Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (4): 725-735.doi: 10.12122/j.issn.1673-4254.2025.04.07
Previous Articles Next Articles
Luyu LIU1,2( ), Maowei GONG1(
), Maowei GONG1( ), Guosong LIAO1,2, Weixing ZHAO1(
), Guosong LIAO1,2, Weixing ZHAO1( ), Qiang FU1(
), Qiang FU1( )
)
Received:2024-12-24
Online:2025-04-20
Published:2025-04-28
Contact:
Weixing ZHAO, Qiang FU
E-mail:luyuliu001@163.com;gongmwei2001@163.com;zwxsjrz@163.com;dr_fuqiang@hotmail.com
Supported by:Luyu LIU, Maowei GONG, Guosong LIAO, Weixing ZHAO, Qiang FU. Hypertension exacerbates postoperative learning and memory impairment in rats possibly due to UCP2 downregulation-mediated mitochondrial dysfunction[J]. Journal of Southern Medical University, 2025, 45(4): 725-735.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.04.07
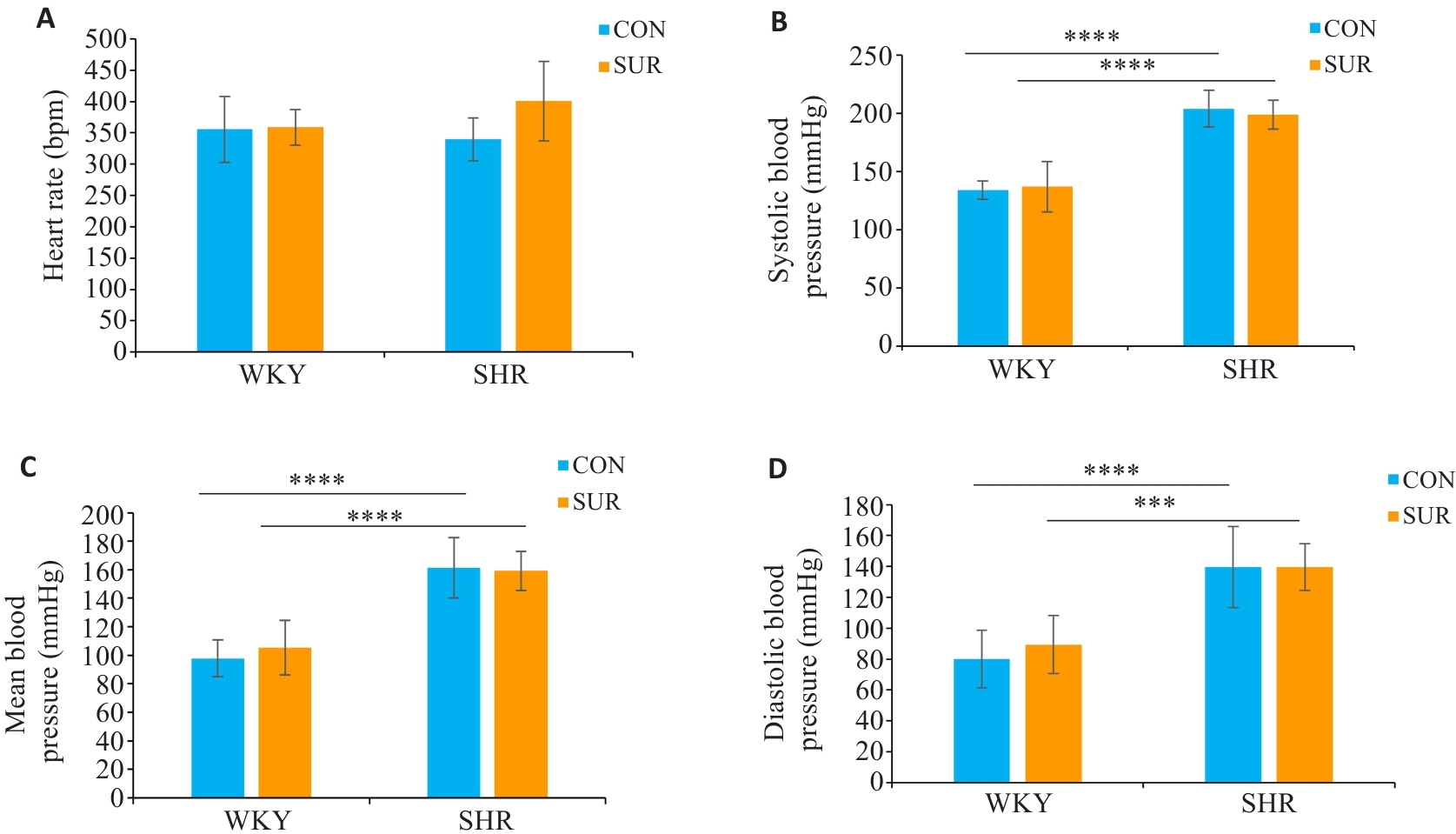
Fig. 1 Comparison of heart rate (A), systolic blood pressure (B), mean arterial pressure (C) and diastolic pressure (D) between SHRs and WKYs before surgery. ***P<0.001, ****P<0.0001.
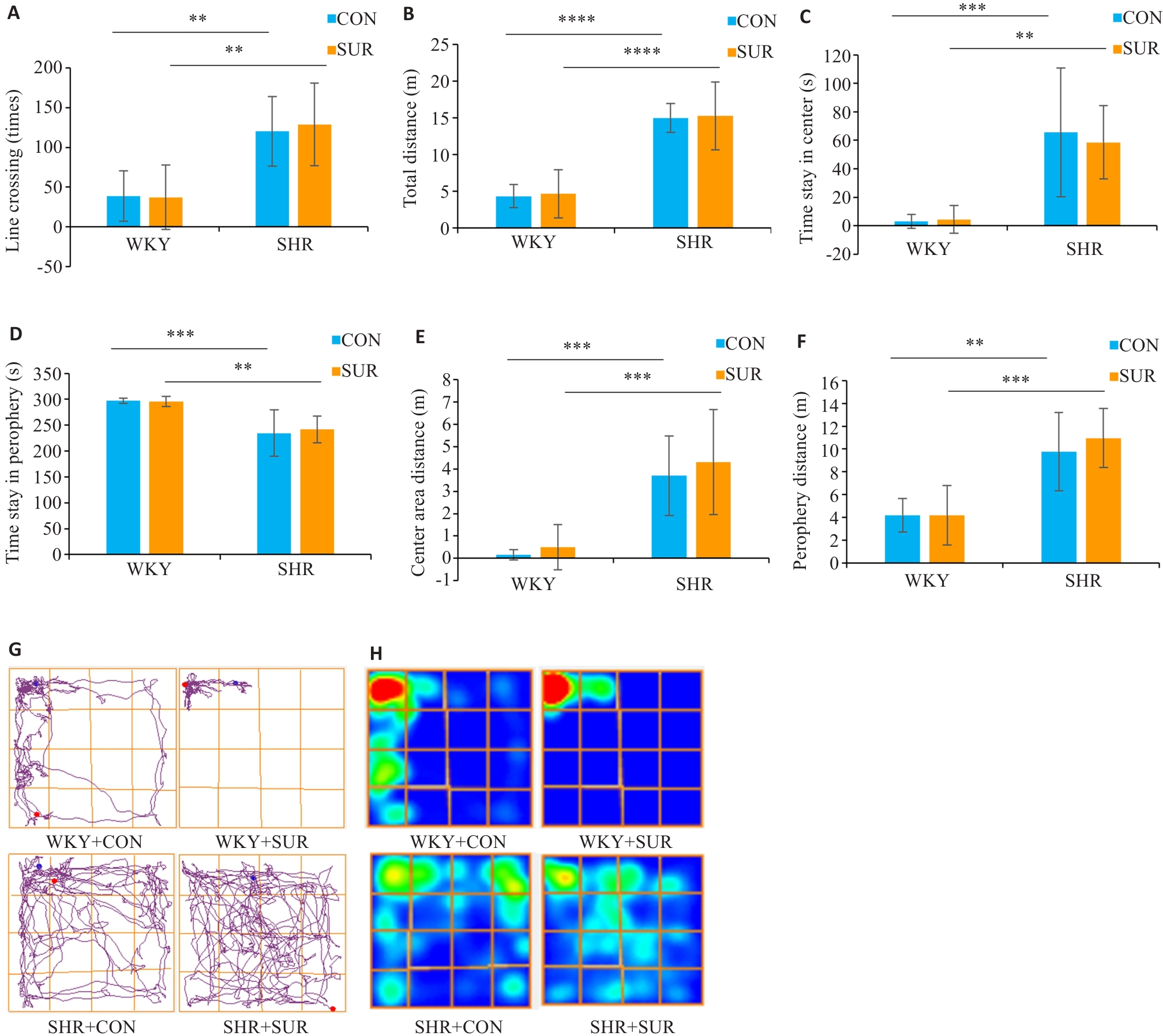
Fig.2 Assessment of hypertension-induced hyperactive behaviors of rats in open field test. A: Performance of line crossing. B: Total moving distance in the test. C: Time spent in center zone. D: Time spent in the periphery zone. E: Center zone moving distance. F: Periphery zone moving distance. G: Representative motion trajectory diagram of the rats in each group. H: Representative motion trajectory heat map of each group. **P<0.01, ***P<0.001, ****P<0.0001.
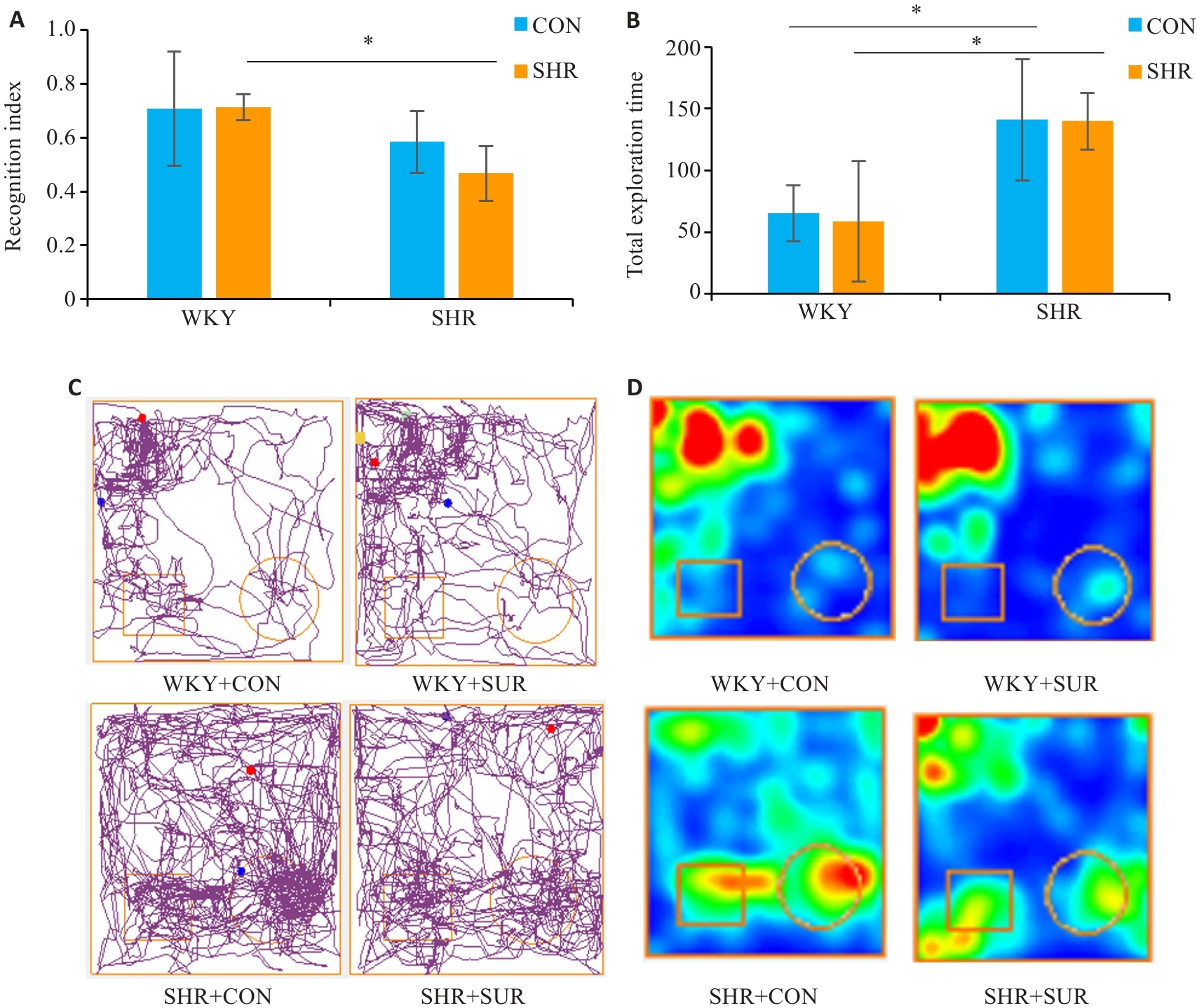
Fig.3 Novel object recognition test for assessing the effect of hypertension on spatial memory impairment of the rats after surgery. A: Novel object recognition index. B: Total exploration times in the test. C: Representative motion trajectory diagrams, where the square represent an old object and the circle represent a novel object. D: Representative motion trajectory heat map. *P<0.05.
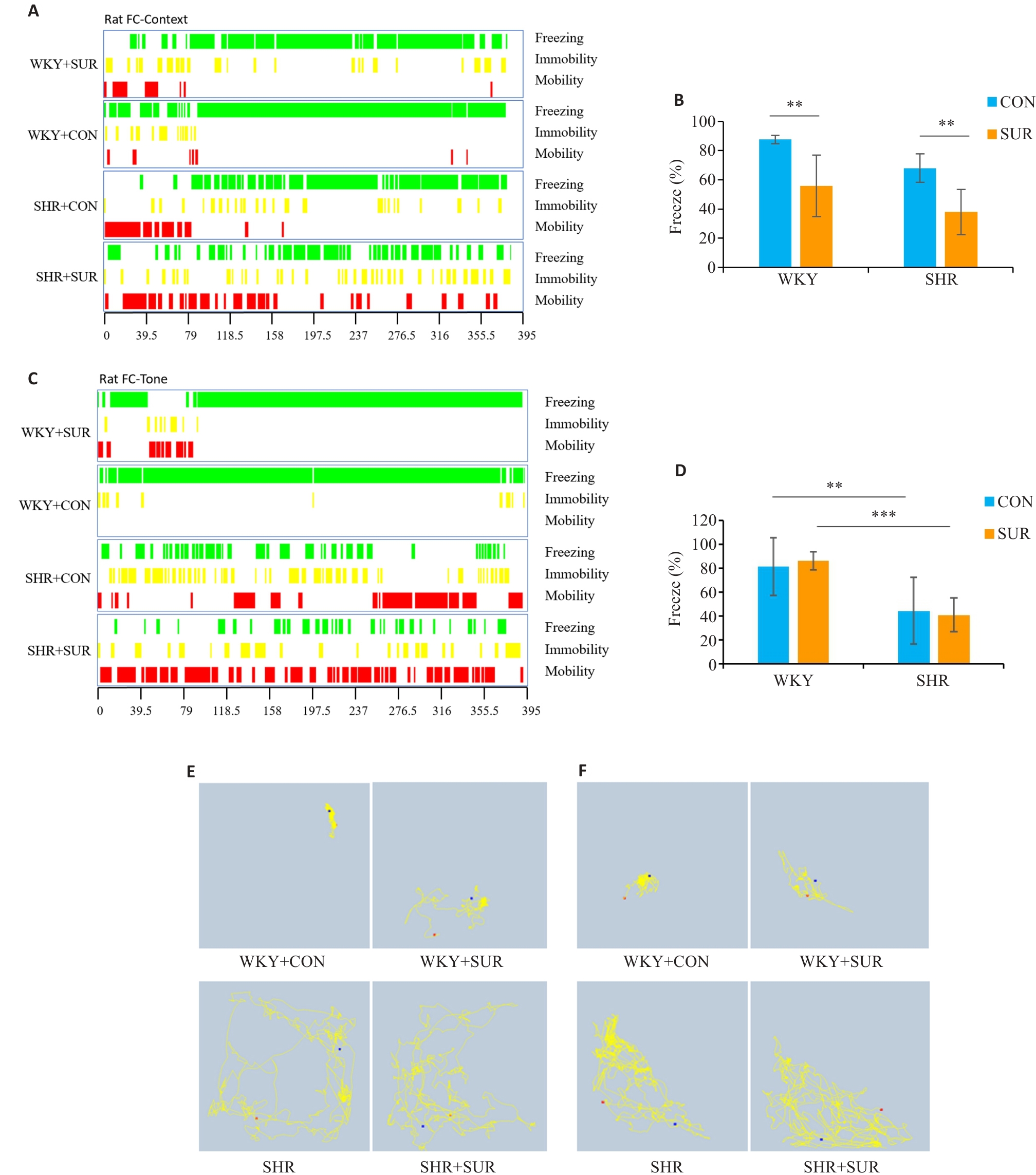
Fig.4 Hypertension exacerbates fear memory impairment in SHRs after surgery. A: FC context-related freezing performance (green indicates freezing, yellow indicates immobility, and red indicates mobility). B: Statistical graph of context-related percent freezing time. C: Tone-related freezing performance. D: Statistical graph of tone-related percent freezing time. E: Representative motion trajectory corresponding to context-related FC test. F: Representative motion trajectory corresponding to tone-related freezing time. **P<0.01, ***P<0.001.
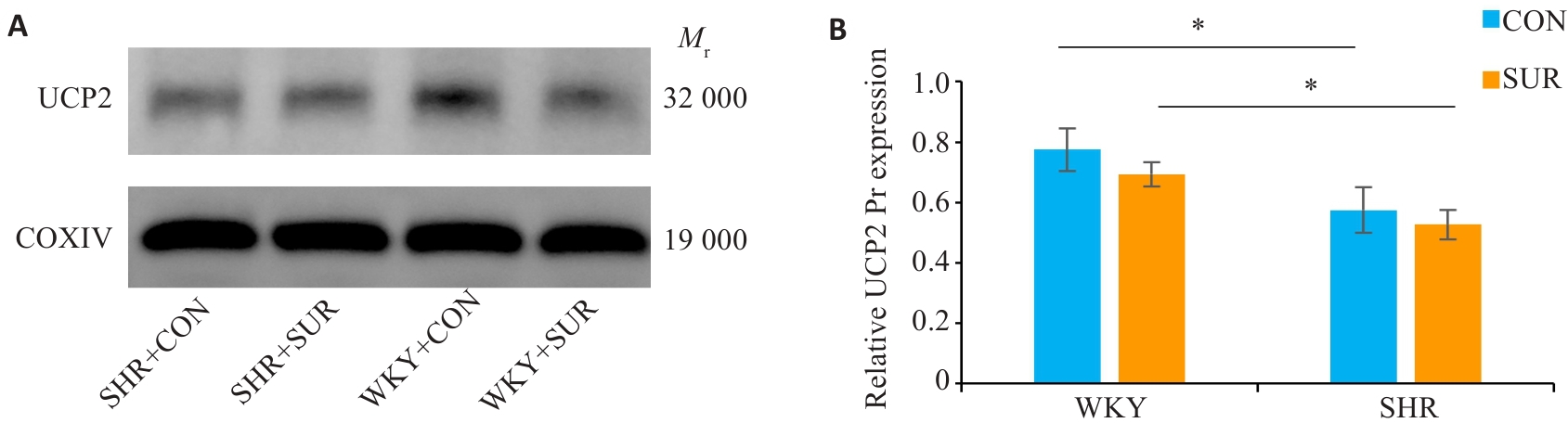
Fig.5 Quantification of mitochondrial UCP2 protein expression in rat hippocampus. A: Western blotting bands of UCP2 protein and internal control COXIV in the mitochondria. B: Statistical graph of mitochondrial UCP2 protein expression in the hippocampus. *P<0.05.
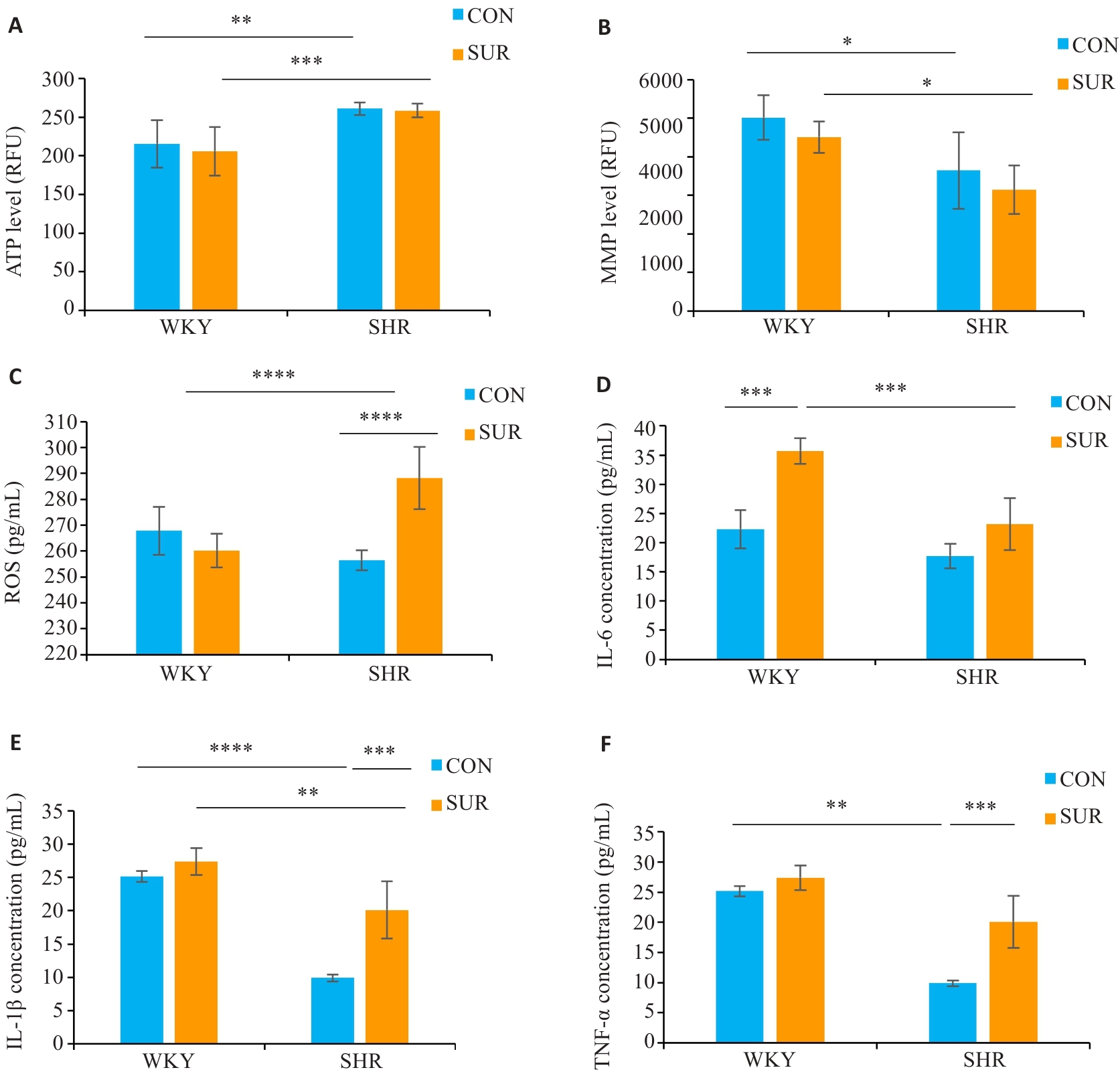
Fig.6 Hypertension aggravates hippocampal mitochondrial dysfunction, oxidative stress, and inflammatory response after surgery. A: Hippocampal ATP level. B: Hippocampal mitochondria membrane potential (MMP). C: Reactive oxygen species level in serum. D: Serum IL-6 level. E: Serum IL-1β level. F: Serum TNF-α level. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
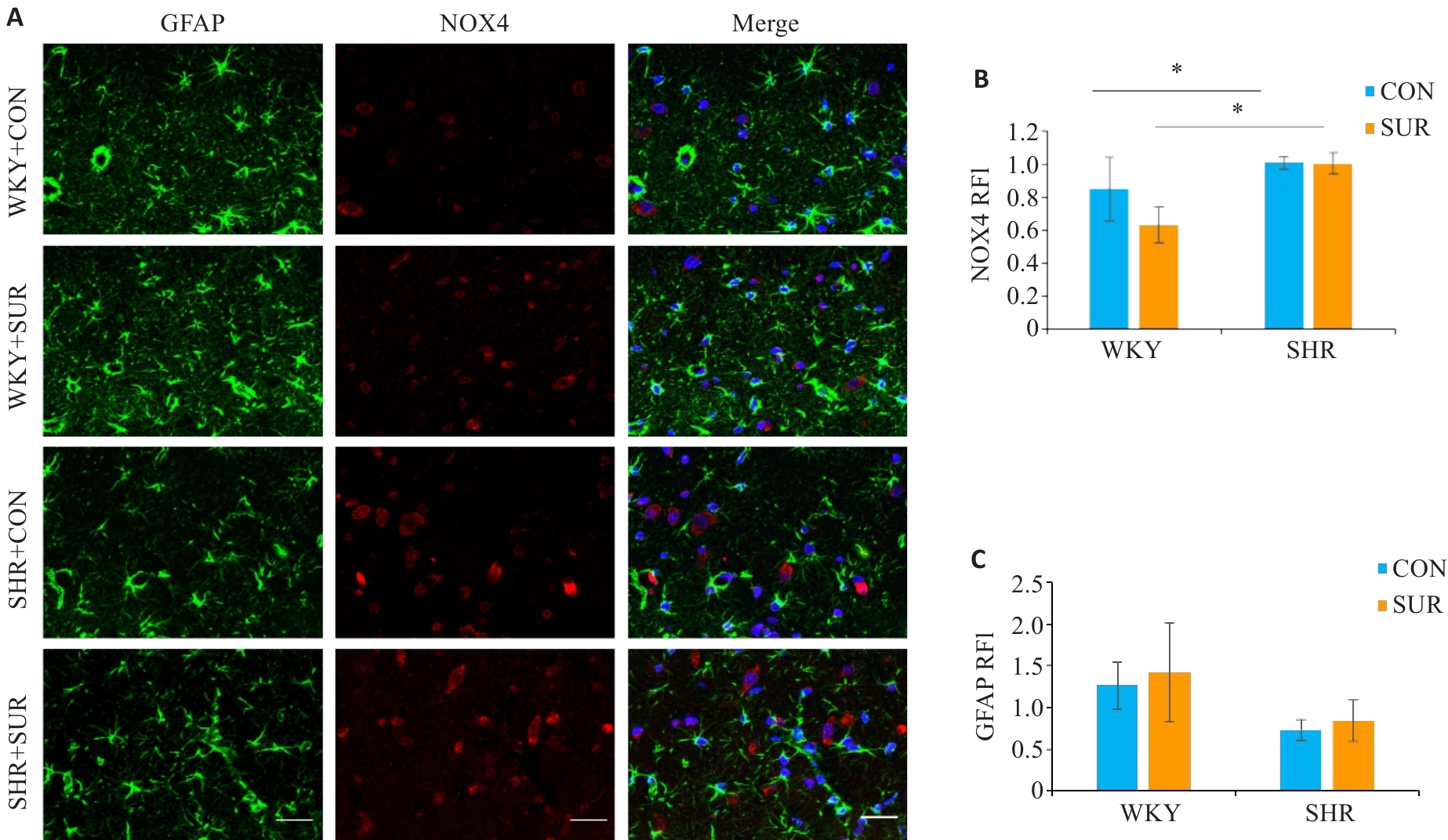
Fig. 7 Immunofluorescence staining of the hippocampus of SHR and WKY rats for detecting expressions of astrocyte markers in the CA1 region. A: Combo graph of immunofluorescence (Scale bar=20 μm; green represents GFAP, and red represents NOX4). B, C: Statistical graphs of NOX4 and GFAP. *P<0.05.
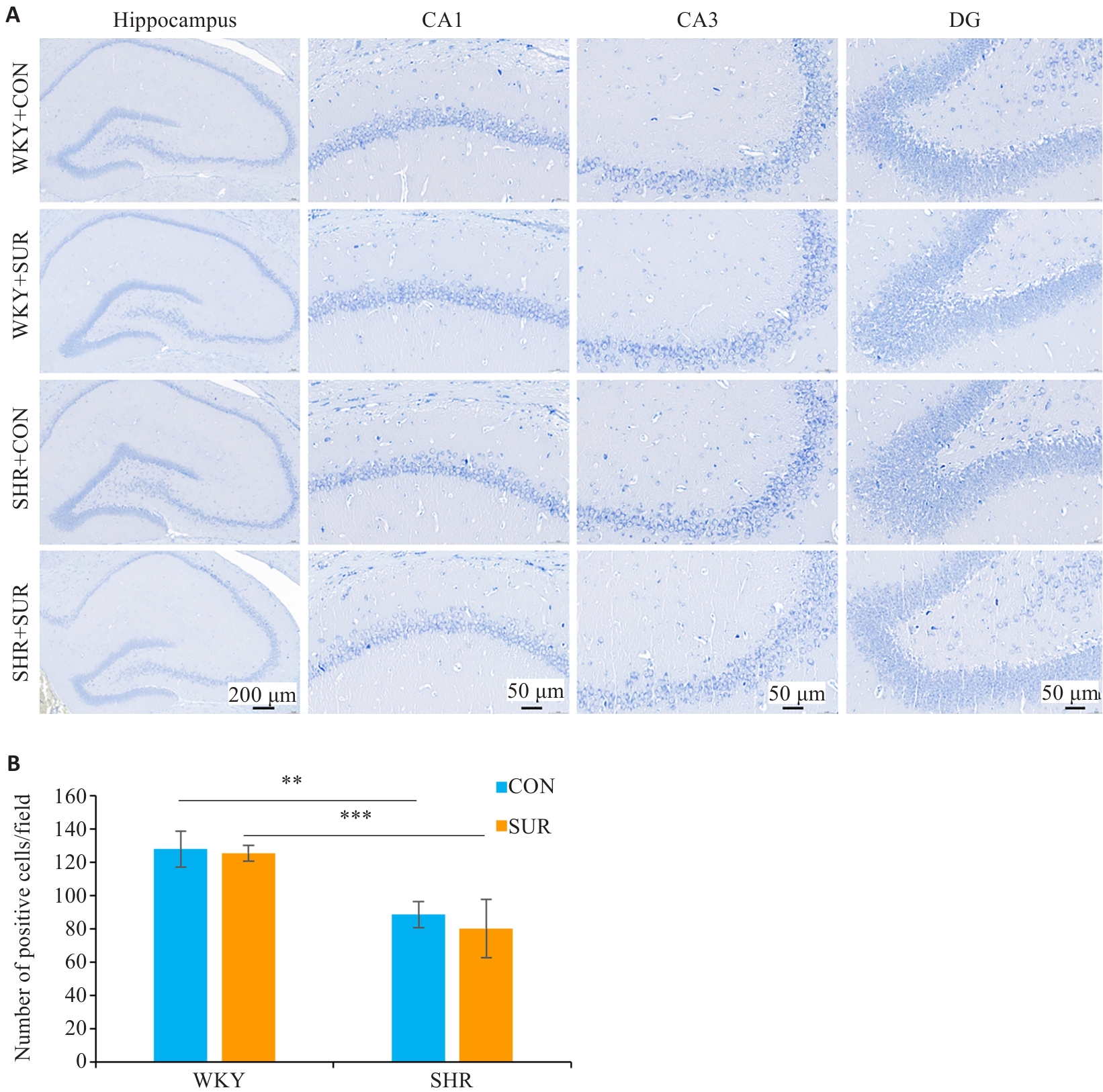
Fig.8 Nissl staining images of different regions in rat hippocampus (A) and comparison of positive cell numbers in the CA1 region among the groups (B). **P<0.01, ***P<0.001
| 1 | Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018[J]. Br J Anaesth, 2018, 121(5): 1005-12. |
| 2 | Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction[J]. Acta Anaesthesiol Scand, 2000, 44(10): 1246-51. |
| 3 | Anand N, Gupta R, Mishra SP, et al. Postoperative cognitive dysfunction: a review[J]. Asian J Anesthesiol, 2024, 62(1): 1-11. |
| 4 | Yang X, Huang X, Li M, et al. Identification of individuals at risk for postoperative cognitive dysfunction (POCD)[J]. Ther Adv Neurol Disord, 2022, 15: 17562864221114356. |
| 5 | Gonzales MM, Garbarino VR, ollet E, et al. Biological aging processes underlying cognitive decline and neurodegenerative disease [J]. J Clin Invest, 20, 132(10):e158453 |
| 6 | Lövdén M, Fratiglioni L, Maria Glymour M, et al. Education and cognitive functioning across the life span[J]. Psychol Sci Public Interest, 2020, 21(1): 6-41. |
| 7 | Feinkohl I, Winterer G, Spies CD, et al. Cognitive reserve and the risk of postoperative cognitive dysfunction[J]. Dtsch Arztebl Int, 2017, 114(7): 110-7. |
| 8 | Butz M, Gerriets T, Sammer G, et al. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: a randomized clinical trial [J]. Eur J Cardiothorac Surg, 20, 62(5):ezac251. |
| 9 | Rappold T, Laflam A, Hori D, et al. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery[J]. Br J Anaesth, 2016, 116(1): 83-9. |
| 10 | Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health[J]. Nat Rev Nephrol, 2021, 17(10): 639-54. |
| 11 | Qiu CX, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia[J]. Lancet Neurol, 2005, 4(8): 487-99. |
| 12 | Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis[J]. JAMA, 2020, 323(19): 1934-44. |
| 13 | Siedlinski M, Carnevale L, Xu X, et al. Genetic analyses identify brain structures related to cognitive impairment associated with elevated blood pressure[J]. Eur Heart J, 2023, 44(23): 2114-25. |
| 14 | Sure VN, Sakamuri SSVP, Sperling JA, et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice[J]. Geroscience, 2018, 40(4): 365-75. |
| 15 | Zong Y, Li H, Liao P, et al. Mitochondrial dysfunction: mechanisms and advances in therapy[J]. Signal Transduct Target Ther, 2024, 9(1): 124. |
| 16 | Wallace DC. Mitochondrial diseases in man and mouse[J]. Science, 1999, 283(5407): 1482-8. |
| 17 | Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival[J]. Nat Rev Neurosci, 2005, 6(11): 829-40. |
| 18 | Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3[J]. Cell Metab, 2005, 2(2): 85-93. |
| 19 | Chan SHH, Wu CA, Wu KLH, et al. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension[J]. Circ Res, 2009, 105(9): 886-96. |
| 20 | Xin J, Shan WR, Li J, et al. Activation of the lateral habenula-ventral tegmental area neural circuit contributes to postoperative cognitive dysfunction in mice[J]. Adv Sci, 2022, 9(22): e2202228. |
| 21 | Zheng B, Lai RC, Li J, et al. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery[J]. Brain Behav Immun, 2017, 61: 365-74. |
| 22 | Yuan HJ, Na WN, Li BZ, et al. Optogenetic cortical spreading depression originating from the primary visual cortex induces migraine-like pain and anxiety behaviors in freely moving C57BL/6J mice[J]. J Headache Pain, 2025, 26(1): 44. |
| 23 | Preveza NJ, Setenet G, Gwin P, et al. Decreases in K63 polyubiquitination in the hippocampus promote the formation of contextual fear memories in both males and females[J]. Hippocampus, 2025, 35(1): e23650. |
| 24 | Yamagishi A, Yonemochi N, Kimura A, et al. AMP-activated protein kinase in the amygdala and hippocampus contributes to enhanced fear memory in diabetic mice[J]. Br J Pharmacol, 2024, 181(24): 5028-40. |
| 25 | Collaborators G 2 DF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019[J]. Lancet Public Health, 2022, 7(2): e105-25. |
| 26 | Chen JXY, Vipin A, Sandhu GK, et al. Blood-brain barrier integrity disruption is associated with both chronic vascular risk factors and white matter hyperintensities[J]. J Prev Alzheimers Dis, 2025, 12(2): 100029. |
| 27 | Feng XM, Valdearcos M, Uchida Y, et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice[J]. JCI Insight, 2017, 2(7): e91229. |
| 28 | Klemmensen MM, Borrowman SH, Pearce C, et al. Mitochondrial dysfunction in neurodegenerative disorders[J]. Neurotherapeutics, 2024, 21(1): e00292. |
| 29 | McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia[J]. Neurology, 2017, 89(24): 2447-54. |
| 30 | Li CL, Zhu YD, Ma YJ, et al. Association of cumulative blood pressure with cognitive decline, dementia, and mortality[J]. J Am Coll Cardiol, 2022, 79(14): 1321-35. |
| 31 | Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American heart association[J]. Hypertension, 2016, 68(6): e67-94. |
| 32 | Jamwal S, Blackburn JK, Elsworth JD. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders[J]. Pharmacol Ther, 2021, 219: 107705. |
| 33 | Ashleigh T, Swerdlow RH, Beal MF. The role of mitochondrial dysfunction in Alzheimer's disease pathogenesis [J]. Alzheimers Dement, 20, 19(1): 333-4. |
| 34 | Yu HY, Yang CW, Chen S, et al. Comparison of the glycopattern alterations of mitochondrial proteins in cerebral cortex between rat Alzheimer's disease and the cerebral ischemia model[J]. Sci Rep, 2017, 7: 39948. |
| 35 | Liu YJ, Sulc J, Auwerx J. Mitochondrial genetics, signalling and stress responses[J]. Nat Cell Biol, 2025 Mar 10.doi: 10.1038/s41556-025-01625-w . |
| 36 | Franco C, Sciatti E, Favero G, et al. Essential Hypertension and Oxidative Stress: Novel Future Perspectives [J]. Int J Mol Sci, 20, 23(22):14489. |
| 37 | Skvarc DR, Berk M, Byrne LK, et al. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies[J]. Neurosci Biobehav Rev, 2018, 84: 116-33. |
| 38 | Liu Y, Yang W, Xue JQ, et al. Neuroinflammation: The central enabler of postoperative cognitive dysfunction[J]. Biomed Pharmacother, 2023, 167: 115582. |
| 39 | Tchekalarova J, Krushovlieva D, Ivanova P, et al. Spontaneously hypertensive rats vs. Wistar Kyoto and Wistar rats: an assessment of anxiety, motor activity, memory performance, and seizure susceptibility[J]. Physiol Behav, 2023, 269: 114268. |
| 40 | Tchekalarova J, Ivanova P, Krushovlieva D. Age-related effects of AT1 receptor antagonist losartan on cognitive decline in spontaneously hypertensive rats[J]. Int J Mol Sci, 2024, 25(13): 7340. |
| 41 | JöHREN O, Golsch C, Ddendorfer A, et al. Differential expression of AT1 receptors in the pituitary and adrenal gland of SHR and WKY [J]. Hypertension, 200, 41(4): 984-90. |
| 42 | Bak J, Pyeon HI, Seok JI, et al. Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation[J]. Behav Brain Res, 2017, 320: 219-24. |
| [1] | Lu ZHANG, Huanzhang DING, Haoran XU, Ke CHEN, Bowen XU, Qinjun YANG, Di WU, Jiabing TONG, Zegeng LI. Shenqi Buzhong Formula ameliorates mitochondrial dysfunction in a rat model of chronic obstructive pulmonary disease by activating the AMPK/SIRT1/PGC-1α pathway [J]. Journal of Southern Medical University, 2025, 45(5): 969-976. |
| [2] | Ming LIAO, Wenhua ZHONG, Ran ZHANG, Juan LIANG, Wentaorui XU, Wenjun WAN, Chao LI Shu WU. Protein C activator derived from snake venom protects human umbilical vein endothelial cells against hypoxia-reoxygenation injury by suppressing ROS via upregulating HIF-1α and BNIP3 [J]. Journal of Southern Medical University, 2025, 45(3): 614-621. |
| [3] | Qiaoyan CAI, Yaoyao XU, Yuxing LIN, Haowei LIN, Junpeng ZHENG, Weixiang ZHANG, Chunyu ZHAO, Yupeng LIN, Ling ZHANG. Qingda Granules alleviate brain damage in spontaneously hypertensive rats by modulating the miR-124/STAT3 signaling axis [J]. Journal of Southern Medical University, 2025, 45(1): 18-26. |
| [4] | Kelei GUO, Yingli LI, Chenguang XUAN, Zijun HOU, Songshan YE, Linyun LI, Liping CHEN, Li HAN, Hua BIAN. Yiqi Yangyin Huazhuo Tongluo Formula alleviates diabetic podocyte injury by regulating miR-21a-5p/FoxO1/PINK1-mediated mitochondrial autophagy [J]. Journal of Southern Medical University, 2025, 45(1): 27-34. |
| [5] | Junping ZHAN, Shuo HUANG, Qingliang MENG, Wei FAN, Huimin GU, Jiakang CUI, Huilian WANG. Buyang Huanwu Decoction reduces mitochondrial autophagy in rheumatoid arthritis synovial fibroblasts in hypoxic culture by inhibiting the BNIP3-PI3K/Akt pathway [J]. Journal of Southern Medical University, 2025, 45(1): 35-42. |
| [6] | Zhiwei ZUO, Qingliang MENG, Jiakang CUI, Kelei GUO, Hua BIAN. An artificial neural network diagnostic model for scleroderma and immune cell infiltration analysis based on mitochondria-associated genes [J]. Journal of Southern Medical University, 2024, 44(5): 920-929. |
| [7] | WANG Zining, YANG Ming, LI Shuanglei, CHI Haitao, WANG Junhui, XIAO Cangsong. A transcriptomic analysis of correlation between mitochondrial function and energy metabolism remodeling in mice with myocardial fibrosis following myocardial infarction [J]. Journal of Southern Medical University, 2024, 44(4): 666-674. |
| [8] | Chengcheng JIANG, Yangyang LI, Kexin DUAN, Tingting ZHAN, Zilong CHEN, Yongxue WANG, Rui ZHAO, Caiyun MA, Yu GUO, Changqing LIU. Parkin deletion affects PINK1/Parkin-mediated mitochondrial autophagy to exacerbate neuroinflammation and accelerate progression of Parkinson's disease in mice [J]. Journal of Southern Medical University, 2024, 44(12): 2359-2366. |
| [9] | Jiawei HU, Fang DU, Lu DING, Luxiang WANG, Weifeng ZHAO. Risk assessment of hepatocellular carcinoma in patients with hepatitis B-related cirrhosis and hypertension: a propensity score matching-based retrospective cohort study [J]. Journal of Southern Medical University, 2024, 44(11): 2243-2249. |
| [10] | Lei WANG, Fenlan BIAN, Feiyang MA, Shu FANG, Zihan LING, Mengran LIU, Hongyan SUN, Chengwen FU, Shiyao NI, Xiaoyang ZHAO, Xinru FENG, Zhengyu SUN, Guoqing LU, Pinfang KANG, Shili WU. Activation of ALDH2 alleviates hypoxic pulmonary hypertension in mice by upregulating the SIRT1/PGC-1α signaling pathway [J]. Journal of Southern Medical University, 2024, 44(10): 1955-1964. |
| [11] | YE Hongwei, ZHANG Yuming, YUN Qi, DU Ruoli, LI Lu, LI Yuping, GAO Qin. Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression [J]. Journal of Southern Medical University, 2024, 44(1): 45-51. |
| [12] | YU Jiachi, LI Ruibing, XIA Tian, WANG Jianan, JIN Jiacheng, YUAN Manqiu, LI Mianyang. PDCD4 knockdown ameliorates lipopolysaccharide- induced endothelial cell damage by improving mitochondrial dynamics [J]. Journal of Southern Medical University, 2024, 44(1): 25-35. |
| [13] | WANG Lian, XIA Yongsheng, ZHANG Zhen, LIU Xinyue, SHI Jinran, WANG Yueyue, LI Jing, ZHNAG Xiaofeng, GENG Zhijun, SONG Xue, ZUO Lugen. High expression of MRPL13 promotes cell cycle progression and proliferation of gastric cancer cells by inhibiting p53 signaling to affect long-term prognosis [J]. Journal of Southern Medical University, 2023, 43(9): 1558-1566. |
| [14] | ZHANG Ningning, QIU Qi, CHEN Yongfeng, SUN Zhengyu, LU Guoqing, WANG Lei, KANG Pingfang, WANG Hongju. Quercetin improves pulmonary arterial hypertension in rats by regulating the HMGB1/RAGE/NF-κB pathway [J]. Journal of Southern Medical University, 2023, 43(9): 1606-1612. |
| [15] | HUANG Yi, Lin Lishan, HUANG Haohua, DONG Hangming. VDAC1 participates in house dust mite- induced asthmatic airway inflammation in mice by inducing ferroptosis of airway epithelial cells [J]. Journal of Southern Medical University, 2023, 43(8): 1333-1338. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||