南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (8): 1743-1750.doi: 10.12122/j.issn.1673-4254.2025.08.18
• • 上一篇
欧泽金1,2( ), 李瀛3, 陈诗3, 王梓译4, 何美仪4, 陈志成3, 唐侍豪1,2, 孟晓静3, 王致1,2(
), 李瀛3, 陈诗3, 王梓译4, 何美仪4, 陈志成3, 唐侍豪1,2, 孟晓静3, 王致1,2( )
)
收稿日期:2025-02-13
出版日期:2025-08-20
发布日期:2025-09-05
通讯作者:
王致
E-mail:ouzejin@smu.edu.cn;zhi_wang@outlook.com
作者简介:欧泽金,副研究员,E-mail: ouzejin@smu.edu.cn
基金资助:
Zejin OU1,2( ), Ying LI3, Shi CHEN3, Ziyi WANG4, Meiyi HE4, Zhicheng CHEN3, Shihao TANG1,2, Xiaojing MENG3, Zhi WANG1,2(
), Ying LI3, Shi CHEN3, Ziyi WANG4, Meiyi HE4, Zhicheng CHEN3, Shihao TANG1,2, Xiaojing MENG3, Zhi WANG1,2( )
)
Received:2025-02-13
Online:2025-08-20
Published:2025-09-05
Contact:
Zhi WANG
E-mail:ouzejin@smu.edu.cn;zhi_wang@outlook.com
摘要:
目的 通过构建斑马鱼敌草快急性中毒模型,探讨铁死亡在敌草快引起的急性肾损伤的作用及分子机制。 方法 采用肾脏标记Tg (Eco.Tshb:EGFP)和中性粒细胞标记Tg(lyz:dsRed2)的转基因斑马鱼构建急性肾损伤模型,设置空白对照组、庆大霉素阳性对照组、敌草快中毒组以及铁死亡抑制剂组,检测斑马鱼肾损伤、炎症反应以及铁死亡相关指标,采用Western blotting检测电压依赖性阴离子选择性通道蛋白1(VDAC1)和线粒体铁蛋白(FTMT)的表达水平。 结果 敌草快引起的急性肾损伤具有明显剂量效应关系,损伤程度与暴露浓度成正比,同时诱导明显的氧化应激和炎症反应。罗丹明代谢法和HE染色发现,肾小球过滤功能随着敌草快暴露浓度增加而下降(P<0.001)。免疫荧光显示,敌草快暴露后斑马鱼肾组织铁死亡标志物GPX4和FTH1的表达水平发生明显变化,而给予铁死亡抑制剂Ferrostatin-1干预后GPX4表达上调(P=0.040),FTH1表达下调(P=0.042),罗丹明B标记葡聚糖代谢率改善(P=0.024)。敌草快暴露引起VDAC1和FTMT表达水平上调(P<0.001),应用铁死亡抑制剂和VDAC1抑制剂VBIT-12后FTMT下调尤为明显。 结论 铁死亡参与敌草快致急性肾损伤的分子机制,并且VDAC1和FTMT参与其中的调控机制,可能是潜在的干预靶点。
欧泽金, 李瀛, 陈诗, 王梓译, 何美仪, 陈志成, 唐侍豪, 孟晓静, 王致. 抑制铁死亡减轻敌草快引起的斑马鱼急性肾损伤的机制[J]. 南方医科大学学报, 2025, 45(8): 1743-1750.
Zejin OU, Ying LI, Shi CHEN, Ziyi WANG, Meiyi HE, Zhicheng CHEN, Shihao TANG, Xiaojing MENG, Zhi WANG. Inhibition of ferroptosis alleviates acute kidney injury caused by diquat in zebrafish[J]. Journal of Southern Medical University, 2025, 45(8): 1743-1750.
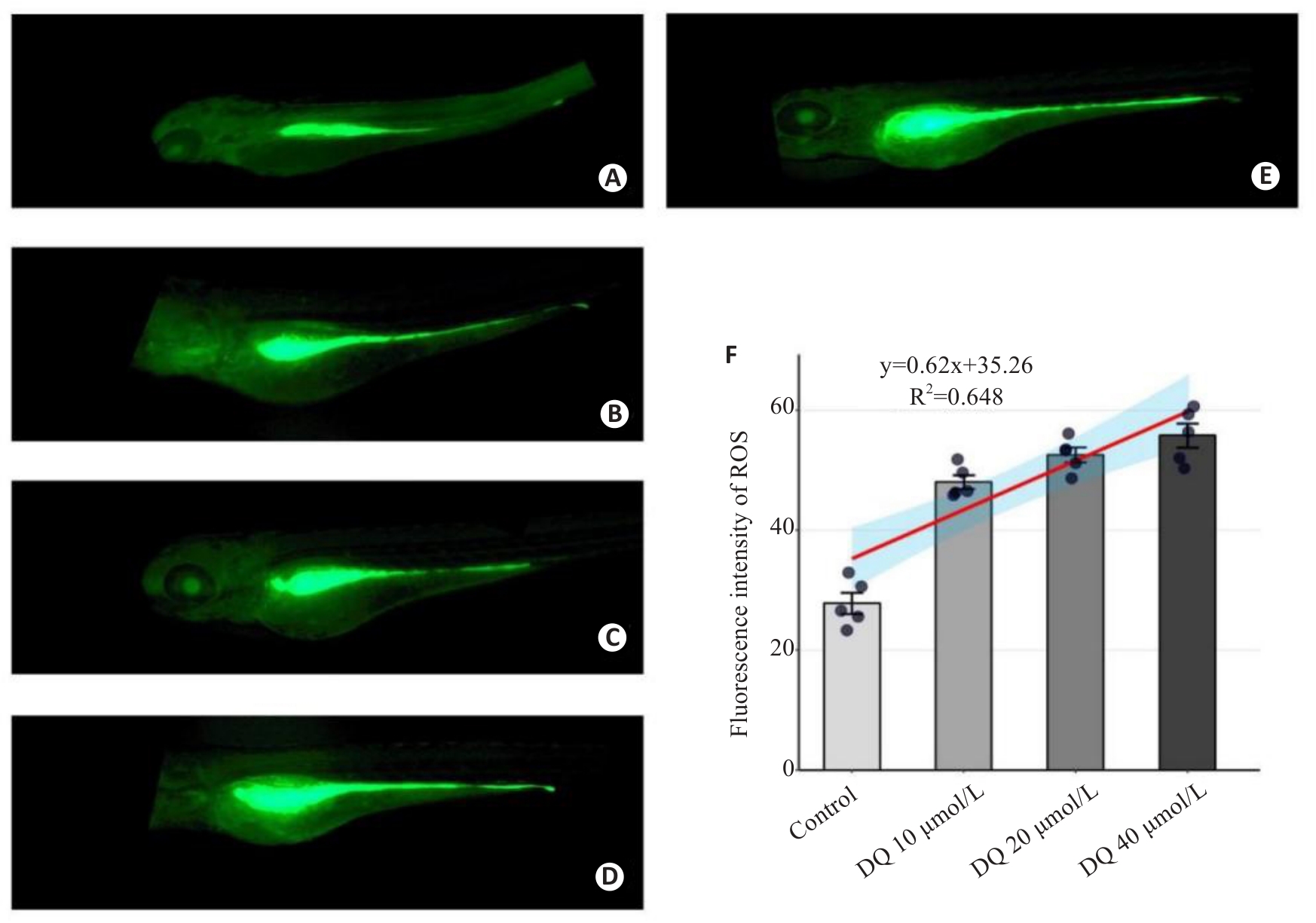
图3 荧光定量分析敌草快暴露对斑马鱼活体ROS水平的影响
Fig.3 Fluorescence quantitative analysis of the effect of diquat on ROS level in live-stained zebrafish (×40). A: Control group. B: Gentamicin-positive group. C: Diquat 10 μmol/L group. D: Diquat 20 μmol/L group. E: Diquat 40 μmol/L group. F: Results of quantitative analysis.
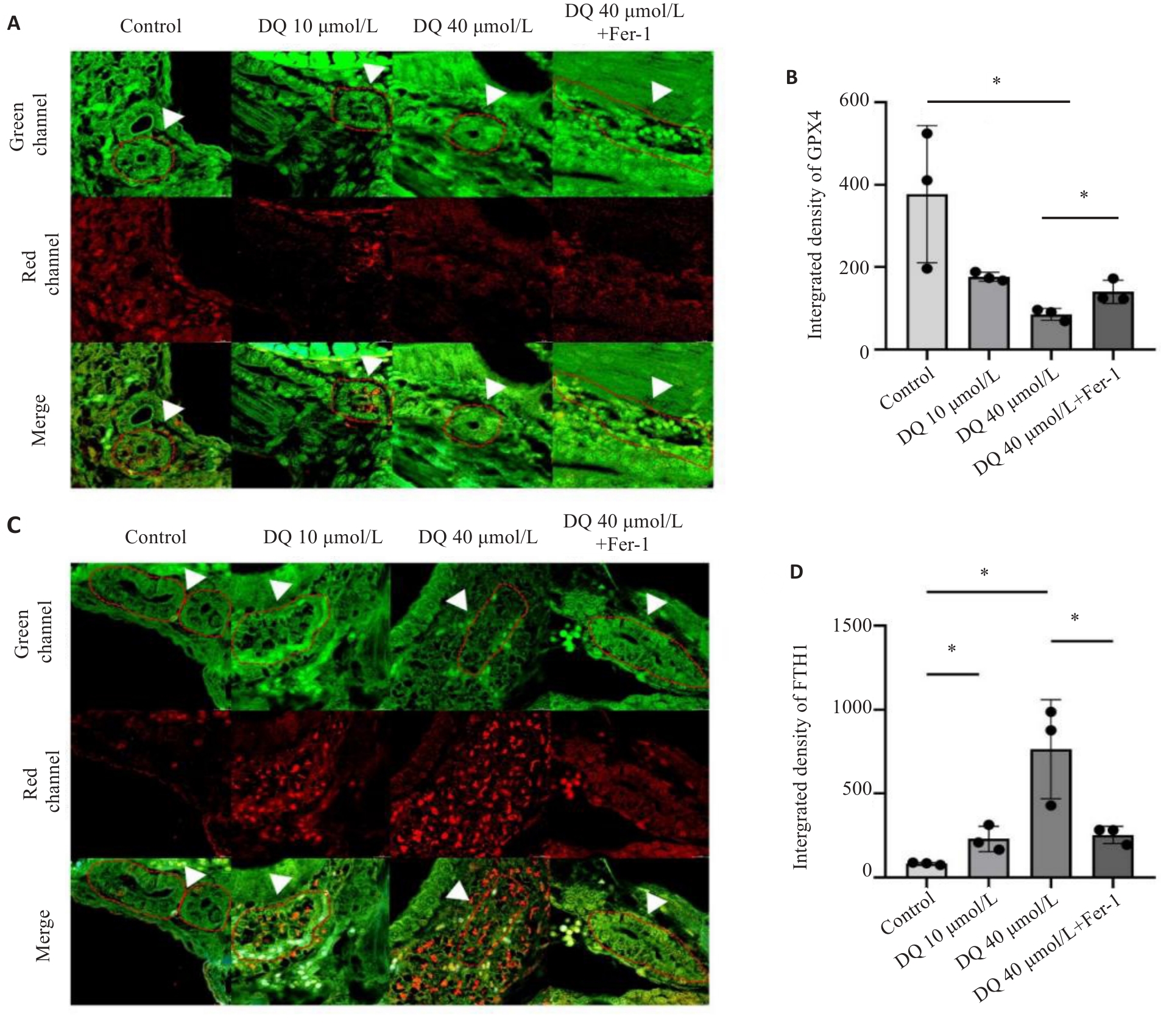
图4 抑制铁死亡对敌草快引起斑马鱼肾组织GPX4和FTH1表达影响
Fig 4 Effect of ferroptosis inhibition on expressions of GPX4 and FTH1 in the kidneys of diquat-exposed zebrafish (×400). A, B: Immunofluorescence and quantitative analysis of GPX4. C,D: Immunofluorescence and quantitative analysis of FTH1. The white arrows indicated the zebrafish kidney tissue. *P<0.05.
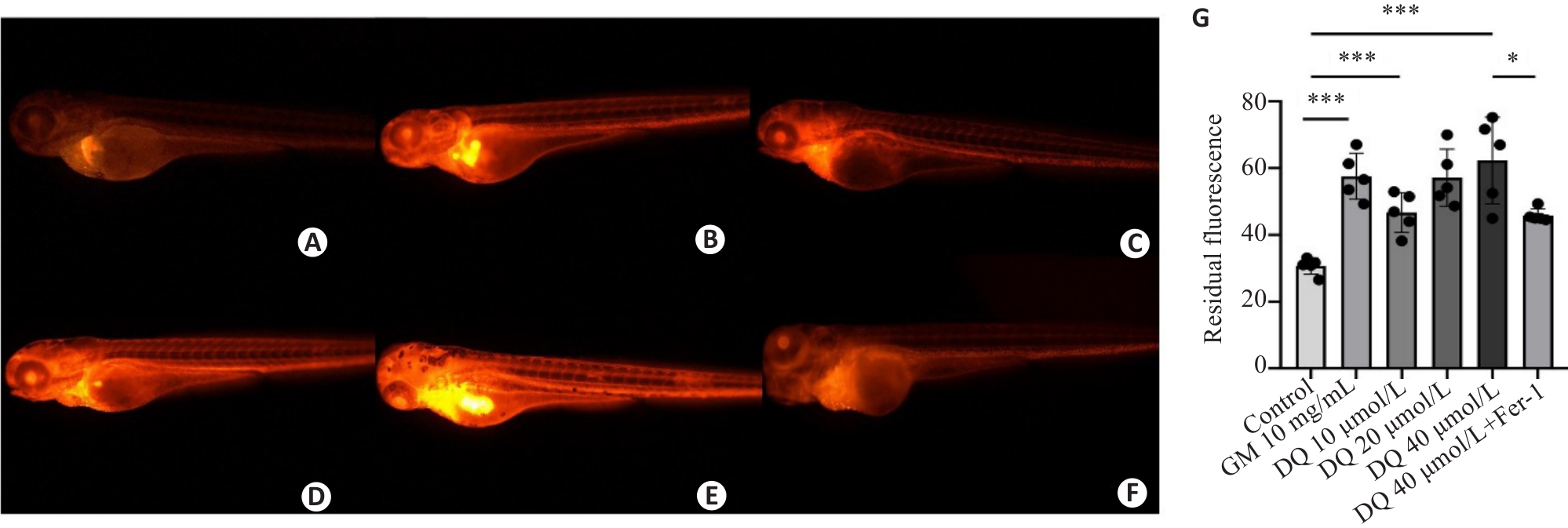
图5 敌草快暴露对斑马鱼肾小球滤过率的影响
Fig.5 Effect of diquat exposure on glomerular filtration rate in zebrafish (×40). The metabolism of rhodamine B-labeled dextran was detected using fluorescence quantification. A: Control group. B: Gentamicin-positive group. C: Diquat 10 μmol/L group. D: Diquat 20 μmol/L group. E: Diquat 40 μmol/L group. F: Diquat 40 μmol/L group+Fer-1 intervention group. G: The results of quantitative analysis. *P<0.05, ***P<0.001.
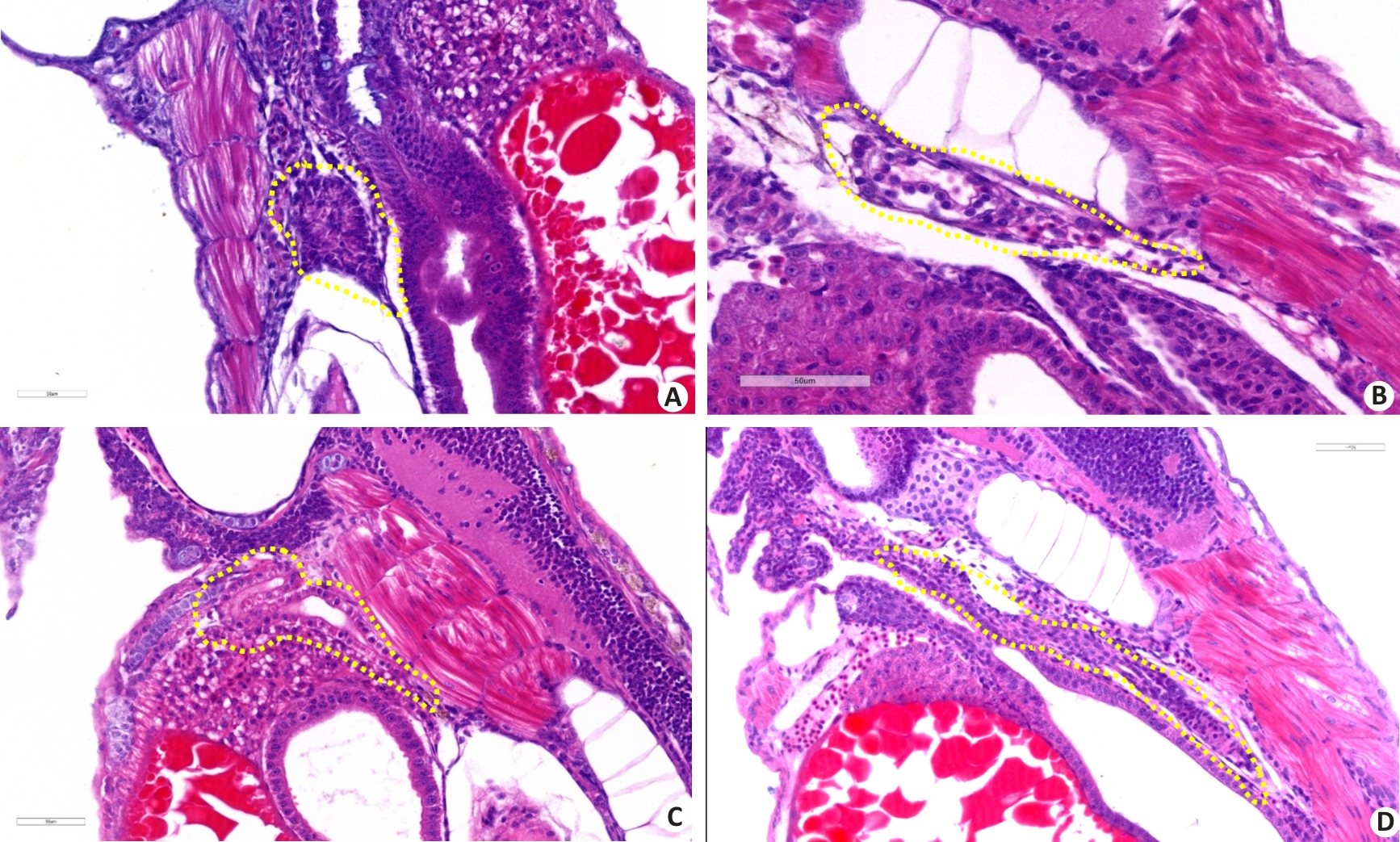
图6 病理学分析敌草快暴露对斑马鱼肾组织损伤的影响
Fig.6 Pathological analysis of the effect of diquat exposure on renal injury in zebrafish (HE staining, ×400). A: Control group. B: Gentamicin-positive group. C: Diquat exposure 40 μmol/L group. D: Diquat 40 μmol/L group+Fer-1 intervention group.
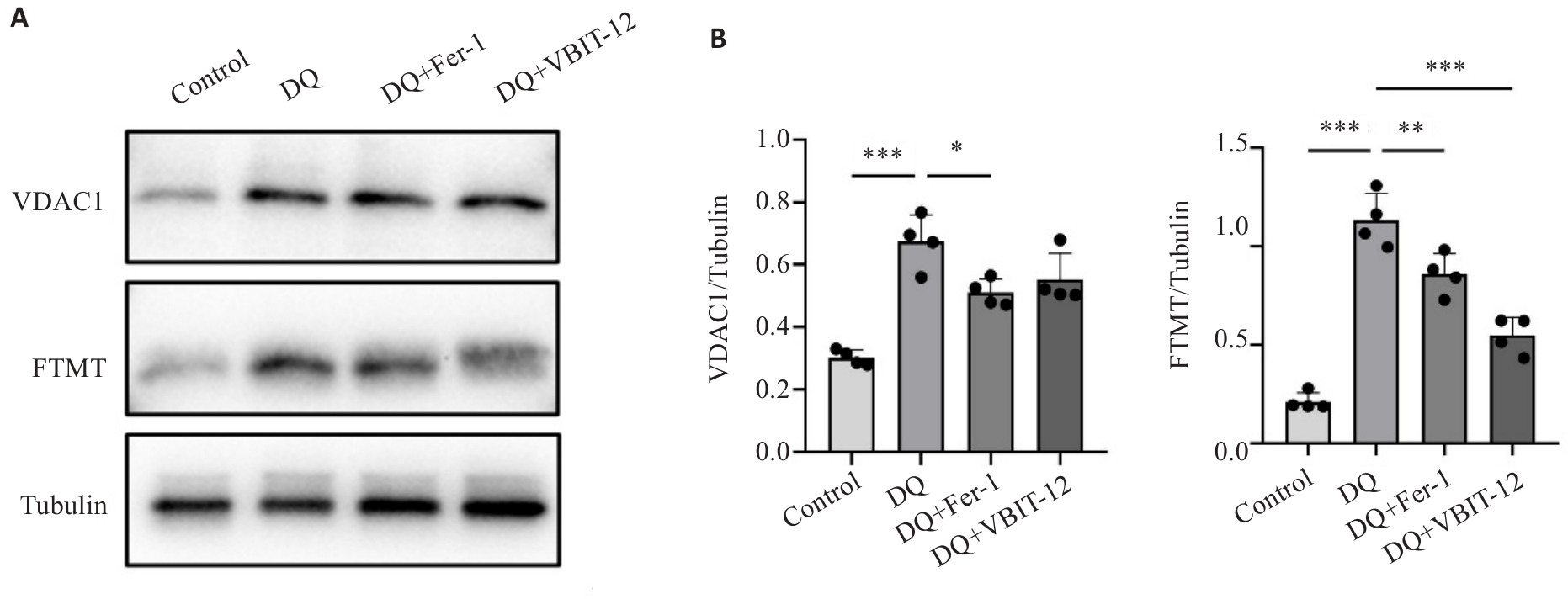
图7 敌草快暴露与干预对VDAC1和FTMT蛋白表达的影响
Fig.7 Effect of diquat exposure and drug interventions on protein expressions of VDAC1 and FTMT. A: Protein expressions of VDAC1 and FTMT detected using Western blotting. B: Quantitative analysis of the protein expressions (n=20). *P<0.05, **P<0.01, ***P<0.001. Each experimental repetition involved 20 larvae.
| [1] | Aloise DM, Memon A, Zaldivar A. Diquat herbicide organo-phosphate poisoning and multi-organ failure: a case report[J]. Cureus, 2022, 14(7): e27241. |
| [2] | 孟 娜, 孙艺青, 刘 亮, 等. 急性敌草快中毒86例临床分析[J]. 中华危重病急救医学, 2022(3): 301-5. doi:10.3760/cma.j.cn121430-20220128-00105 |
| [3] | 敌草快中毒诊断与治疗专家共识组. 急性敌草快中毒诊断与治疗专家共识[J]. 中华急诊医学杂志, 2020, 10(29): 1282-302. |
| [4] | Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment[J]. Hum Exp Toxicol, 2018, 37(11): 1131-60. doi:10.1177/0960327118765330 |
| [5] | Zeng DH, Chen XH, Li Y, et al. Clinical and pathological characteristics of acute kidney injury caused by diquat poisoning[J]. Clin Toxicol (Phila), 2023, 61(9): 705-8. doi:10.1080/15563650.2023.2262113 |
| [6] | Vohra R, Salazar A, Cantrell FL, et al. The poison pen: bedside diagnosis of urinary diquat[J]. J Med Toxicol, 2010, 6(1): 35-6. doi:10.1007/s13181-010-0033-6 |
| [7] | Chen YC, Ou ZJ, Zhang RC, et al. Case report: Successful outcome of a young patient with rhabdomyolysis and shock caused by diquat poisoning[J]. Front Med (Lausanne), 2023, 10: 1116912. doi:10.3389/fmed.2023.1116912 |
| [8] | Yin J, Liu MF, Ren WK, et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets[J]. PLoS One, 2015, 10(4): e0122893. doi:10.1371/journal.pone.0122893 |
| [9] | Jović-Stosić J, Babić G, Todorović V. Fatal diquat intoxication[J]. Vojnosanit Pregl, 2009, 66(6): 477-81. doi:10.2298/vsp0906477j |
| [10] | Suleiman SA, Stevens JB. Bipyridylium herbicide toxicity: effects of paraquat and diquat on isolated rat hepatocytes[J]. J Environ Pathol Toxicol Oncol, 1987, 7(3): 73-84. doi:10.3109/08923978709035231 |
| [11] | Lai KM, Wang JJ, Lin SY, et al. Sensing of mitochondrial DNA by ZBP1 promotes RIPK3-mediated necroptosis and ferroptosis in response to diquat poisoning[J]. Cell Death Differ, 2024, 31(5): 635-50. doi:10.1038/s41418-024-01279-5 |
| [12] | von Mässenhausen A, Tonnus W, Himmerkus N, et al. Phenytoin inhibits necroptosis[J]. Cell Death Dis, 2018, 9(3): 359. doi:10.1038/s41419-018-0394-3 |
| [13] | Qin QY, Yu NJ, Gu YX, et al. Inhibiting multiple forms of cell death optimizes ganglion cells survival after retinal ischemia reperfusion injury[J]. Cell Death Dis, 2022, 13(5): 507. doi:10.1038/s41419-022-04911-9 |
| [14] | Wu YZ, Cui SQ, Wang WJ, et al. Kidney and lung injury in rats following acute diquat exposure[J]. Exp Ther Med, 2022, 23(4): 275. doi:10.3892/etm.2022.11201 |
| [15] | Scindia Y, Dey P, Thirunagari A, et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis[J]. J Am Soc Nephrol, 2015, 26(11): 2800-14. doi:10.1681/asn.2014101037 |
| [16] | 黄 奕, 林丽珊, 黄浩华, 等. VDAC1通过诱导气道上皮细胞铁死亡参与屋尘螨诱导的哮喘小鼠气道炎症[J]. 南方医科大学学报, 2023, 43(8): 1333-8. |
| [17] | Kim J, Gupta R, Blanco LP, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease[J]. Science, 2019, 366(6472): 1531-6. doi:10.1126/science.aav4011 |
| [18] | Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome[J]. Nature, 2013, 496(7446): 498-503. |
| [19] | McCampbell KK, Springer KN, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney[J]. J Vis Exp, 2014(90): e51644. doi:10.3791/51644-v |
| [20] | Diep CQ, Peng ZZ, Ukah TK, et al. Development of the zebrafish mesonephros[J]. Genesis, 2015, 53(3/4): 257-69. doi:10.1002/dvg.22846 |
| [21] | Outtandy P, Russell C, Kleta R, et al. Zebrafish as a model for kidney function and disease[J]. Pediatr Nephrol, 2019, 34(5): 751-62. doi:10.1007/s00467-018-3921-7 |
| [22] | Xu X, Wei Y, Hua HW, et al. Glycine alleviated intestinal injury by inhibiting ferroptosis in piglets challenged with diquat[J]. Animals (Basel), 2022, 12(22): 3071. doi:10.3390/ani12223071 |
| [23] | Chen YN, Zhang H, Li Y, et al. Pterostilbene confers protection against diquat-induced intestinal damage with potential regulation of redox status and ferroptosis in broiler chickens[J]. Oxid Med Cell Longev, 2023, 2023: 8258354. doi:10.1155/2023/8258354 |
| [24] | Cheng JY, Yang L, Zhang ZL, et al. Diquat causes mouse testis injury through inducing heme oxygenase-1-mediated ferroptosis in spermatogonia[J]. Ecotoxicol Environ Saf, 2024, 280: 116562. doi:10.1016/j.ecoenv.2024.116562 |
| [25] | Cui SQ, Zhang XX, Wang C, et al. Study on the therapeutic effect of glucocorticoids on acute kidney injury in rats exposed to diquat[J]. Biomed Pharmacother, 2023, 166: 115310. doi:10.1016/j.biopha.2023.115310 |
| [26] | Gorgulho R, Jacinto R, Lopes SS, et al. Usefulness of zebrafish larvae to evaluate drug-induced functional and morphological renal tubular alterations[J]. Arch Toxicol, 2018, 92(1): 411-23. doi:10.1007/s00204-017-2063-1 |
| [27] | Drechsel DA, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in Parkinsonism[J]. Toxicol Sci, 2009, 112(2): 427-34. doi:10.1093/toxsci/kfp223 |
| [28] | Qu J, Pei H, Li XZ, et al. Erythrocyte membrane biomimetic EGCG nanoparticles attenuate renal injury induced by diquat through the NF-κB/NLRP3 inflammasome pathway[J]. Front Pharmacol, 2024, 15: 1414918. doi:10.3389/fphar.2024.1414918 |
| [29] | Huang SF, Lin SR, Zhou SL, et al. Soluble thrombomodulin alleviates Diquat-induced acute kidney injury by inhibiting the HMGB1/IκBα/NF‑κB signalling pathway[J]. Food Chem Toxicol, 2023, 178: 113871. doi:10.1016/j.fct.2023.113871 |
| [30] | Liu Y, Yuan JM, Xi WS, et al. Lactiplantibacillus plantarum ameliorated morphological damage and barrier dysfunction and reduced apoptosis and ferroptosis in the jejunum of oxidatively stressed piglets[J]. Animals (Basel), 2024, 14(22): 3335. doi:10.3390/ani14223335 |
| [31] | Chen KY, Tang YH, Lan LH, et al. Autophagy mediated FTH1 degradation activates gasdermin E dependent pyroptosis contributing to diquat induced kidney injury[J]. Food Chem Toxicol, 2024, 184: 114411. doi:10.1016/j.fct.2023.114411 |
| [32] | Wu LZ, Luo ZW, Luo FL, et al. Edaravone inhibits neuronal ferroptosis and alleviates acute Central nervous system injury induced by diquat via enhancement of METTL14-mediated m6A methylation of Aldh1l1[J]. Free Radic Res, 2025, 59(3): 274-88. doi:10.1080/10715762.2025.2482774 |
| [33] | Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis[J]. Proc Natl Acad Sci USA, 2014, 111(47): 16836-41. doi:10.1073/pnas.1415518111 |
| [34] | Sun MF, Zhu L, Chen X. The role of ferroptosis in renal injury induced by diquat[J]. Chin J Indust Hygiene Occupat Dis, 2025, 43(1): 14-24. |
| [35] | Yang YT, Lin QS, Zhu XY, et al. Activation of lipophagy is required for RAB7 to regulate ferroptosis in sepsis-induced acute kidney injury[J]. Free Radic Biol Med, 2024, 218: 120-31. doi:10.1016/j.freeradbiomed.2024.04.213 |
| [36] | Cao YX, Liu XW, Guo CJ, et al. Biomimetic reactive oxygen/nitrogen nanoscavengers inhibit "ferroptosis storm" and modulate immune targeting for acute kidney injury[J]. J Control Release, 2025, 379: 59-76. doi:10.1016/j.jconrel.2025.01.006 |
| [37] | Karmi O, Marjault HB, Bai F, et al. A VDAC1-mediated NEET protein chain transfers [2Fe-2S] clusters between the mitochondria and the cytosol and impacts mitochondrial dynamics[J]. Proc Natl Acad Sci USA, 2022, 119(7): e2121491119. doi:10.1073/pnas.2121491119 |
| [38] | Shao FY, Han JY, Tian ZY, et al. Synergistic ROS generation and directional overloading of endogenous calcium induce mito-chondrial dysfunction in living cells[J]. Biomaterials, 2023, 301: 122284. doi:10.1016/j.biomaterials.2023.122284 |
| [39] | Niu BL, Lei XH, Xu QL, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury[J]. Cell Biol Toxicol, 2022, 38(3): 505-30. doi:10.1007/s10565-021-09624-x |
| [40] | Yang J, Lu X, Hao JL, et al. VSTM2L protects prostate cancer cells against ferroptosis via inhibiting VDAC1 oligomerization and maintaining mitochondria homeostasis[J]. Nat Commun, 2025, 16(1): 1160. doi:10.1038/s41467-025-56494-6 |
| [41] | Drysdale J, Arosio P, Invernizzi R, et al. Mitochondrial ferritin: a new player in iron metabolism[J]. Blood Cells Mol Dis, 2002, 29(3): 376-83. doi:10.1006/bcmd.2002.0577 |
| [42] | Wang YQ, Chang SY, Wu Q, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis[J]. Front Aging Neurosci, 2016, 8: 308. doi:10.3389/fnagi.2016.00308 |
| [43] | Qian B, Jiang RJ, Song JL, et al. Organophosphorus flame retardant TDCPP induces neurotoxicity via mitophagy-related ferroptosis in vivo and in vitro [J]. Chemosphere, 2022, 308(Pt 2): 136345. doi:10.1016/j.chemosphere.2022.136345 |
| [1] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [2] | 张梦影, 赵晨玲, 田丽伟, 余郭芳, 杨文明, 董婷. 肝豆扶木汤通过GPX4/ACSL4/ALOX15通路抑制铁死亡改善Wilson病小鼠的肝脏脂肪变性[J]. 南方医科大学学报, 2025, 45(7): 1471-1478. |
| [3] | 王子皓, 陶丽丽, 邹碧清, 安胜利. 重症监护病房急性肾损伤患者首次24 h动脉氧分压与死亡率相关:基于MIMIC-IV数据库[J]. 南方医科大学学报, 2025, 45(5): 1056-1062. |
| [4] | 张柳攀, 石晓彤, 李露兰, 师瑞, 安胜利, 曾振华. 合并急性肾损伤的危重症患者输注白蛋白后血清白蛋白水平与28 d死亡率的相关性[J]. 南方医科大学学报, 2025, 45(5): 1074-1081. |
| [5] | 张安邦, 孙秀颀, 庞博, 吴远华, 时靖宇, 张宁, 叶涛. 电针预处理通过调节肠道-大脑轴及Nrf2/HO-1信号通路抑制铁死亡减轻大鼠脑缺血再灌注损伤[J]. 南方医科大学学报, 2025, 45(5): 911-920. |
| [6] | 张林落, 李长青, 皇玲玲, 周学平, 娄媛媛. 梓醇扶正制毒配伍从SLC7A11/GPX4通路抑制铁死亡减轻雷公藤甲素肝毒性[J]. 南方医科大学学报, 2025, 45(4): 810-818. |
| [7] | 季春斐, 左宗超, 王钧, 李妙男. N-乙酰神经氨酸中通过抑制Nrf2轴促进缺氧/复氧损伤的H9C2心肌细胞发生铁死亡[J]. 南方医科大学学报, 2025, 45(1): 72-79. |
| [8] | 陈凯, 孟兆菲, 闵静婷, 王佳慧, 李正红, 高琴, 胡俊锋. 姜黄素通过抑制TXNIP/TRX-1/GPX4通路介导的铁死亡减轻脓毒症小鼠肺损伤[J]. 南方医科大学学报, 2024, 44(9): 1805-1813. |
| [9] | 欧阳明子, 崔佳琦, 王慧, 梁正, 皮大锦, 陈利国, 陈前军, 吴迎朝. 开心散通过减轻前额叶皮质铁死亡缓解小鼠的阿霉素化疗性抑郁[J]. 南方医科大学学报, 2024, 44(8): 1441-1449. |
| [10] | 张银亮, 骆泽谭, 赵睿, 赵娜, 徐志东, 奥迪, 丛古一, 刘新宇, 郑海伦. 血根碱通过调控STUB1/GPX4诱导直肠癌细胞发生铁死亡[J]. 南方医科大学学报, 2024, 44(8): 1537-1544. |
| [11] | 王元国, 张鹏. 铁死亡抑制基因在食管癌中的高表达分析[J]. 南方医科大学学报, 2024, 44(7): 1389-1396. |
| [12] | 何华星, 刘璐琳, 刘颖茵, 陈纳川, 孙素霞. 丁酸钠与索拉非尼可能通过YAP诱导铁死亡协同抑制肝癌细胞增殖[J]. 南方医科大学学报, 2024, 44(7): 1425-1430. |
| [13] | 任智先, 周倍贤, 王林鑫, 李菁, 张荣平, 潘锡平. 5-羟基-6,7-二甲氧基黄酮抑制流感病毒诱导A549细胞炎症反应和铁死亡的作用及机制[J]. 南方医科大学学报, 2024, 44(6): 1070-1078. |
| [14] | 张方圆, 刘刚. 右美托咪定通过激活Nrf2/HO-1/GPX4通路抑制肾小管上皮细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(6): 1135-1140. |
| [15] | 王南, 石斌, 马小兰, 吴伟超, 曹佳. FMRP通过激活RAS/MAPK信号通路抑制结直肠肿瘤细胞的铁死亡[J]. 南方医科大学学报, 2024, 44(5): 885-893. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||