南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (9): 1685-1695.doi: 10.12122/j.issn.1673-4254.2024.09.08
纪凯1,2( ), 于冠宇2, 周乐其2, 张天帅2, 凌潜龙1, 满文江1,2, 朱冰1(
), 于冠宇2, 周乐其2, 张天帅2, 凌潜龙1, 满文江1,2, 朱冰1( ), 张卫2(
), 张卫2( )
)
收稿日期:2024-05-19
出版日期:2024-09-20
发布日期:2024-10-31
通讯作者:
朱冰,张卫
E-mail:1361679354@qq.com;bbmczhubing@163.com;weizhang2000cn@163.com
作者简介:纪 凯,在读硕士研究生,E-mail: 1361679354@qq.com
基金资助:
Kai JI1,2( ), Guanyu YU2, Leqi ZHOU2, Tianshuai ZHANG2, Qianlong LING1, Wenjiang MAN1,2, Bing ZHU1(
), Guanyu YU2, Leqi ZHOU2, Tianshuai ZHANG2, Qianlong LING1, Wenjiang MAN1,2, Bing ZHU1( ), Wei ZHANG2(
), Wei ZHANG2( )
)
Received:2024-05-19
Online:2024-09-20
Published:2024-10-31
Contact:
Bing ZHU, Wei ZHANG
E-mail:1361679354@qq.com;bbmczhubing@163.com;weizhang2000cn@163.com
Supported by:摘要:
目的 通过生物信息学和细胞实验探究HNRNP A1在结直肠癌中的临床意义及其在肿瘤组织中的表达情况。 方法 使用HPA、TIMER和GEPIA数据库,分析HNRNP A1在结直肠癌组织中的表达水平,并检验HNRNP A1与Ki-67/VEGFA在结直肠癌中表达的相关性。使用Kaplan-Meier Plotter数据库评估HNRNP A1 mRNA水平与结直肠癌患者生存率之间的联系。通过基因富集途径分析,预测HNRNP A1在结直肠癌中的潜在生物学作用。通过免疫组织化学(IHC)和Western blotting技术检测HNRNP A1在结直肠癌及其癌旁组织中的蛋白表达。利用TIMER数据库网站对HNRNP A1在免疫浸润细胞中的表达进行预测。使用慢病毒敲低RKO/Caco2细胞中HNRNP A1的表达;通过CCK-8实验检测细胞增殖,使用克隆形成实验检测HNRNP A1对细胞增殖能力的影响;利用细胞划痕实验和Transwell迁移实验评估两组细胞(RKO/Caco2-nc、RKO/Caco2-sh)的迁移能力。最后验证HNRNP A1小分子抑制剂(VPC-80051)对肿瘤细胞增殖的影响。 结果 在结直肠癌(CRC)肿瘤组织中HNRNP A1的表达显著上调并与患者的不良预后显著相关(P<0.01)。TIMER数据库的分析结果指出,HNRNP A1与肿瘤微环境中的免疫细胞之间存在一定的相关性。根据GEPIA数据库的分析,CRC组织中HNRNP A1、MKI67和VEGFA的表达均较高(P<0.05),且HNRNP A1与这两者之间存在正相关关系。通过Kaplan-Meier Plotter进行的生存分析表明,在CRC中,HNRNP A1的高表达预示着较差的总生存期(P=0.0081)和无进展生存期(P=0.012)。基因富集通路分析的数据显示,HNRNP A1可能参与到多个与CRC进展相关的生物途径中。HNRNP A1影响RKO/Caco2细胞的增殖和迁移能力,对照组(RKO/Caco2-nc)的增殖能力、克隆形成能力和迁移能力均优于实验组(RKO/Caco2-sh),差异有统计学意义(P<0.05);HNRNP A1小分子抑制剂(VPC-80051)可以有效抑制结直肠癌增殖活性,并具有时间和浓度依赖性;IHC显示HNRNP A1在结直肠癌中高表达,且与肿瘤分期有密切关系(P<0.0001)。 结论 HNRNP A1基因在CRC组织中表达较高,并可调节细胞的增殖和迁移能力,与不良预后密切相关,同时HNRNP A1小分子抑制剂(VPC-80051)也可以抑制结直肠癌细胞的增殖,因而可作为CRC治疗过程中新的潜在治疗靶点。
纪凯, 于冠宇, 周乐其, 张天帅, 凌潜龙, 满文江, 朱冰, 张卫. HNRNPA1基因在结直肠癌组织中高表达及其潜在的诊断和治疗价值[J]. 南方医科大学学报, 2024, 44(9): 1685-1695.
Kai JI, Guanyu YU, Leqi ZHOU, Tianshuai ZHANG, Qianlong LING, Wenjiang MAN, Bing ZHU, Wei ZHANG. HNRNPA1 gene is highly expressed in colorectal cancer: its prognostic implications and potential as a therapeutic target[J]. Journal of Southern Medical University, 2024, 44(9): 1685-1695.
| Gene | Primerse quence (5'-3') | |
|---|---|---|
| Forward | Reverse | |
| HNRNP A1 | TCAGAGTCTCCTAAAGAGCCC | ACCTTGTGTGGCCTTGCAT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
表1 RT-PCR对差异表达mRNA的引物序列
Tab.1 Primer sequence for qRT-PCR of HNRNP A1
| Gene | Primerse quence (5'-3') | |
|---|---|---|
| Forward | Reverse | |
| HNRNP A1 | TCAGAGTCTCCTAAAGAGCCC | ACCTTGTGTGGCCTTGCAT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
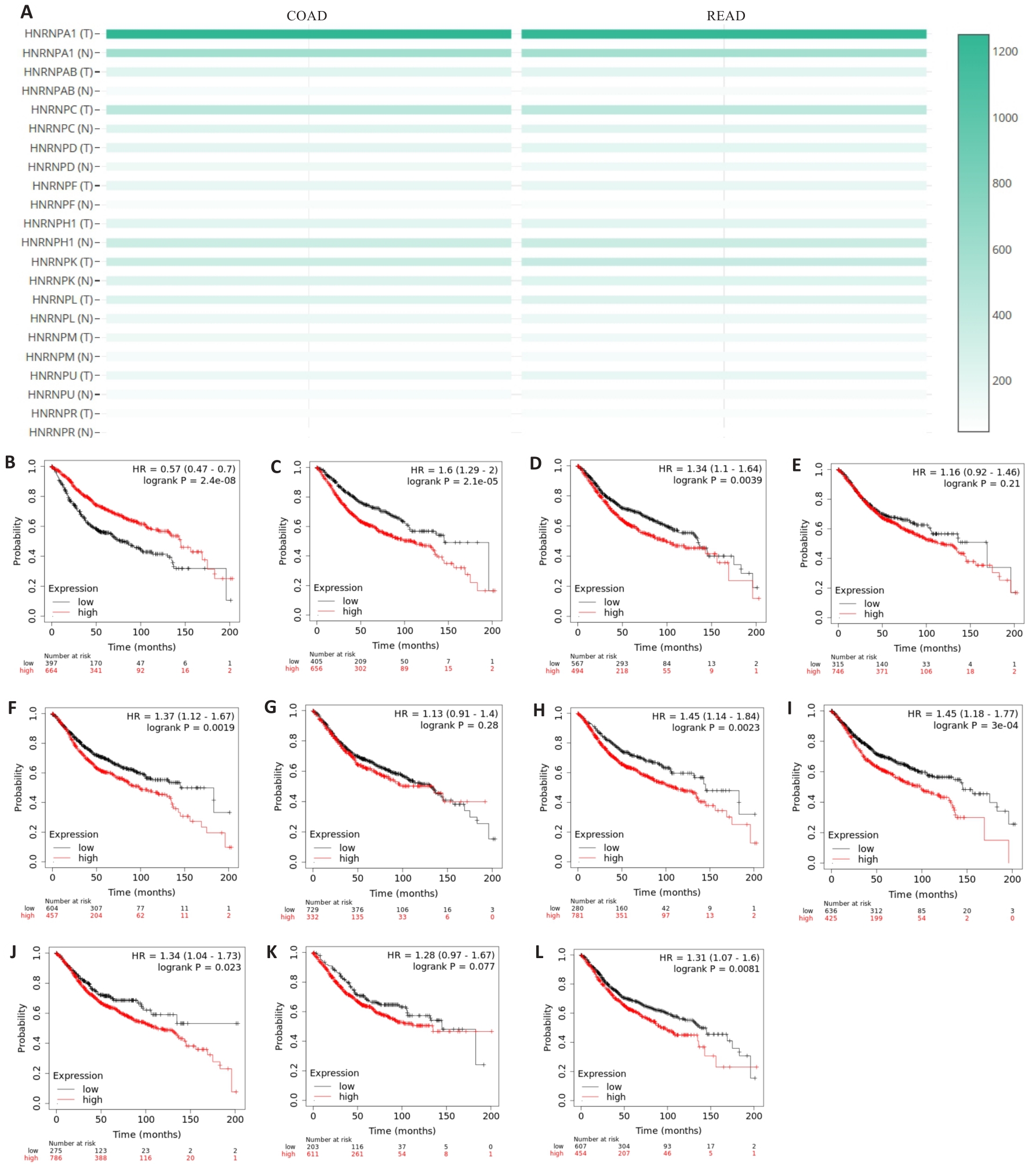
图1 HNRNP家族成员在结直肠癌中的表达及预后
Fig.1 Expression of HNRNP family members in colorectal cancer and their association with the patients' prognosis. A: Expression of HNRNP family members in colorectal cancer. B-L: Prognostic profile of HNRNP family members in colorectal cancer.

图2 HNRNP A1在细胞模型及人体细胞中的定位
Fig.2 Localization of HNRNPA1 in the cell model and human cells. A: Expression of HNRNP A1 in the cell model. B: Expression of HNRNPA1 in human cells detected using immunofluorescence assay.

图3 HNRNP A1在各种组织和免疫细胞中的表达
Fig.3 Expression of HNRNP A1 in different tissues and immune cells. A: Expression of HNRNP A1 in different tissues. B: Expression of HNRNP A1 in immune cells. C: Expression of HNRNP A1 in the core cells.

图4 HNRNP A1在结直肠癌和癌旁组织中的表达情况
Fig. 4 Expression of HNRNP A1 in colorectal cancer and adjacent tissues. A: Expression of HNRNP A1 in different tumors in TIMER 2.0 database. B: Expression of HNRNP A1 in colorectal cancer in GEPIA database. C: Immunohistochemical analysis of SPHK1 expression in colorectal cancer tissue and adjacent tissue.

图5 HNRNP A1与MKI67/VEGFA相关性与患者预后的关系
Fig.5 Correlation of HNRNP A1 with MKI67 and VEGFA expressions and prognosis of colorectal cancer patients. A: Correlation between HNRNP A1 and MKI67 expression. B: Correlation between HNRNP A1 and VEGFA expression. C: Expression of MKI67 in CRC tissue. D: Expression of VEGFA in colorectal cancer tissue. E: Expression of HNRNPA1 in COAD based on individual cancer stages. F: Expression of HNRNPA1 in READ based on individual cancer stages. G: Overall survival of colorectal cancer patients. H: Post progression survival in colorectal cancer patients.

图6 结直肠癌中HNRNP A1与免疫浸润水平的相关性
Fig.6 Correlation between HNRNP A1 and immune infiltration level in colorectal cancer. A: Expression of HNRNP A1 correlates with immune cell infiltration in colon cancer microenvironment. B: Expression of HNRNP A1 correlates with immune cell infiltration in rectal cancer microenvironment.

图7 HNRNP A1的通路富集结果
Fig.7 Enrichment analysis of HNRNP A1 pathways. A: GOterm analysis of BP. B: GOterm analysis of CC. C: GOterm analysis of MF. D: KEGGterm analysis result.

图8 HNRNP A1对CRC细胞的增殖、迁移的影响
Fig.8 HNRNP A1 promotes proliferation and migration of colorectal cancer cells. A, B, G, H: Relative expression level of HNRNPA1 in RKO cells. C, I: CCK-8 assay for assessing cell proliferation. D, J: Clone formation assay. E, K: Wound-healing assay for assessing cell migration (Scale bar: 200 μm). F, L: Transwell assay for assessing cell migration (Scale bar: 50 μm). M, N: Effect of VPC-80051 at different concentrations on cell proliferation. O: Viability of the cells treated with 10 μmol/L VPC-80051 at different time points. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
| Parameters | Clinical case characteristics | |
|---|---|---|
| Case | Rate (%) | |
| Gender | ||
| Male | 29 | 59.2 |
| Female | 20 | 40.8 |
| Age (year) | ||
| ≥60 | 32 | 65.3 |
| <60 | 17 | 34.7 |
| Pathologic stage | ||
| Tis | 2 | 4.08 |
| Ⅰ+Ⅱ | 25 | 51.02 |
| Ⅲ+Ⅳ | 22 | 44.9 |
| T stage | ||
| T1+T2 | 14 | 29.79 |
| T3+T4 | 33 | 70.21 |
| N stage | ||
| N0 | 25 | 53.19 |
| N1+N2 | 22 | 46.81 |
表 2 HNRNP A1表达与临床病例特点的关系
Tab.2 Relationship between HNRNP A1 expression and clinical characteristics of the patients
| Parameters | Clinical case characteristics | |
|---|---|---|
| Case | Rate (%) | |
| Gender | ||
| Male | 29 | 59.2 |
| Female | 20 | 40.8 |
| Age (year) | ||
| ≥60 | 32 | 65.3 |
| <60 | 17 | 34.7 |
| Pathologic stage | ||
| Tis | 2 | 4.08 |
| Ⅰ+Ⅱ | 25 | 51.02 |
| Ⅲ+Ⅳ | 22 | 44.9 |
| T stage | ||
| T1+T2 | 14 | 29.79 |
| T3+T4 | 33 | 70.21 |
| N stage | ||
| N0 | 25 | 53.19 |
| N1+N2 | 22 | 46.81 |
| 1 | Xi Y, Xu PF. Global colorectal cancer burden in 2020 and projections to 2040[J]. Transl Oncol, 2021, 14(10): 101174. |
| 2 | Ahmad R, Singh JK, Wunnava A, et al. Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review)[J]. Int J Mol Med, 2021, 47(3): 14. |
| 3 | Klimeck L, Heisser T, Hoffmeister M, et al. Colorectal cancer: a health and economic problem[J]. Best Pract Res Clin Gastroenterol, 2023, 66: 101839. |
| 4 | Li QY, Zhao HX, Dong WW, et al. RAB27A promotes the proliferation and invasion of colorectal cancer cells[J]. Sci Rep, 2022, 12(1): 19359. |
| 5 | Siculella L, Giannotti L, Di Chiara Stanca B, et al. A comprehensive understanding of hnRNP A1 role in cancer: new perspectives on binding with noncoding RNA[J]. Cancer Gene Ther, 2023, 30(3): 394-403. |
| 6 | 杨宏广, 童春梅, 邓惠敏. hnRNP A1的功能研究进展[J]. 生物化学与生物物理进展, 2021, 48(10): 1146-56. |
| 7 | Liu X, Ishizuka T, Bao HL, et al. Structure-dependent binding of hnRNPA1 to telomere RNA[J]. J Am Chem Soc, 2017, 139(22): 7533-9. |
| 8 | Li YX, Yang Y, Ma Q, et al. HNRNPK/CLCN3 axis facilitates the progression of LUAD through CAF-tumor interaction[J]. Int J Biol Sci, 2022, 18(16): 6084-101. |
| 9 | Li MY, Yang XJ, Zhang GX, et al. Heterogeneous nuclear ribonucleoprotein K promotes the progression of lung cancer by inhibiting the p53-dependent signaling pathway[J]. Thorac Cancer, 2022, 13(9): 1311-21. |
| 10 | Jiang RQ, Su GF, Chen X, et al. Esculetin inhibits endometrial cancer proliferation and promotes apoptosis via hnRNPA1 to down-regulate BCLXL and XIAP[J]. Cancer Lett, 2021, 521: 308-21. |
| 11 | Xu HR, Li P, Wang XY, et al. Emerging roles of hnRNP A2B1 in cancer and inflammation[J]. Int J Biol Macromol, 2022, 221: 1077-92. |
| 12 | Möller K, Wecker AL, Höflmayer D, et al. Upregulation of the heterogeneous nuclear ribonucleoprotein hnRNPA1 is an independent predictor of early biochemical recurrence in TMPRSS2: ERG fusion-negative prostate cancers[J]. Virchows Arch, 2020, 477(5): 625-36. |
| 13 | Ma YL, Peng JY, Zhang P, et al. Heterogeneous nuclear ribonucleoprotein A1 is identified as a potential biomarker for colorectal cancer based on differential proteomics technology[J]. J Proteome Res, 2009, 8(10): 4525-35. |
| 14 | Ghosh M, Singh M. RGG-box in hnRNPA1 specifically recognizes the telomere G-quadruplex DNA and enhances the G-quadruplex unfolding ability of UP1 domain[J]. Nucleic Acids Res, 2018, 46(19): 10246-61. |
| 15 | Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review[J]. JAMA, 2021, 325(7): 669-85. |
| 16 | Liu Y, Shi SL. The roles of hnRNP A2/B1 in RNA biology and disease[J]. Wiley Interdiscip Rev RNA, 2021, 12(2): e1612. |
| 17 | Xie W, Zhu HC, Zhao M, et al. Crucial roles of different RNA-binding hnRNP proteins in Stem Cells[J]. Int J Biol Sci, 2021, 17(3): 807-17. |
| 18 | Dutta K, Kravtsov V, Oleynikova K, et al. Analyzing the effects of single nucleotide polymorphisms on hnRNPA2/B1 protein stability and function: insights for anticancer therapeutic design[J]. ACS Omega, 2024, 9(5): 5485-95. |
| 19 | Low YH, Asi Y, Foti SC, et al. Heterogeneous nuclear ribonucleoproteins: implications in neurological diseases[J]. Mol Neurobiol, 2021, 58(2): 631-46. |
| 20 | Kim HJ, Kim NC, Wang YD, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS[J]. Nature, 2013, 495(7442): 467-73. |
| 21 | Xia AL, Yuan WW, Wang Q, et al. The cancer-testis lncRNA lnc-CTHCC promotes hepatocellular carcinogenesis by binding hnRNP K and activating YAP1 transcription[J]. Nat Cancer, 2022, 3(2): 203-18. |
| 22 | Gao R, Yu Y, Inoue A, et al. Heterogeneous nuclear ribonucleoprotein K (hnRNP-K) promotes tumor metastasis by induction of genes involved in extracellular matrix, cell movement, and angiogenesis[J]. J Biol Chem, 2013, 288(21): 15046-56. |
| 23 | Zhu HE, Li T, Shi SN, et al. ESCO2 promotes lung adenocarcinoma progression by regulating hnRNPA1 acetylation[J]. J Exp Clin Cancer Res, 2021, 40(1): 64. |
| 24 | Zhou JM, Jiang H, Yuan T, et al. High hnRNP AB expression is associated with poor prognosis in patients with colorectal cancer[J]. Oncol Lett, 2019, 18(6): 6459-68. |
| 25 | Li H, Liu JW, Shen SX, et al. Pan-cancer analysis of alternative splicing regulator heterogeneous nuclear ribonucleoproteins (hnRNPs) family and their prognostic potential[J]. J Cell Mol Med, 2020, 24(19): 11111-9. |
| 26 | Shi X, Ran L, Liu Y, et al. Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway[J]. Oncol Rep, 2018, 39(3): 939-50. |
| 27 | Zhang PS, Ji DH, Hu XH, et al. Oncogenic heterogeneous nuclear ribonucleoprotein D-like promotes the growth of human colon cancer SW620 cells via its regulation of cell-cycle[J]. Acta Biochim Biophys Sin, 2018, 50(9): 880-7. |
| 28 | Yao P, Wu JB, Lindner D, et al. Interplay between miR-574-3p and hnRNP L regulates VEGFA mRNA translation and tumorigenesis[J]. Nucleic Acids Res, 2017, 45(13): 7950-64. |
| 29 | Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy[J]. Front Immunol, 2022, 13: 954992. |
| 30 | Khan S, Kwak YT, Peng L, et al. NLRP12 downregulates the Wnt/β-catenin pathway via interaction with STK38 to suppress colorectal cancer[J]. J Clin Invest, 2023, 133(19): e166295. |
| 31 | Bradley RK, Anczuków O. RNA splicing dysregulation and the hallmarks of cancer[J]. Nat Rev Cancer, 2023, 23(3): 135-55. |
| 32 | Wan LD, Yu WY, Shen EH, et al. SRSF6-regulated alternative splicing that promotes tumour progression offers a therapy target for colorectal cancer[J]. Gut, 2019, 68(1): 118-29. |
| 33 | Wen ZL, Lian LY, Ding H, et al. LncRNA ANCR promotes hepatocellular carcinoma metastasis through upregulating HNRNPA1 expression[J]. RNA Biol, 2020, 17(3): 381-94. |
| 34 | Carabet LA, Leblanc E, Lallous N, et al. Computer-aided discovery of small molecules targeting the RNA splicing activity of hnRNP A1 in castration-resistant prostate cancer[J]. Molecules, 2019, 24(4): 763. |
| [1] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [2] | 庞金龙, 赵新丽, 张振, 王豪杰, 周星琦, 杨玉梅, 李姗姗, 常小强, 李锋, 李娴. 皮肤黑色素瘤中MMRN2高表达促进肿瘤细胞的侵袭和迁移并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1479-1489. |
| [3] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [4] | 王康, 李海宾, 余靖, 孟源, 张虹丽. ELFN1高表达是结肠癌的预后生物标志物并促进结肠癌细胞的增殖转移[J]. 南方医科大学学报, 2025, 45(7): 1543-1553. |
| [5] | 翁诺舟, 谭彬, 曾文涛, 古家宇, 翁炼基, 郑克鸿. 过表达RGL1通过激活CDC42/RAC1复合体上调运动型黏着斑组装促进结直肠癌转移[J]. 南方医科大学学报, 2025, 45(5): 1031-1038. |
| [6] | 马振南, 刘福全, 赵雪峰, 张晓微. DTX2促进奥沙利铂耐药的结直肠癌细胞增殖、侵袭和上皮间质转化[J]. 南方医科大学学报, 2025, 45(4): 829-836. |
| [7] | 张毅, 沈昱, 万志强, 陶嵩, 柳亚魁, 王栓虎. CDKN3高表达促进胃癌细胞的迁移和侵袭:基于调控p53/NF-κB信号通路和抑制胃癌细胞凋亡[J]. 南方医科大学学报, 2025, 45(4): 853-861. |
| [8] | 高志, 吴傲, 胡仲翔, 孙培养. 类风湿性关节炎中氧化应激与免疫浸润的生物信息学分析[J]. 南方医科大学学报, 2025, 45(4): 862-870. |
| [9] | 庆顺杰, 沈智勇. 过表达己糖激酶2通过激活JAK/STAT途径促进结直肠癌细胞的增殖、迁移和侵袭并调节肿瘤免疫微环境[J]. 南方医科大学学报, 2025, 45(3): 542-553. |
| [10] | 黄晴晴, 张文静, 张小凤, 王炼, 宋雪, 耿志军, 左芦根, 王月月, 李静, 胡建国. 高表达MYO1B促进胃癌细胞增殖、迁移和侵袭并与患者的不良预后有关[J]. 南方医科大学学报, 2025, 45(3): 622-631. |
| [11] | 李华莉, 宋婷, 刘嘉雯, 李永宝, 姜兆静, 窦文, 周凌宏. 预后导向的肺癌调强放疗计划优化新方法[J]. 南方医科大学学报, 2025, 45(3): 643-649. |
| [12] | 宋雪, 陈悦, 张敏, 张诺, 左芦根, 李静, 耿志军, 张小凤, 王月月, 王炼, 胡建国. GPSM2在胃癌组织中高表达并通过促进肿瘤细胞的增殖影响患者预后[J]. 南方医科大学学报, 2025, 45(2): 229-238. |
| [13] | 唐天威, 李路安, 陈源汉, 张丽, 徐丽霞, 李志莲, 冯仲林, 张辉林, 华瑞芳, 叶智明, 梁馨苓, 李锐钊. 高血清胱抑素C水平是IgA肾病不良预后的独立危险因素[J]. 南方医科大学学报, 2025, 45(2): 379-386. |
| [14] | 许怀文, 翁丽, 薛鸿. CXCL12可作为2型糖尿病合并慢性阻塞性肺疾病的潜在治疗靶点[J]. 南方医科大学学报, 2025, 45(1): 100-109. |
| [15] | 陈晓睿, 魏青政, 张宗亮, 原江水, 宋卫青. 过表达带电多泡体蛋白2B基因抑制肾透明细胞癌细胞的增殖[J]. 南方医科大学学报, 2025, 45(1): 126-136. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||