南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (10): 2160-2170.doi: 10.12122/j.issn.1673-4254.2025.10.12
孙陛航1( ), 郭煜君1, 祁玉麟2, 姚丹1, 陈文直1(
), 郭煜君1, 祁玉麟2, 姚丹1, 陈文直1( ), 陈念芝1(
), 陈念芝1( )
)
收稿日期:2025-07-11
出版日期:2025-10-20
发布日期:2025-10-24
通讯作者:
陈文直,陈念芝
E-mail:2023111943@stu.cqmu.edu.cn;chenwz@haifu.com.cn;saber930607@163.com
作者简介:孙陛航,在读硕士研究生,E-mail: 2023111943@stu.cqmu.edu.cn
基金资助:
Bihang SUN1( ), Yujun GUO1, Yulin QI2, Dan YAO1, Wenzhi CHEN1(
), Yujun GUO1, Yulin QI2, Dan YAO1, Wenzhi CHEN1( ), Nianzhi CHEN1(
), Nianzhi CHEN1( )
)
Received:2025-07-11
Online:2025-10-20
Published:2025-10-24
Contact:
Wenzhi CHEN, Nianzhi CHEN
E-mail:2023111943@stu.cqmu.edu.cn;chenwz@haifu.com.cn;saber930607@163.com
摘要:
目的 基于铁死亡探讨冬凌草甲素(Ori)对胰腺癌的影响,以及低强度脉冲超声(LIPUS)在协同增效中的作用机制。 方法 体外实验首先分为对照组、不同浓度的Ori组,通过CCK-8法检测不同浓度Ori对PANC-1增殖活力的影响。其次实验分为对照组、Ori组,通过流式细胞术检测细胞内Fe2+、ROS含量,试剂盒检测细胞内二醛(MDA)、而谷胱甘肽(GSH)和三磷酸腺苷(ATP)浓度,Western blotting检测细胞内GPX4、Nrf2、HO-1蛋白表达。随后分为对照组、铁死亡抑制剂组(Fer-1)、Ori组以及Ori联合Fer-1组,通过CCK-8法检测各组对PANC-1增殖活力的影响。最后分为对照组、Ori组、LIPUS组及联合组,通过Western blotting检测细胞内PIEZO1蛋白表达。体内实验构建C57BL/6J小鼠胰腺癌模型,实验分组分为对照组、Ori组、LIPUS组及联合组,通过检测皮下移植瘤小鼠肿瘤的苏木精-伊红染色(HE)、免疫组织化学Ki67染色评估肿瘤组织病理变化及细胞增殖活性,Western blotting及免疫荧光染色(IF)检测小鼠肿瘤内GPX4表达。 结果 与对照组相比,Ori可以抑制PANC-1细胞增殖(P<0.001),细胞内Fe2+、ROS、MDA升高(P<0.05),GSH和ATP下降(P<0.001)。与对照组相比,Ori组GPX4蛋白表达下降(P<0.01),HO-1、Nrf2蛋白表达升高(P<0.05)。与Ori组相比,Ori联合LIPUS组细胞活力下降(P<0.01)。比较各组皮下移植瘤小鼠肿瘤发现,与Ori组相比,Ori联合LIPUS组进一步抑制肿瘤的生长(P<0.01)。Ki67染色表明联合组相较于Ori组细胞增殖活性更弱。与Ori组相比,联合组GPX4蛋白表达及荧光强度均更低(P<0.05)。与对照组相比,LIPUS组和Ori组PIEZO1蛋白表达均升高(P<0.005),与Ori组相比,联合组PIEZO1蛋白表达升高更为显著(P<0.01)。 结论 LIPUS协同Ori通过激活PIEZO1,通过Nrf2/HO-1/GPX4通路促进胰腺癌细胞铁死亡。
孙陛航, 郭煜君, 祁玉麟, 姚丹, 陈文直, 陈念芝. 低强度脉冲超声协同冬凌草甲素通过激活PIEZO1诱导胰腺癌细胞铁死亡的机制[J]. 南方医科大学学报, 2025, 45(10): 2160-2170.
Bihang SUN, Yujun GUO, Yulin QI, Dan YAO, Wenzhi CHEN, Nianzhi CHEN. Low-intensity pulsed ultrasound and oridonin synergistically induce ferroptosis of pancreatic cancer cells by activating PIEZO1 via the Nrf2/HO-1/GPX4 pathway[J]. Journal of Southern Medical University, 2025, 45(10): 2160-2170.
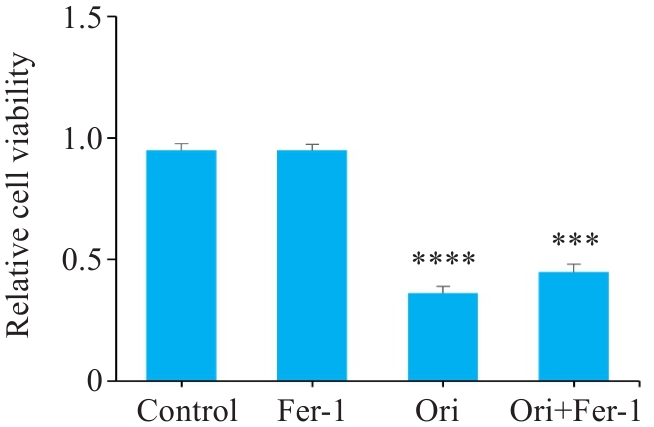
图4 Ori联合Fer-1对PANC-1活力的影响
Fig.4 Effect of Ori combined with Fer-1 on proliferation of PANC-1 cells (n=6). ****P<0.001 vs Control, ***P<0.005 vs Ori Group.
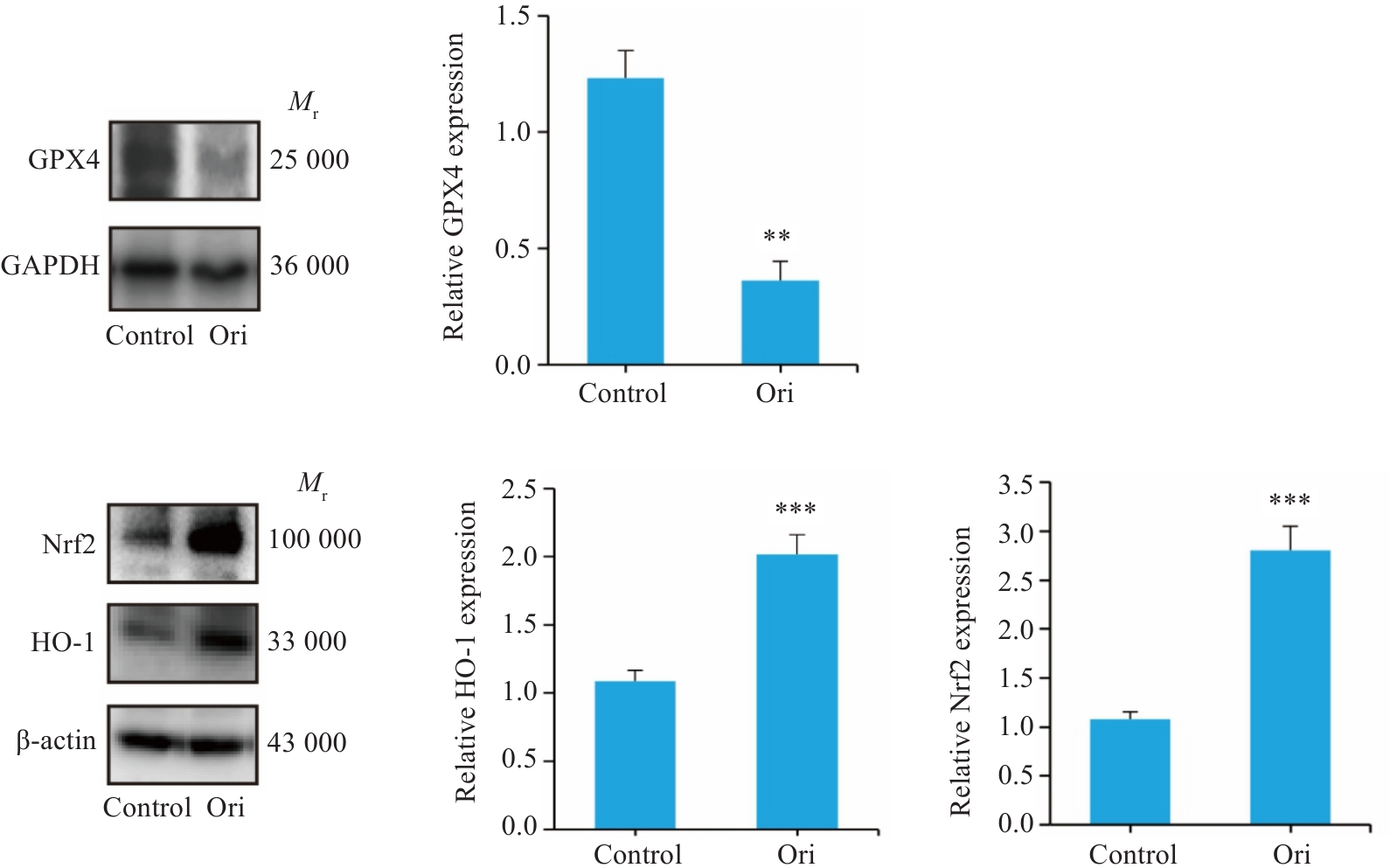
图6 Ori处理后PANC-1细胞内GPX4、Nrf2、HO-1蛋白表达
Fig.6 Effect of Ori on intracellular GPX4, Nrf2, and HO-1 expressions in PANC-1 cells (n=3). **P<0.001, ***P<0.005 vs Control.
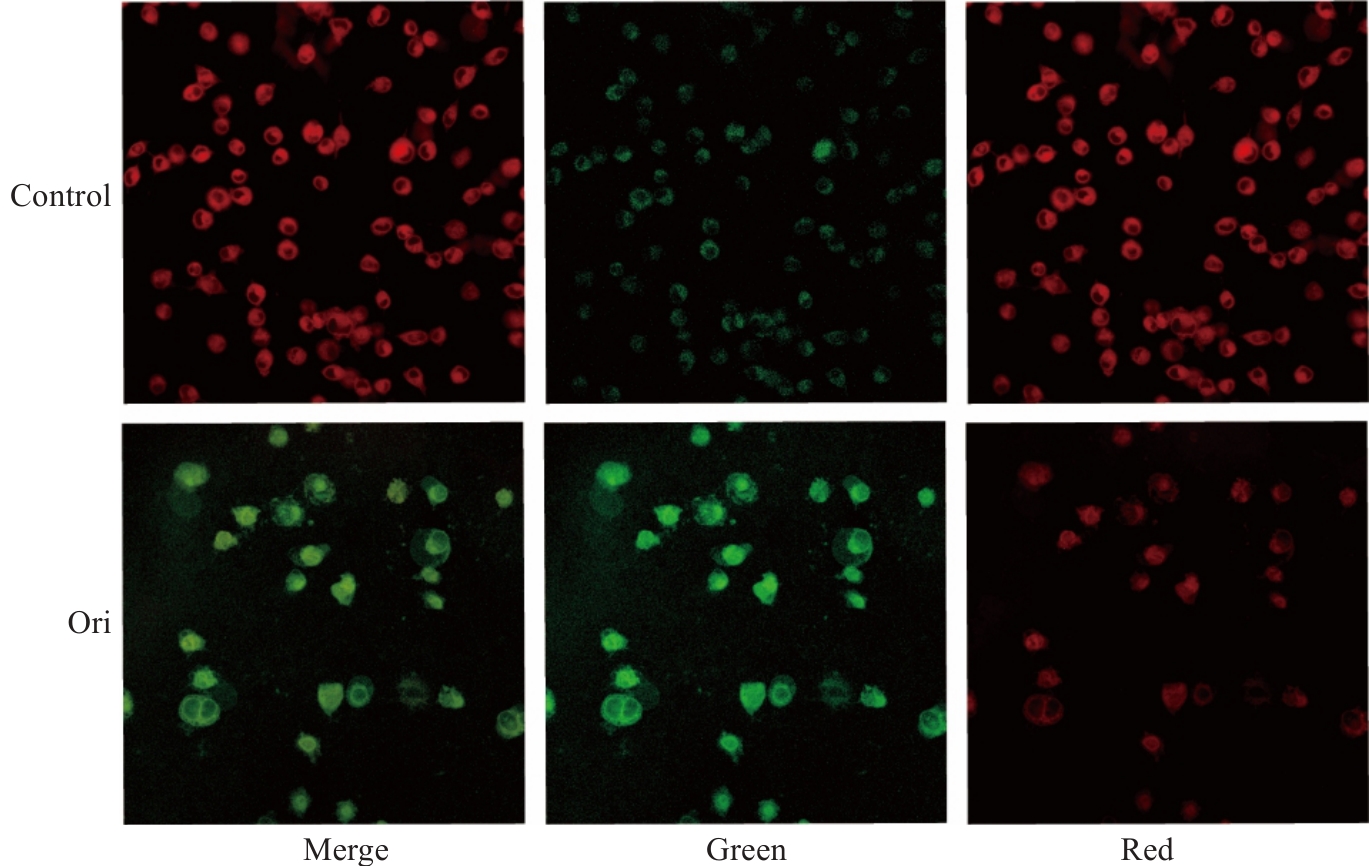
图7 Ori处理后PANC-1细胞内C11-BODIPY标记荧光强度
Fig.7 Fluorescence intensity of C11-BODIPY labeling in PANC-1 cells after Ori treatment (Original magnification: ×40) (n=3).
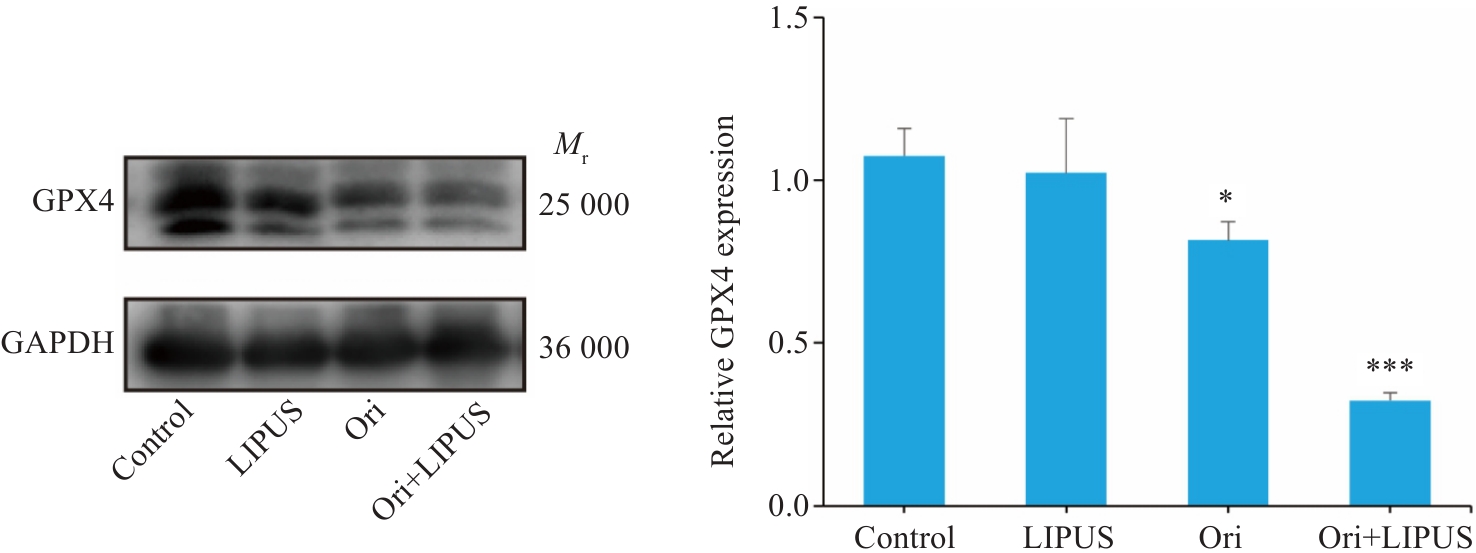
图9 Ori联合LIPUS处理后小鼠肿瘤内GPX4表达
Fig.9 Effect of Ori combined with LIPUS on intra-tumoral GPX4 expression in the mouse models (n=3). *P<0.05 vs Control,***P<0.005 vs Ori.
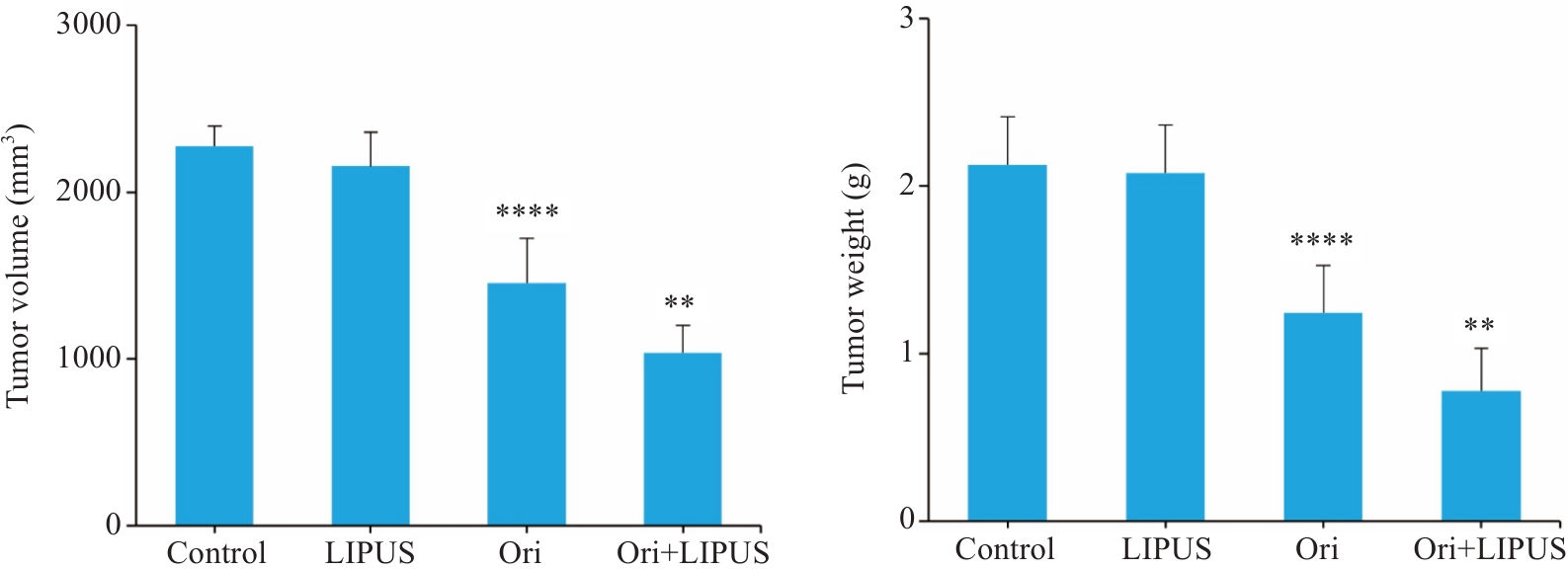
图12 Ori联合LIPUS对胰腺癌小鼠肿瘤体积及体质量影响
Fig.12 Effect of LIPUS combined with Ori on tumor volume and weight in the tumor-bearing mice (n=8). ****P<0.001 vs Control, **P<0.01 vs Ori.
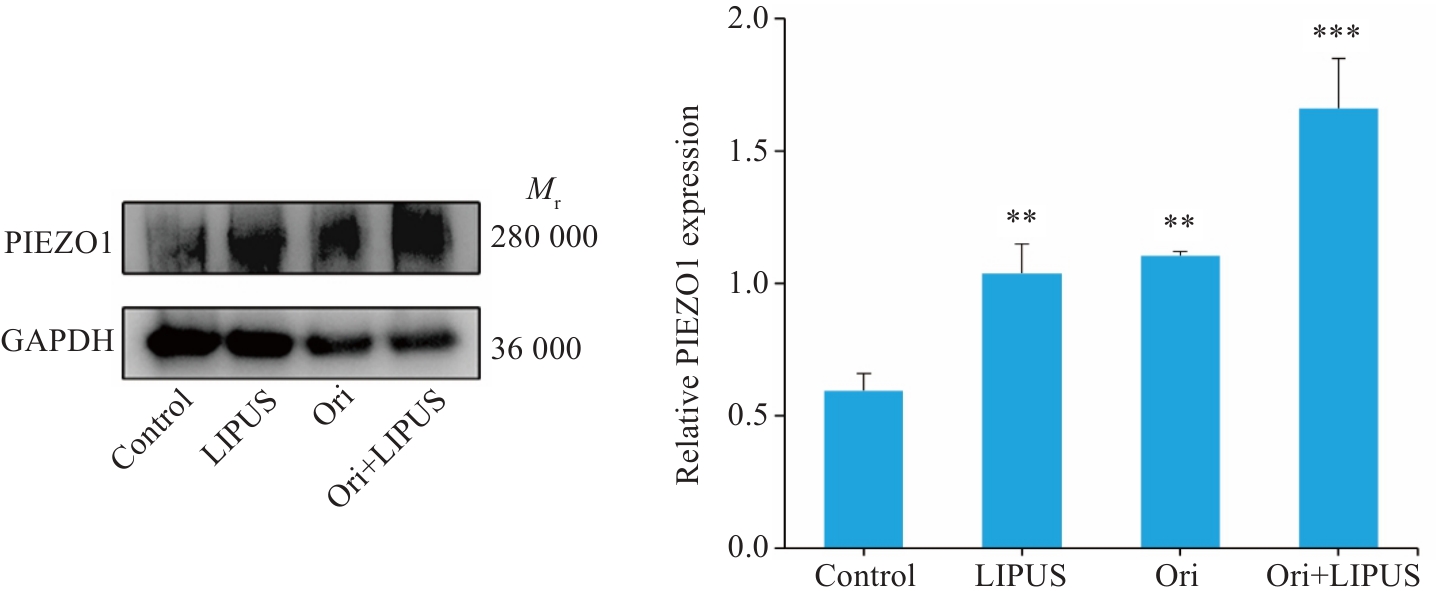
图15 Ori联合LIPUS处理后PANC-1细胞内PIEZO1蛋白表达
Fig.15 Effect of Ori combined with LIPUS on intracellular PIEZO1 expression (n=3). **P<0.005 vs Control; ***P<0.001 vs Ori.
| [1] | 易丽夏, 方涵露, 李婧怡, 等. 2022年全球和中国胰腺癌发病及死亡分析[J]. 海军军医大学学报, 2024, 45(12): 1470-7. |
| [2] | Zheng RS, Zhang SW, Zeng HM, et al. Cancer incidence and mortality in China, 2016[J]. J Natl Cancer Cent, 2022, 2(1): 1-9. doi:10.1016/j.jncc.2022.02.002 |
| [3] | Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, et al. Pancreatic cancer: advances and challenges[J]. Cell, 2023, 186(8): 1729-54. doi:10.1016/j.cell.2023.02.014 |
| [4] | 中国抗癌协会胰腺癌专业委员会, 虞先濬, 徐 近. 中国抗癌协会胰腺癌整合诊治指南(精简版)[J]. 中国肿瘤临床, 2023, 50(10): 487-96. |
| [5] | Rohatgi S, Jagannathan JP, Rosenthal MH, et al. Vascular toxicity associated with chemotherapy and molecular targeted therapy: what should a radiologist know[J]? AJR Am J Roentgenol, 2014, 203(6): 1353-62. doi:10.2214/ajr.13.11967 |
| [6] | 韦天夫, 周 琪, 胡凤林, 等. 慢性胰腺炎炎癌转化中医理论浅析[J]. 时珍国医国药, 2020, 31(5): 1192-4. |
| [7] | 魏小曼, 李 柳, 王俊壹, 等. 癌毒病机理论辨治胰腺癌探讨[J]. 中华中医药杂志, 2022, 37(4): 2062-5. |
| [8] | 刘延泽, 陈士林, 马 培, 等. 中草药中发现新抗癌药物的途径[J]. 现代药物与临床, 2012, 27(4): 323-37. |
| [9] | 高世勇, 唐博琪, 魏新瑞, 等. 冬凌草的本草考证、化学成分、药理作用及其临床应用[J]. 中草药, 2023, 54(19): 6543-54. |
| [10] | Li X, Zhang CT, Ma W, et al. Oridonin: a review of its pharmacology, pharmacokinetics and toxicity[J]. Front Pharmacol, 2021, 12: 645824. doi:10.3389/fphar.2021.645824 |
| [11] | Zhang CL, Wu LJ, Zuo HJ, et al. Cytochrome c release from oridonin-treated apoptotic A375-S2 cells is dependent on p53 and extracellular signal-regulated kinase activation[J]. J Pharmacol Sci, 2004, 96(2): 155-63. doi:10.1254/jphs.fpj04008x |
| [12] | Huang J, Wu LJ, Tashiro SI, et al. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways[J]. J Pharmacol Sci, 2008, 107(4): 370-9. doi:10.1254/jphs.08044fp |
| [13] | Liu Y, Song Z, Liu YJ, et al. Identification of ferroptosis as a novel mechanism for antitumor activity of natural product derivative a2 in gastric cancer[J]. Acta Pharm Sin B, 2021, 11(6): 1513-25. doi:10.1016/j.apsb.2021.05.006 |
| [14] | Ye SY, Hu XY, Sun SW, et al. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1[J]. Eur J Pharmacol, 2024, 974: 176620. doi:10.1016/j.ejphar.2024.176620 |
| [15] | Cai MR, Fu TT, Zhu RY, et al. An iron-based metal-organic framework nanoplatform for enhanced ferroptosis and oridonin delivery as a comprehensive antitumor strategy[J]. Acta Pharm Sin B, 2024, 14(9): 4073-86. doi:10.1016/j.apsb.2024.05.015 |
| [16] | 倪 琰, 郭晓冬, 王静霞, 等. 胰腺癌中医治疗评述[J]. 中国中医基础医学杂志, 2023, 29(12): 2117-22. |
| [17] | Yang X, Liu YQ, Wang Z, et al. Ferroptosis as a new tool for tumor suppression through lipid peroxidation[J]. Commun Biol, 2024, 7(1): 1475. doi:10.1038/s42003-024-07180-8 |
| [18] | De Leon-Oliva D, Boaru DL, Minaya-Bravo AM, et al. Improving understanding of ferroptosis: Molecular mechanisms, connection with cellular senescence and implications for aging[J]. Heliyon, 2024, 10(21): e39684. doi:10.1016/j.heliyon.2024.e39684 |
| [19] | Chen AQ, Huang HF, Fang SM, et al. ROS: a “booster” for chronic inflammation and tumor metastasis[J]. Biochim Biophys Acta BBA Rev Cancer, 2024, 1879(6): 189175. doi:10.1016/j.bbcan.2024.189175 |
| [20] | Su ZY, Liu YQ, Wang L, et al. Regulation of SLC7A11 as an unconventional checkpoint in tumorigenesis through ferroptosis[J]. Genes Dis, 2024, 12(1): 101254. doi:10.1016/j.gendis.2024.101254 |
| [21] | Zhu LP, Leng DL, Guo ZA, et al. Self-catalyzed nitric oxide nano complexes induce ferroptosis for cancer immunotherapy[J]. J Control Release, 2025, 377: 524-39. doi:10.1016/j.jconrel.2024.11.048 |
| [22] | 李泽彦, 李国东, 孙 硕, 等. 雷公藤红素诱导人胰腺癌PANC-1细胞铁死亡的机制研究[J]. 中国病理生理杂志, 2024, 40(6): 1062-9. |
| [23] | Liu X, Xu JM, Zhou J, et al. Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance[J]. Genes Dis, 2020, 8(4): 448-62. doi:10.1016/j.gendis.2020.06.010 |
| [24] | Nomikou N, Li YS, McHale AP. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy[J]. Cancer Lett, 2010, 288(1): 94-8. doi:10.1016/j.canlet.2009.06.028 |
| [25] | Gong YP, Wang ZG, Dong GF, et al. Low-intensity focused ultrasound mediated localized drug delivery for liver tumors in rabbits[J]. Drug Deliv, 2016, 23(7): 2280-9. doi:10.3109/10717544.2014.972528 |
| [26] | 孙亚超, 王 昊, 商慧娟, 等. 低强度脉冲超声研究现状及趋势的可视化分析[J]. 联勤军事医学, 2024, 38(1): 75-84. |
| [27] | 王敏丹, 刘 莉, 崔夏莲, 等. 冬凌草甲素对食管鳞状细胞癌细胞内谷胱甘肽代谢网络影响的研究[J]. 上海中医药杂志, 2024, 58(7): 77-82, 94. |
| [28] | Ru Q, Li YS, Chen L, et al. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects[J]. Signal Transduct Target Ther, 2024, 9(1): 271. doi:10.1038/s41392-024-01969-z |
| [29] | Kwon MY, Park E, Lee SJ, et al. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death[J]. Oncotarget, 2015, 6(27): 24393-403. doi:10.18632/oncotarget.5162 |
| [30] | O’Rourke SA, Shanley LC, Dunne A. The Nrf2-HO-1 system and inflammaging[J]. Front Immunol, 2024, 15: 1457010. doi:10.3389/fimmu.2024.1457010 |
| [31] | Zhu YT, Zhang JC, Liu QN, et al. Semen Cuscutae-Fructus Lycii attenuates Tripterygium glycosides-induced spermatogenesis dysfunction by inhibiting oxidative stress-mediated ferroptosis via the Nrf2/HO-1 pathway[J]. Phytomedicine, 2024, 135: 156221. doi:10.1016/j.phymed.2024.156221 |
| [32] | Du HF, Wu JW, Zhu YS, et al. Fucoxanthin induces ferroptosis in cancer cells via downregulation of the Nrf2/HO-1/GPX4 pathway[J]. Molecules, 2024, 29(12): 2832. doi:10.3390/molecules29122832 |
| [33] | Acheta J, Stephens SBZ, Belin S, et al. Therapeutic low-intensity ultrasound for peripheral nerve regeneration - a schwann cell perspective[J]. Front Cell Neurosci, 2022, 15: 812588. doi:10.3389/fncel.2021.812588 |
| [34] | Xu MS, Wang L, Wu SM, et al. Review on experimental study and clinical application of low-intensity pulsed ultrasound in inflammation[J]. Quant Imaging Med Surg, 2021, 11(1): 443-62. doi:10.21037/qims-20-680 |
| [35] | Tardoski S, Gineyts E, Ngo J, et al. Low-intensity ultrasound promotes clathrin-dependent endocytosis for drug penetration into tumor cells[J]. Ultrasound Med Biol, 2015, 41(10): 2740-54. doi:10.1016/j.ultrasmedbio.2015.06.006 |
| [36] | Lentacker I, De Cock I, Deckers R, et al. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms[J]. Adv Drug Deliv Rev, 2014, 72: 49-64. doi:10.1016/j.addr.2013.11.008 |
| [37] | Jin JQ, Fan ZF, Long YL, et al. Matrine induces ferroptosis in cervical cancer through activation of piezo1 channel[J]. Phytomedicine, 2024, 122: 155165. doi:10.1016/j.phymed.2023.155165 |
| [38] | Wang SY, Li WW, Zhang PF, et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx[J]. J Adv Res, 2022, 41: 63-75. doi:10.1016/j.jare.2022.01.004 |
| [39] | Xiang ZQ, Zhang PF, Jia CW, et al. Piezo1 channel exaggerates ferroptosis of nucleus pulposus cells by mediating mechanical stress-induced iron influx[J]. Bone Res, 2024, 12(1): 20. doi:10.1038/s41413-024-00317-9 |
| [1] | 何榕茂, 方泽扬, 张芸芸, 吴友谅, 梁世秀, 计涛, 陈科全, 王斯琪. 铁死亡相关基因对溃疡性结肠炎具有诊断预测价值[J]. 南方医科大学学报, 2025, 45(9): 1927-1937. |
| [2] | 云琦, 杜若丽, 贺玉莹, 张贻欣, 王佳慧, 叶红伟, 李正红, 高琴. 肉桂酸通过抑制TLR4减轻阿霉素诱导的小鼠心肌损伤铁死亡的发生[J]. 南方医科大学学报, 2025, 45(9): 1946-1958. |
| [3] | 欧泽金, 李瀛, 陈诗, 王梓译, 何美仪, 陈志成, 唐侍豪, 孟晓静, 王致. 抑制铁死亡减轻敌草快引起的斑马鱼急性肾损伤的机制[J]. 南方医科大学学报, 2025, 45(8): 1743-1750. |
| [4] | 李军仪, 陈思源, 谢力遥, 王劲, 程奥, 张绍伟, 林继瑜, 方志涵, 潘一锐, 崔翀鹤, 陈庚鑫, 张超, 李栎. 益智仁提取物谷甾醇通过抑制铁死亡中的ETS-5基因表达延长秀丽隐杆线虫的寿命[J]. 南方医科大学学报, 2025, 45(8): 1751-1757. |
| [5] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [6] | 张梦影, 赵晨玲, 田丽伟, 余郭芳, 杨文明, 董婷. 肝豆扶木汤通过GPX4/ACSL4/ALOX15通路抑制铁死亡改善Wilson病小鼠的肝脏脂肪变性[J]. 南方医科大学学报, 2025, 45(7): 1471-1478. |
| [7] | 张安邦, 孙秀颀, 庞博, 吴远华, 时靖宇, 张宁, 叶涛. 电针预处理通过调节肠道-大脑轴及Nrf2/HO-1信号通路抑制铁死亡减轻大鼠脑缺血再灌注损伤[J]. 南方医科大学学报, 2025, 45(5): 911-920. |
| [8] | 张林落, 李长青, 皇玲玲, 周学平, 娄媛媛. 梓醇扶正制毒配伍从SLC7A11/GPX4通路抑制铁死亡减轻雷公藤甲素肝毒性[J]. 南方医科大学学报, 2025, 45(4): 810-818. |
| [9] | 富丽萍, 袁立霞, 王杰, 陈学蓝, 柯桂芝, 黄煜, 杨心仪, 刘刚. 近十年低强度脉冲超声在肌骨疾病治疗中的应用进展[J]. 南方医科大学学报, 2025, 45(3): 661-668. |
| [10] | 谢梦瑶, 杨敏, 李欣, 杜永洪. 低强度脉冲超声联合制霉菌素可协同抑制兔阴道白色念珠菌生物被膜感染[J]. 南方医科大学学报, 2025, 45(2): 296-303. |
| [11] | 郭涛, 陈柏霖, 石金沙, 匡显锋, 余腾跃, 魏嵩, 刘雄, 肖蓉, 李娟娟. 天麻素通过激活GPX4/SLC7A11/FTH1信号抑制铁死亡减轻新生小鼠缺氧缺血性脑损伤[J]. 南方医科大学学报, 2025, 45(10): 2071-2081. |
| [12] | 张思雨, 冉林武, 曾瑾, 王玉炯. 产气荚膜梭菌Beta1毒素通过P2X7-Ca2+轴诱导巨噬细胞焦亡和铁死亡[J]. 南方医科大学学报, 2025, 45(10): 2126-2134. |
| [13] | 王锦帼, 马扬, 李赵鑫, 何丽妃, 黄英泽, 范晓明. PDZ结合激酶作为胰腺癌潜在预后标志物:从泛癌分析到胰腺癌验证[J]. 南方医科大学学报, 2025, 45(10): 2210-2222. |
| [14] | 季春斐, 左宗超, 王钧, 李妙男. N-乙酰神经氨酸中通过抑制Nrf2轴促进缺氧/复氧损伤的H9C2心肌细胞发生铁死亡[J]. 南方医科大学学报, 2025, 45(1): 72-79. |
| [15] | 陈凯, 孟兆菲, 闵静婷, 王佳慧, 李正红, 高琴, 胡俊锋. 姜黄素通过抑制TXNIP/TRX-1/GPX4通路介导的铁死亡减轻脓毒症小鼠肺损伤[J]. 南方医科大学学报, 2024, 44(9): 1805-1813. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||