 2022, 113(6): 983-9. |
| 34 | 孙 永, 史兆博, 刘美香, 等. 山药多糖对脓毒症大鼠心肌损伤及JAK2/STAT3信号通路的影响[J]. 中国动脉硬化杂志, 2022, 30(8): 669-75. |
| 35 | Jiang JX, Zhang D, Liu W, et al. Overexpression of NLRP12 enhances macrophage immune response and alleviates herpes simplex keratitis[J]. Front Cell Infect Microbiol, 2024, 14: 1416105. |
| [1] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [2] | 周海忆, 何斯怡, 韩瑞芳, 关永格, 董丽娟, 宋阳. 艾灸通过调控miR-223-3p/NLRP3焦亡通路修复薄型子宫内膜[J]. 南方医科大学学报, 2025, 45(7): 1380-1388. |
| [3] | 王立明, 陈宏睿, 杜燕, 赵鹏, 王玉洁, 田燕歌, 刘新光, 李建生. 益气滋肾方通过抑制PI3K/Akt/NF-κB通路改善小鼠慢性阻塞性肺疾病的炎症反应[J]. 南方医科大学学报, 2025, 45(7): 1409-1422. |
| [4] | 朱胤福, 李怡燃, 王奕, 黄颖而, 龚昆翔, 郝文波, 孙玲玲. 桂枝茯苓丸活性成分常春藤皂苷元通过抑制JAK2/STAT3通路抑制宫颈癌细胞的生长[J]. 南方医科大学学报, 2025, 45(7): 1423-1433. |
| [5] | 何丽君, 陈晓菲, 闫陈昕, 师林. 扶正化积汤治疗非小细胞肺癌的分子机制:基于网络药理学及体外实验验证[J]. 南方医科大学学报, 2025, 45(6): 1143-1152. |
| [6] | 李国永, 黎仁玲, 刘艺婷, 柯宏霞, 李菁, 王新华. 牛蒡子治疗小鼠病毒性肺炎后肺纤维化的机制:基于代谢组学、网络药理学和实验验证方法[J]. 南方医科大学学报, 2025, 45(6): 1185-1199. |
| [7] | 管丽萍, 颜燕, 卢心怡, 李智峰, 高晖, 曹东, 侯晨曦, 曾靖宇, 李欣怡, 赵洋, 王俊杰, 方会龙. 复方积雪草减轻小鼠日本血吸虫引起的肝纤维化:通过调控TLR4/MyD88通路抑制炎症-纤维化级联反应[J]. 南方医科大学学报, 2025, 45(6): 1307-1316. |
| [8] | 唐培培, 谈勇, 殷燕云, 聂晓伟, 黄菁宇, 左文婷, 李玉玲. 调周滋阴方治疗早发性卵巢功能不全的疗效、安全性及作用机制[J]. 南方医科大学学报, 2025, 45(5): 929-941. |
| [9] | 卞芬兰, 倪诗垚, 赵鹏, 戚毛男星, 唐碧, 王洪巨, 康品方, 刘进军. 积雪草苷通过抑制NLRP3炎症体介导的细胞焦亡减轻大鼠心肌缺血再灌注损伤[J]. 南方医科大学学报, 2025, 45(5): 977-985. |
| [10] | 梁晓涛, 熊一凡, 刘雪琪, 梁小珊, 朱晓煜, 谢炜. 活血疏风颗粒通过抑制TLR4/NF-κB通路改善慢性偏头痛小鼠的中枢敏化[J]. 南方医科大学学报, 2025, 45(5): 986-994. |
| [11] | 孙亚磊, 罗萌, 郭长胜, 高静, 苏凯奇, 陈立典, 冯晓东. 穗花杉双黄酮通过抑制细胞焦亡减轻小鼠急性肺损伤[J]. 南方医科大学学报, 2025, 45(4): 692-701. |
| [12] | 朱正望, 王琳琳, 赵静涵, 马瑞雪, 余雨春, 蔡庆春, 王兵, 朱平生, 苗明三. 退黄合剂通过调控法尼醇X受体抑制NLRP3炎症小体改善α-萘异硫氰酸酯诱导的大鼠胆汁淤积[J]. 南方医科大学学报, 2025, 45(4): 718-724. |
| [13] | 冉念东, 刘杰, 徐剑, 张永萍, 郭江涛. 黑骨藤正丁醇萃取成分治疗大鼠阿尔茨海默病的药效学及作用机制[J]. 南方医科大学学报, 2025, 45(4): 785-798. |
| [14] | 黄菊, 殷丽霞, 牛民主, 耿志军, 左芦根, 李静, 胡建国. 紫花前胡苷通过抑制肠上皮细胞焦亡改善2,4,6-三硝基苯磺酸诱导的小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(2): 261-268. |
| [15] | 徐皓男, 张放, 黄钰莹, 姚其盛, 管悦琴, 陈浩. 百蕊草通过调节肠道菌群和调控EGFR/PI3K/Akt信号通路改善小鼠抗生素相关性腹泻[J]. 南方医科大学学报, 2025, 45(2): 285-295. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||