Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (10): 2223-2230.doi: 10.12122/j.issn.1673-4254.2025.10.18
Shuxian LIN1( ), Lina GUO1, Yan MA2, Yao XIONG3, Yingxi HE1, Xinzhu XU1, Wen SHENG1, Suhua XU1, Feng QIU4(
), Lina GUO1, Yan MA2, Yao XIONG3, Yingxi HE1, Xinzhu XU1, Wen SHENG1, Suhua XU1, Feng QIU4( )
)
Received:2025-01-11
Online:2025-10-20
Published:2025-10-24
Contact:
Feng QIU
E-mail:369207901@qq.com;QFSFL@126.com
Shuxian LIN, Lina GUO, Yan MA, Yao XIONG, Yingxi HE, Xinzhu XU, Wen SHENG, Suhua XU, Feng QIU. Lactobacillus plantarum ZG03 alleviates oxidative stress via its metabolites short-chain fatty acids[J]. Journal of Southern Medical University, 2025, 45(10): 2223-2230.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.10.18
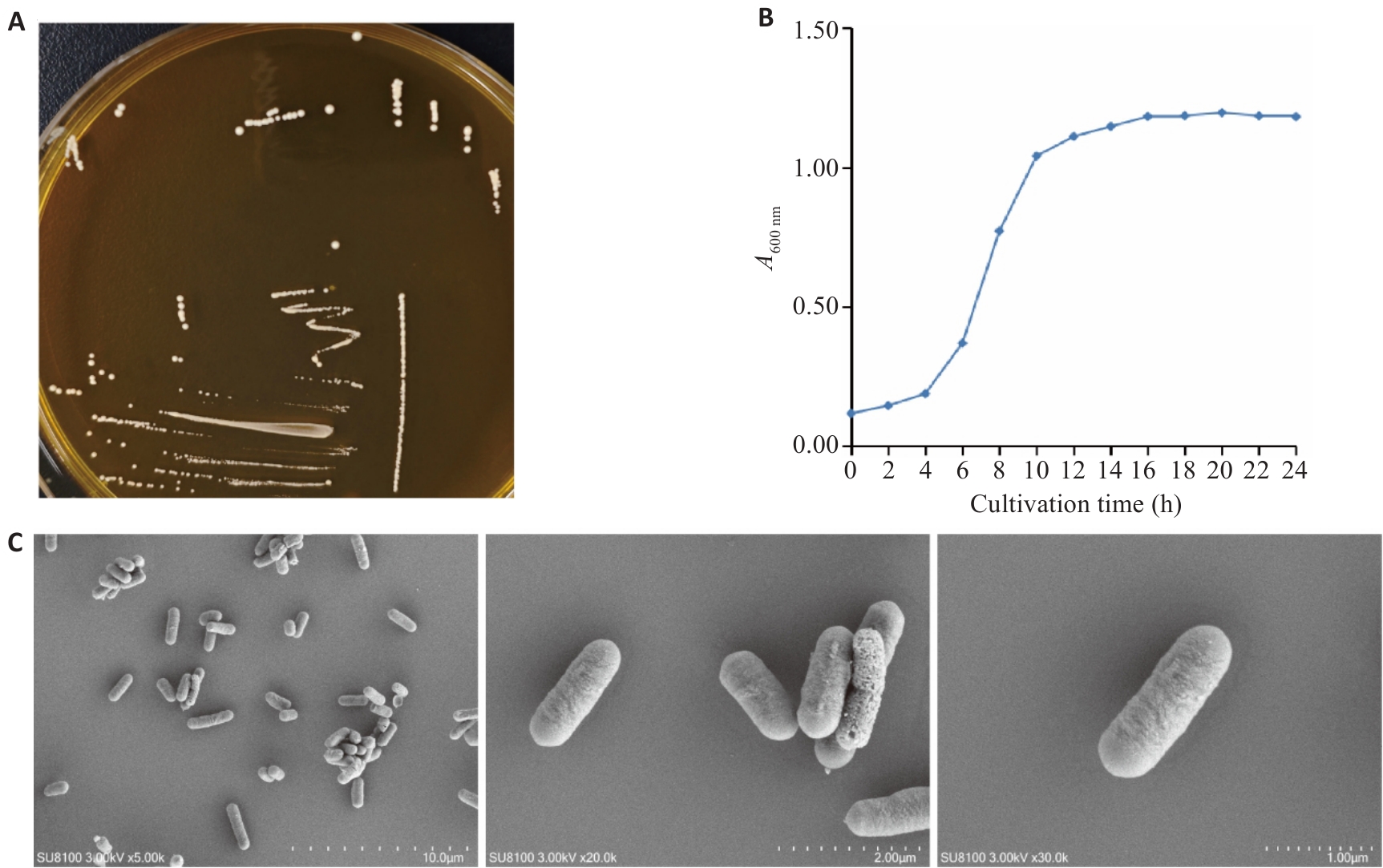
Fig.1 Growth characteristics of L. plantarum ZG03. A: Growth curve of ZG03. B: Colony morphology of ZG03. C: Field emission scanning electron microscopy (FESEM) images of ZG03.
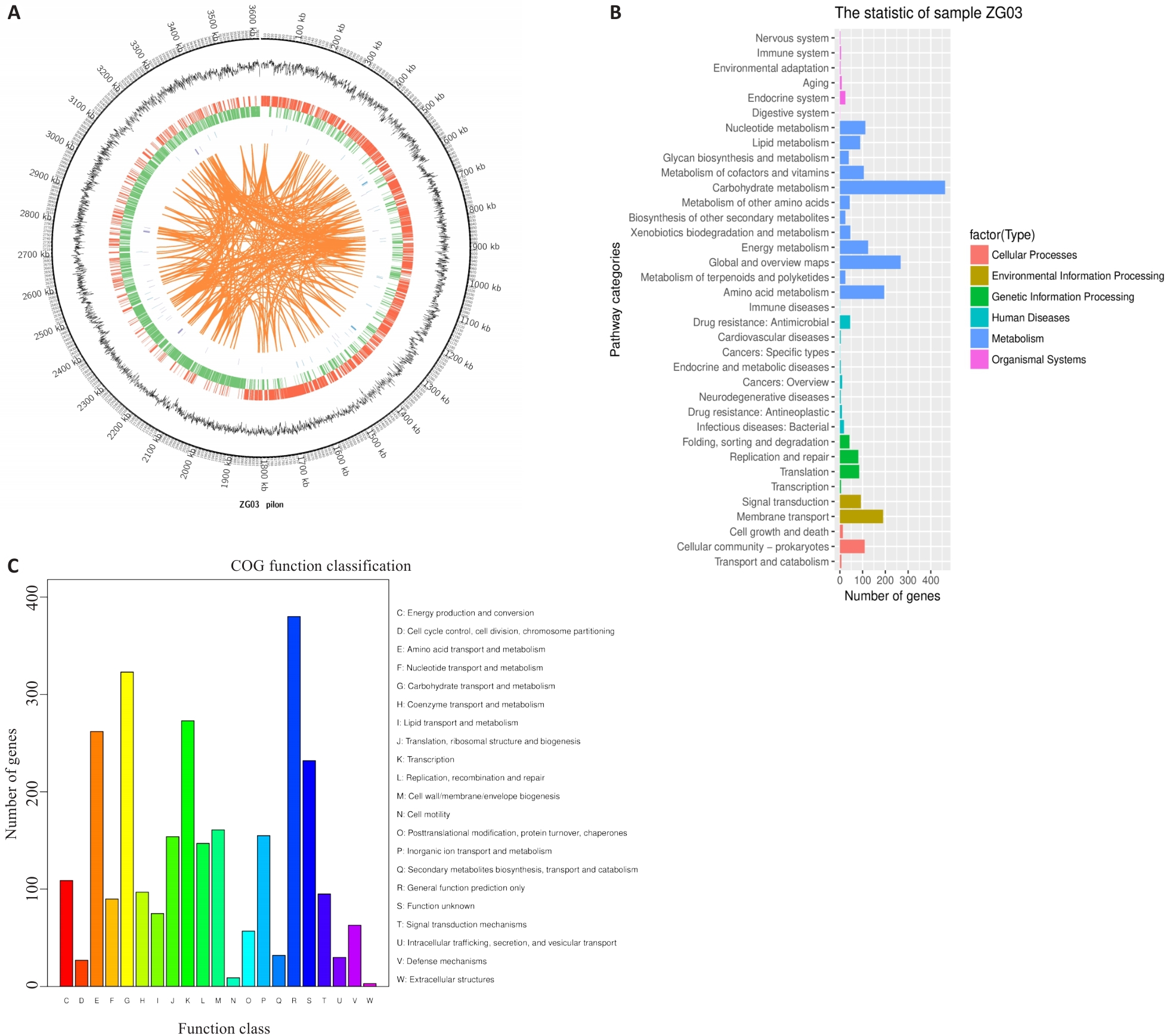
Fig.2 Genomic characteristics of L. plantarum ZG03. A: Genomic circular map (from outer to inner circles): The first circle represents genomic position information; the second circle shows GC content; the third circle indicates protein-coding genes on the positive strand (marked in red); the fourth circle denotes protein-coding genes on the negative strand (marked in green); the fifth circle displays non-coding elements on the positive strand (marked in blue); the sixth circle represents non-coding elements on the negative strand (marked in purple); and the seventh circle shows long fragment repeat sequences within the genome (marked in orange). B: KEGG enrichment analysis. C: COG enrichment analysis.
| Feature | Value |
|---|---|
| Size (bp) | 3 613 757 |
| G+C content (%) | 46.42 |
| Coding region (%) | 80.70 |
| Total genes | 3 354 |
| RNA genes | 123 |
| rRNA genes | 16 |
| tRNA genes | 70 |
| Protein-coding genes | 3 231 |
| Protein coding genes with enzymes | 1 099 |
| Genes with signal petides | 176 |
| Genes with transmembrane helices | 911 |
Tab.1 Genomic information of L. plantarum ZG03
| Feature | Value |
|---|---|
| Size (bp) | 3 613 757 |
| G+C content (%) | 46.42 |
| Coding region (%) | 80.70 |
| Total genes | 3 354 |
| RNA genes | 123 |
| rRNA genes | 16 |
| tRNA genes | 70 |
| Protein-coding genes | 3 231 |
| Protein coding genes with enzymes | 1 099 |
| Genes with signal petides | 176 |
| Genes with transmembrane helices | 911 |
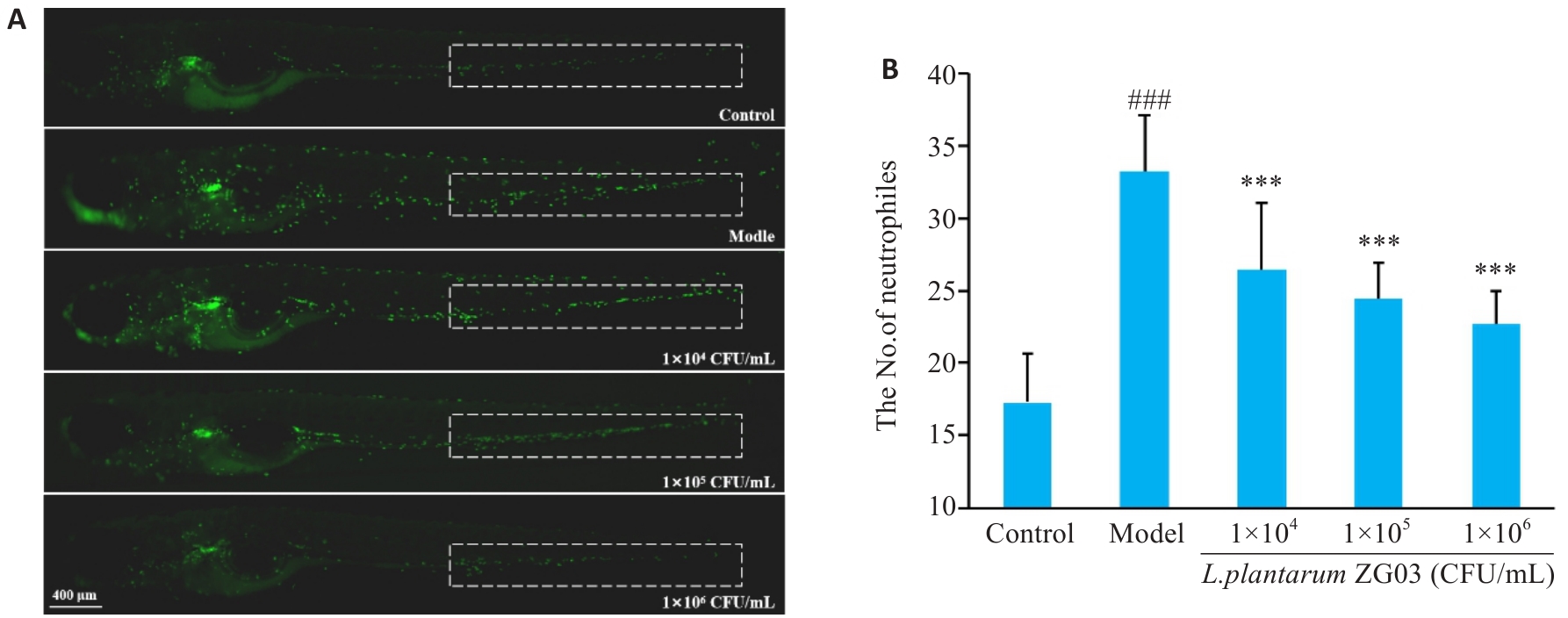
Fig.4 Number of neutrophils in the caudal hematopoietic tissue of zebrafish (n=10). A: Representative images of neutrophils (dashed box) in zebrafish. B: Quantitative analysis of neutrophil numbers in zebrafish. ###P<0.001 vs control group; ***P<0.001 vs model group.
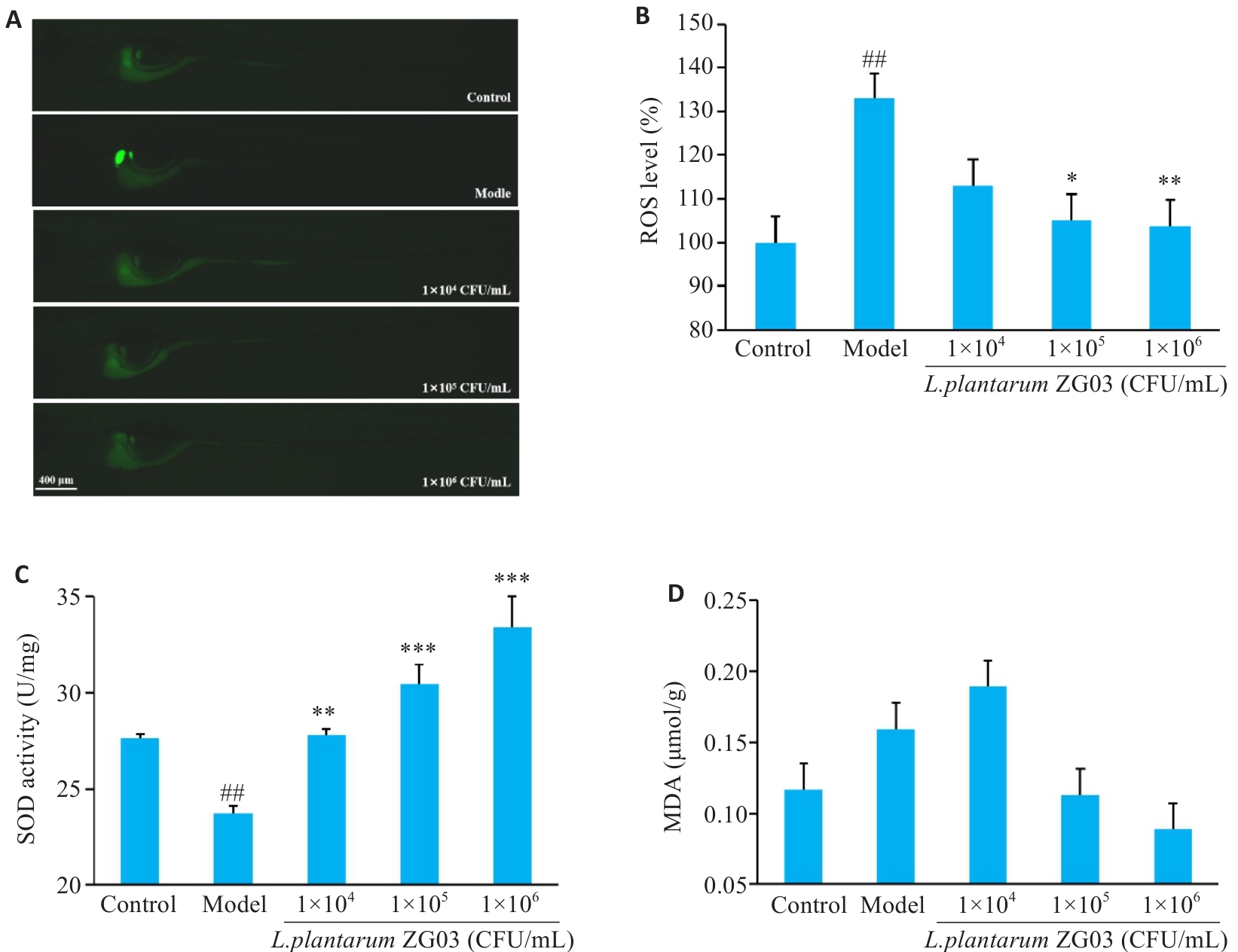
Fig.5 Effect of L. plantarum ZG03 on glucose-induced oxidative stress in zebrafish. A: Representative images of ROS staining in zebrafish. B: Statistical analysis of relative ROS levels (n=6). C: SOD activity in zebrafish (n=3). D: MDA levels in zebrafish (n=3). ##P<0.01 vs control group; *P<0.05, **P<0.01, ***P<0.001 vs model group.
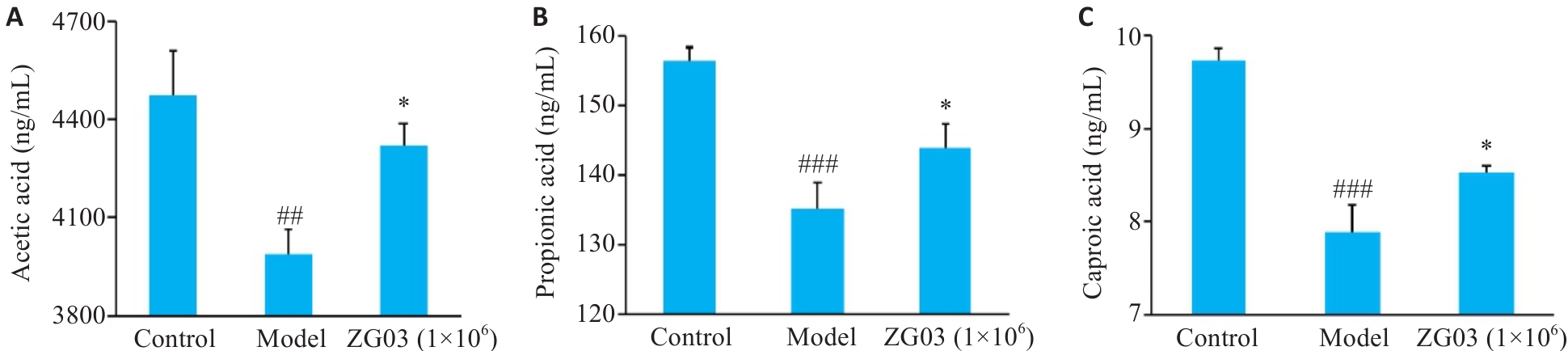
Fig.6 Content of short-chain fatty acid metabolites in zebrafish (n=3). A: Acetic acid content in zebrafish. B: Propionic acid content in zebrafish. C: Hexanoic acid content in zebrafish. ##P<0.01, ###P<0.001vs control group; *P<0.05 vs model group.
| [1] | Sies H. Oxidative stress: a concept in redox biology and medicine[J]. Redox Biol, 2015, 4: 180-3. doi:10.1016/j.redox.2015.01.002 |
| [2] | Sies H. Oxidative stress: concept and some practical aspects[J]. Antioxidants (Basel), 2020, 9(9): 852. doi:10.3390/antiox9090852 |
| [3] | Shaito A, Aramouni K, Assaf R, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases[J]. Front Biosci (Landmark Ed), 2022, 27(3): 105. doi:10.31083/j.fbl2703105 |
| [4] | Mani S, Dubey R, Lai IC, et al. Oxidative stress and natural antioxidants: back and forth in the neurological mechanisms of Alzheimer’s disease[J]. J Alzheimers Dis, 2023, 96(3): 877-912. doi:10.3233/jad-220700 |
| [5] | Houldsworth A. Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants[J]. Brain Commun, 2024, 6(1): fcad356. doi:10.1093/braincomms/fcad356 |
| [6] | Ryan A, Murphy M, Godson C, et al. Diabetes mellitus and apoptosis: inflammatory cells[J]. Apoptosis, 2009, 14(12): 1435-50. doi:10.1007/s10495-009-0340-z |
| [7] | Zhang PJ, Li T, Wu XY, et al. Oxidative stress and diabetes: antioxidative strategies[J]. Front Med, 2020, 14(5): 583-600. doi:10.1007/s11684-019-0729-1 |
| [8] | Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development[J]. Lancet, 2012, 379(9833): 2279-90. doi:10.1016/s0140-6736(12)60283-9 |
| [9] | Liu YY, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment[J]. J Clin Pharmacol, 2018, 58(): S164-79. doi:10.1002/jcph.1121 |
| [10] | Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review[J]. Gut Microbes, 2020, 12(1): 1801944. doi:10.1080/19490976.2020.1801944 |
| [11] | 尹龙杰, 张 雨, 陈舒焕, 等. 鼠李糖乳杆菌GG对大鼠脂多糖应激下抗氧化能力、免疫功能和肠道健康的影响[J]. 饲料工业, 2024, 45(9): 93-100. |
| [12] | 周先容, 谭 仟, 母健菲, 等. 泡菜源乳酸菌的分离筛选及其对小鼠氧化应激水平的改善作用[J]. 现代食品科技, 2020, 36(9): 17-25. |
| [13] | 李 月, 闫 薇, 姜 斌, 等. 植物乳植杆菌H8对小鼠氧化损伤的作用机制[J]. 食品科学技术学报, 2024, 42(5): 93-103. |
| [14] | Wang L, Zhao ZJ, Zhao L, et al. Lactobacillus plantarum DP189 reduces α-SYN aggravation in MPTP-induced Parkinson’s disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder[J]. J Agric Food Chem, 2022, 70(4): 1163-73. doi:10.1021/acs.jafc.1c07711 |
| [15] | He J, Zhang PW, Shen LY, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism[J]. Int J Mol Sci, 2020, 21(17): 6356. doi:10.3390/ijms21176356 |
| [16] | Xu R, Wang T, Ding FF, et al. Lactobacillus plantarum ameliorates high-carbohydrate diet-induced hepatic lipid accumulation and oxidative stress by upregulating uridine synthesis[J]. Antioxidants (Basel), 2022, 11(7): 1238. doi:10.3390/antiox11071238 |
| [17] | Li ZH, Shi YQ, Zhang XH, et al. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo zebrafish assays[J]. Front Pharmacol, 2020, 11: 287. doi:10.3389/fphar.2020.00287 |
| [18] | Zhao WC, Chen YN, Hu N, et al. The uses of zebrafish (Danio rerio) as an in vivo model for toxicological studies: a review based on bibliometrics[J]. Ecotoxicol Environ Saf, 2024, 272: 116023. doi:10.1016/j.ecoenv.2024.116023 |
| [19] | Hu CW, Sun L, Chen JQ, et al. Advantages of the zebrafish tumor xenograft model: the evaluation of efficacy in cancer therapy and the application to the study of lncRNAs[J]. Front Immunol, 2024, 15: 1483192. doi:10.3389/fimmu.2024.1483192 |
| [20] | Zhang Y, Xia Q, Wang JB, et al. Progress in using zebrafish as a toxicological model for traditional Chinese medicine[J]. J Ethnopharmacol, 2022, 282: 114638. doi:10.1016/j.jep.2021.114638 |
| [21] | Chowdhury S, Saikia SK. Use of zebrafish as a model organism to study oxidative stress: a review[J]. Zebrafish, 2022, 19(5): 165-76. doi:10.1089/zeb.2021.0083 |
| [22] | Subba R, Fasciolo G, Geremia E, et al. Simultaneous induction of systemic hyperglycaemia and stress impairs brain redox homeostasis in the adult zebrafish[J]. Arch Biochem Biophys, 2024, 759: 110101. doi:10.1016/j.abb.2024.110101 |
| [23] | Li YQ, Chen QQ, Liu YN, et al. High glucose-induced ROS-accumulation in embryo-larval stages of zebrafish leads to mitochondria-mediated apoptosis[J]. Apoptosis, 2022, 27(7/8): 509-20. doi:10.1007/s10495-022-01731-2 |
| [24] | Weinberg Sibony R, Segev O, Dor S, et al. Overview of oxidative stress and inflammation in diabetes[J]. J Diabetes, 2024, 16(10): e70014. doi:10.1111/1753-0407.70014 |
| [25] | He BL, Hu TG, Wu H. Phenotypic screening of novel probiotics with potential anti-neuroinflammation activity based on cell and zebrafish models[J]. Food Biosci, 2023, 55: 102949. doi:10.1016/j.fbio.2023.102949 |
| [26] | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism[J]. Gut Microbes, 2016, 7(3): 189-200. doi:10.1080/19490976.2015.1134082 |
| [27] | Jeong JJ, Ganesan R, Jin YJ, et al. Multi-strain probiotics alleviate loperamide-induced constipation by adjusting the microbiome, serotonin, and short-chain fatty acids in rats[J]. Front Microbiol, 2023, 14: 1174968. doi:10.3389/fmicb.2023.1174968 |
| [28] | Cuciniello R, Di Meo F, Filosa S, et al. The antioxidant effect of dietary bioactives arises from the interplay between the physiology of the host and the gut microbiota: involvement of short-chain fatty acids[J]. Antioxidants (Basel), 2023, 12(5): 1073. doi:10.3390/antiox12051073 |
| [29] | Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity[J]. Nat Rev Immunol, 2024, 24(8): 577-95. doi:10.1038/s41577-024-01014-8 |
| [30] | Wang YM, Dilidaxi D, Wu YC, et al. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice[J]. Biomed Pharmacother, 2020, 125: 109914. doi:10.1016/j.biopha.2020.109914 |
| [31] | Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, et al. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study[J]. Clin Nutr, 2017, 36(1): 85-92. doi:10.1016/j.clnu.2015.11.011 |
| [1] | Yu ZHANG, Yinqi HU, Peipei LI, Xiao SHI, Wei XU, Jianpeng HU. Naoluo Xintong Decoction promotes proliferation of rat brain microvascular endothelial cells after oxygen-glucose deprivation by activating the HIF-1α/VEGF signaling pathway [J]. Journal of Southern Medical University, 2025, 45(9): 1980-1988. |
| [2] | Jingxian WANG, Zijing REN, Peiyang ZHOU. S1PR5 activation or overexpression enhances barrier function of mouse brain microvascular endothelial cells against OGD/R injury by modulating oxidative stress [J]. Journal of Southern Medical University, 2025, 45(7): 1451-1459. |
| [3] | Ruimin HAN, Manke ZHAO, Junfang YUAN, Zhenhong SHI, Zhen WANG, Defeng WANG. Live combined Bacillus subtilis and Enterococcus faecium improves glucose and lipid metabolism in type 2 diabetic mice with circadian rhythm disruption via the SCFAs/GPR43/GLP-1 pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1490-1497. |
| [4] | Xiaoxiang ZHANG, Ying TIAN, Lilan FU, Yin ZHANG, Ye DONG, Fei XIE, Li CHEN, Yanchao HUANG, Hubing WU, Jianer TAN. 68Ga-DOTATATE and 18F-FDG PET/CT dual-modality imaging enhances precision of staging and treatment decision for gastroenteropancreatic neuroendocrine neoplasms [J]. Journal of Southern Medical University, 2025, 45(6): 1212-1219. |
| [5] | Xinrui HOU, Zhendong ZHANG, Mingyuan CAO, Yuxin DU, Xiaoping WANG. Salidroside inhibits proliferation of gastric cancer cells by regulating the miR-1343-3p-OGDHL/PDHB glucose metabolic axis [J]. Journal of Southern Medical University, 2025, 45(6): 1226-1239. |
| [6] | Anbang ZHANG, Xiuqi SUN, Bo PANG, Yuanhua WU, Jingyu SHI, Ning ZHANG, Tao YE. Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury in rats by inhibiting ferroptosis through the gut-brain axis and the Nrf2/HO-1 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(5): 911-920. |
| [7] | Zhi GAO, Ao WU, Zhongxiang HU, Peiyang SUN. Bioinformatics analysis of oxidative stress and immune infiltration in rheumatoid arthritis [J]. Journal of Southern Medical University, 2025, 45(4): 862-870. |
| [8] | Ming LIAO, Wenhua ZHONG, Ran ZHANG, Juan LIANG, Wentaorui XU, Wenjun WAN, Chao LI Shu WU. Protein C activator derived from snake venom protects human umbilical vein endothelial cells against hypoxia-reoxygenation injury by suppressing ROS via upregulating HIF-1α and BNIP3 [J]. Journal of Southern Medical University, 2025, 45(3): 614-621. |
| [9] | Pengwei HUANG, Jie CHEN, Jinhu ZOU, Xuefeng GAO, Hong CAO. Quercetin mitigates HIV-1 gp120-induced rat astrocyte neurotoxicity via promoting G3BP1 disassembly in stress granules [J]. Journal of Southern Medical University, 2025, 45(2): 304-312. |
| [10] | XU Xinzhu, Lina GUO, Kangdi ZHENG, Yan MA, Shuxian LIN, Yingxi HE, Wen SHENG, Suhua XU, Feng QIU. Lacticaseibacillus paracasei E6 improves vinorelbine-induced immunosuppression in zebrafish through its metabolites acetic acid and propionic acid [J]. Journal of Southern Medical University, 2025, 45(2): 331-339. |
| [11] | Tao GUO, Bolin CHEN, Jinsha SHI, Xianfeng KUANG, Tengyue YU, Song WEI, Xiong LIU, Rong XIAO, Juanjuan LI. Gastrodin inhibits ferroptosis to alleviate hypoxic-ischemic brain damage in neonatal mice by activating GPX4/SLC7A11/FTH1 signaling [J]. Journal of Southern Medical University, 2025, 45(10): 2071-2081. |
| [12] | Wei LUO, Yuhang WANG, Yansong LIU, Yuanyuan WANG, Lei AI. High glucose induces pro-inflammatory polarization of macrophages by inhibiting immune-responsive gene 1 expression [J]. Journal of Southern Medical University, 2025, 45(1): 1-9. |
| [13] | Yuming ZHANG, Shicheng XIA, Linlin ZHANG, Mengxi CHEN, Xiaojing LIU, Qin GAO, Hongwei YE. Protective effect of Lonicerae japonicae flos extract against doxorubicin-induced liver injury in mice [J]. Journal of Southern Medical University, 2024, 44(8): 1571-1581. |
| [14] | Jing XIAO, Ying LI, Min FANG, Hong GONG, Wen LI, Chunyan ZHANG, Fangyao CHEN, Yan ZHANG, Tuo HAN. Triglyceride-glucose index in non-obese individuals: its association with and predictive value for non-alcoholic fatty liver disease [J]. Journal of Southern Medical University, 2024, 44(7): 1266-1271. |
| [15] | Zhijun REN, Jianxin DIAO, Yiting WANG. Xionggui Decoction alleviates heart failure in mice with myocardial infarction by inhibiting oxidative stress-induced cardiomyocyte apoptosis [J]. Journal of Southern Medical University, 2024, 44(7): 1416-1424. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||