南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (5): 913-919.doi: 10.12122/j.issn.1673-4254.2024.05.13
夏勇生1,2( ), 王炼1,2, 陈孝华1,2, 张雨路3, 孙奥飞3, 陈德利1(
), 王炼1,2, 陈孝华1,2, 张雨路3, 孙奥飞3, 陈德利1( )
)
收稿日期:2024-01-23
出版日期:2024-05-20
发布日期:2024-06-06
通讯作者:
陈德利
E-mail:xiayongsheng0818@163.com;13965295950@139.com
作者简介:夏勇生,在读硕士研究生,E-mail: xiayongsheng0818@163.com
基金资助:
Yongsheng XIA1,2( ), Lian WANG1,2, Xiaohua CHEN1,2, Yulu ZHANG3, Aofei SUN3, Deli CHEN1(
), Lian WANG1,2, Xiaohua CHEN1,2, Yulu ZHANG3, Aofei SUN3, Deli CHEN1( )
)
Received:2024-01-23
Online:2024-05-20
Published:2024-06-06
Contact:
Deli CHEN
E-mail:xiayongsheng0818@163.com;13965295950@139.com
摘要:
目的 探讨TSR2核糖体成熟因子在胃癌中的表达情况及其与胃癌恶性演进的相关性,并分析其潜在的作用机制。 方法 纳入105例胃癌患者资料,分析TSR2在胃癌组织中表达水平及其对胃癌恶性进展、术后5年生存率的影响;GO及KEGG富集分析预测TSR2的生物学功能及可能的作用机制;通过慢病毒转染技术上调和下调TSR2在胃癌细胞系(MGC-803)的表达水平,并采用CCK-8、Transwell评估其对MGC-803细胞增殖、侵袭及迁移的影响;Western blot检测p-PI3K、p-AKT表达。 结果 TSR2在胃癌组织的表达水平显著低于癌旁组织(P<0.001),且TSR2的表达水平与CEA、CA19-9、T分期及N分期相关(P<0.05)。单因素联合多因素分析显示,TSR2低表达(P=0.020)、CEA≥5 μg/L(P=0.021)、CA19-9≥37 kU/L(P=0.001)、T3~T4分期(P=0.039)和N2~N3分期(P=0.027)是独立影响胃癌患者施行根治术后5年生存率的风险因子。生存分析结果显示,TSR2表达水平与胃癌患者术后5年生存率呈正相关(P<0.001)。生物信息学富集分析预测TSR2的功能可能与PI3K/AKT信号通路相关。CCK-8和Transwell实验结果显示,上调TSR2可抑制胃癌细胞的增殖、迁移和侵袭(P<0.05),下调则反之(P<0.05)。Western blot结果显示过表达TSR2可下调胃癌细胞中磷脂肌醇3激酶(PI3K)和蛋白激酶B(AKT)的磷酸化,敲低则反之(P<0.05)。 结论 TSR2在胃癌组织中低表达并影响患者预后,其可能与下调PI3K/AKT信号通路抑制胃癌细胞的增殖、侵袭与迁移有关。
夏勇生, 王炼, 陈孝华, 张雨路, 孙奥飞, 陈德利. 过表达TSR2通过下调PI3K/AKT信号通路抑制胃癌细胞的增殖和侵袭[J]. 南方医科大学学报, 2024, 44(5): 913-919.
Yongsheng XIA, Lian WANG, Xiaohua CHEN, Yulu ZHANG, Aofei SUN, Deli CHEN. TSR2 overexpression inhibits proliferation and invasion of gastric cancer cells by downregulating the PI3K/AKT signaling pathway[J]. Journal of Southern Medical University, 2024, 44(5): 913-919.
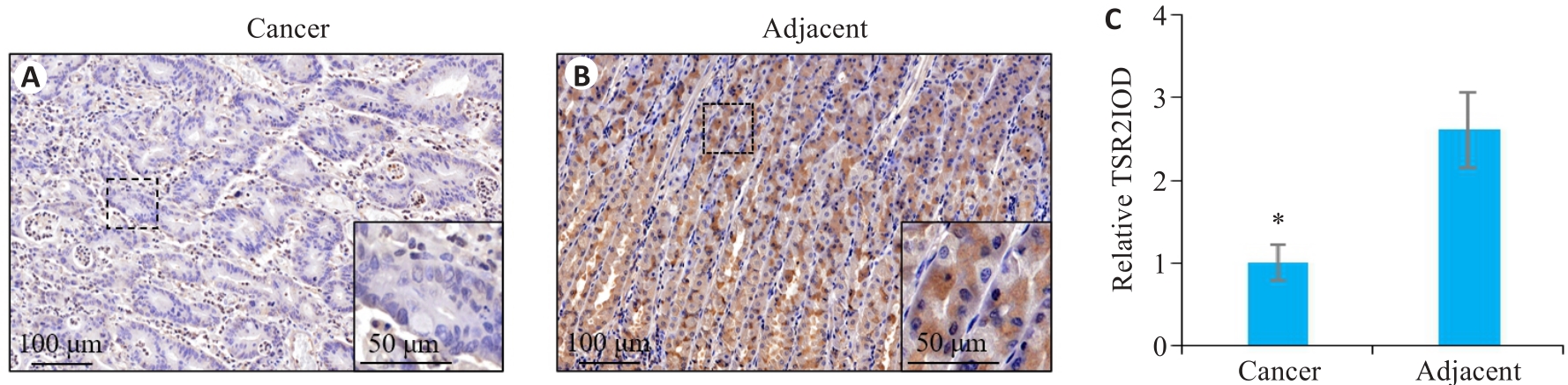
图1 TSR2在胃癌及癌旁组织中的表达情况
Fig.1 Expression of TSR2 in gastric cancer and adjacent tissues. A: TSR2 is lowly expressed in gastric cancer tissue. B: TSR2 is highly expressed in adjacent tissue. C: Relative IOD value of TSR2 (n=105). *P<0.05 vs adjacent tissues.
| Factors | n | TSR2 expression | χ 2 | P | |
|---|---|---|---|---|---|
| Low (n=53) | High (n=52) | ||||
| Gender | 0.023 | 0.880 | |||
| Male | 74 | 37 (50.0%) | 37 (50.0%) | ||
| Female | 31 | 16 (51.6%) | 15 (48.4%) | ||
| Age (year) | 0.015 | 0.903 | |||
| <60 | 41 | 21 (51.2%) | 20 (48.8%) | ||
| ≥60 | 64 | 32 (50.0%) | 32 (50.0%) | ||
| CEA (μg/L) | 6.940 | 0.008 | |||
| <5 | 49 | 18 (36.7%) | 31 (63.3%) | ||
| ≥5 | 56 | 35 (62.5%) | 21 (37.5%) | ||
| CA19-9 (kU/L) | 17.720 | <0.001 | |||
| <37 | 47 | 13 (27.7%) | 34 (72.3%) | ||
| ≥37 | 58 | 40 (69.0%) | 18 (31.0%) | ||
| Tumor size (cm) | 2.134 | 0.144 | |||
| <5 | 49 | 21 (42.9%) | 28 (57.1%) | ||
| ≥5 | 56 | 32 (57.1%) | 24 (42.9%) | ||
| Histological type | 1.177 | 0.278 | |||
| Adenocarcinoma | 66 | 36 (54.5%) | 30 (45.5%) | ||
| Other | 39 | 17 (43.6%) | 22 (56.4%) | ||
| T stage | 11.675 | 0.001 | |||
| 1-2 | 53 | 18 (34.0%) | 35 (66.0%) | ||
| 3-4 | 52 | 35 (67.3%) | 17 (32.7%) | ||
| N stage | 13.414 | <0.001 | |||
| 0-1 | 60 | 21 (35.0%) | 39 (65.0%) | ||
| 2-3 | 45 | 32 (71.1%) | 13 (28.9%) | ||
表1 胃癌组织中TSR2的表达量与恶性进展参数的关系
Tab.1 Correlation between TSR2 expression and progression of gastric cancer [n (%)]
| Factors | n | TSR2 expression | χ 2 | P | |
|---|---|---|---|---|---|
| Low (n=53) | High (n=52) | ||||
| Gender | 0.023 | 0.880 | |||
| Male | 74 | 37 (50.0%) | 37 (50.0%) | ||
| Female | 31 | 16 (51.6%) | 15 (48.4%) | ||
| Age (year) | 0.015 | 0.903 | |||
| <60 | 41 | 21 (51.2%) | 20 (48.8%) | ||
| ≥60 | 64 | 32 (50.0%) | 32 (50.0%) | ||
| CEA (μg/L) | 6.940 | 0.008 | |||
| <5 | 49 | 18 (36.7%) | 31 (63.3%) | ||
| ≥5 | 56 | 35 (62.5%) | 21 (37.5%) | ||
| CA19-9 (kU/L) | 17.720 | <0.001 | |||
| <37 | 47 | 13 (27.7%) | 34 (72.3%) | ||
| ≥37 | 58 | 40 (69.0%) | 18 (31.0%) | ||
| Tumor size (cm) | 2.134 | 0.144 | |||
| <5 | 49 | 21 (42.9%) | 28 (57.1%) | ||
| ≥5 | 56 | 32 (57.1%) | 24 (42.9%) | ||
| Histological type | 1.177 | 0.278 | |||
| Adenocarcinoma | 66 | 36 (54.5%) | 30 (45.5%) | ||
| Other | 39 | 17 (43.6%) | 22 (56.4%) | ||
| T stage | 11.675 | 0.001 | |||
| 1-2 | 53 | 18 (34.0%) | 35 (66.0%) | ||
| 3-4 | 52 | 35 (67.3%) | 17 (32.7%) | ||
| N stage | 13.414 | <0.001 | |||
| 0-1 | 60 | 21 (35.0%) | 39 (65.0%) | ||
| 2-3 | 45 | 32 (71.1%) | 13 (28.9%) | ||
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank χ2 | P | HR | 95% CI | P | |
| Gender (male vs female) | 1.451 | 0.228 | - | - | - |
| Age (≥60 years vs <60 years) | 1.356 | 0.244 | - | - | - |
| TSR2 expression (low vs high) | 31.840 | <0.001 | 0.418 | 0.201-0.871 | 0.020 |
| CEA (≥5μg/L vs <5 μg/L) | 18.367 | <0.001 | 2.173 | 1.126-4.192 | 0.021 |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 25.491 | <0.001 | 3.144 | 1.559-6.342 | 0.001 |
| Tumor size (≥5 cm vs <5 cm) | 2.163 | 0.141 | - | - | - |
| Histological type(adenocarcinoma vs other) | 0.001 | 0.982 | - | - | - |
| T stage (T3-T4vs T1-T2) | 21.658 | <0.001 | 1.991 | 1.034-3.832 | 0.039 |
| N stage (N2-N3vs N0-N1) | 26.362 | <0.001 | 2.106 | 1.090-4.066 | 0.027 |
表2 影响胃癌患者预后的危险因素
Tab.2 Risk factors affecting prognosis of patients with gastric cancer
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank χ2 | P | HR | 95% CI | P | |
| Gender (male vs female) | 1.451 | 0.228 | - | - | - |
| Age (≥60 years vs <60 years) | 1.356 | 0.244 | - | - | - |
| TSR2 expression (low vs high) | 31.840 | <0.001 | 0.418 | 0.201-0.871 | 0.020 |
| CEA (≥5μg/L vs <5 μg/L) | 18.367 | <0.001 | 2.173 | 1.126-4.192 | 0.021 |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 25.491 | <0.001 | 3.144 | 1.559-6.342 | 0.001 |
| Tumor size (≥5 cm vs <5 cm) | 2.163 | 0.141 | - | - | - |
| Histological type(adenocarcinoma vs other) | 0.001 | 0.982 | - | - | - |
| T stage (T3-T4vs T1-T2) | 21.658 | <0.001 | 1.991 | 1.034-3.832 | 0.039 |
| N stage (N2-N3vs N0-N1) | 26.362 | <0.001 | 2.106 | 1.090-4.066 | 0.027 |
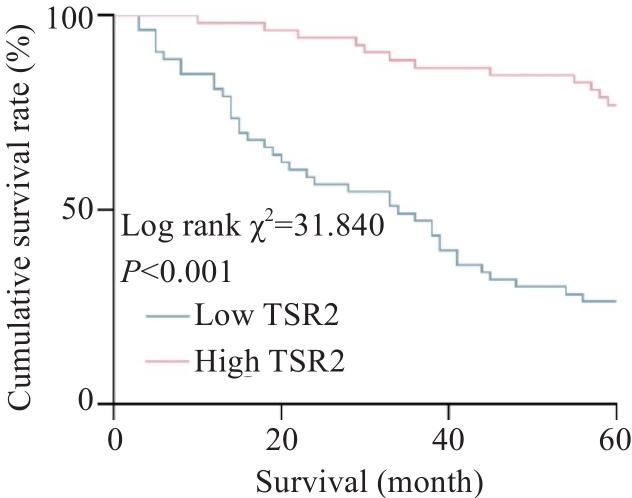
图2 胃癌组织中TSR2表达水平对患者术后5年生存率的影响
Fig.2 Influence of TSR2 expression level in gastric cancer tissue on 5-year survival rate of the patients after surgery.
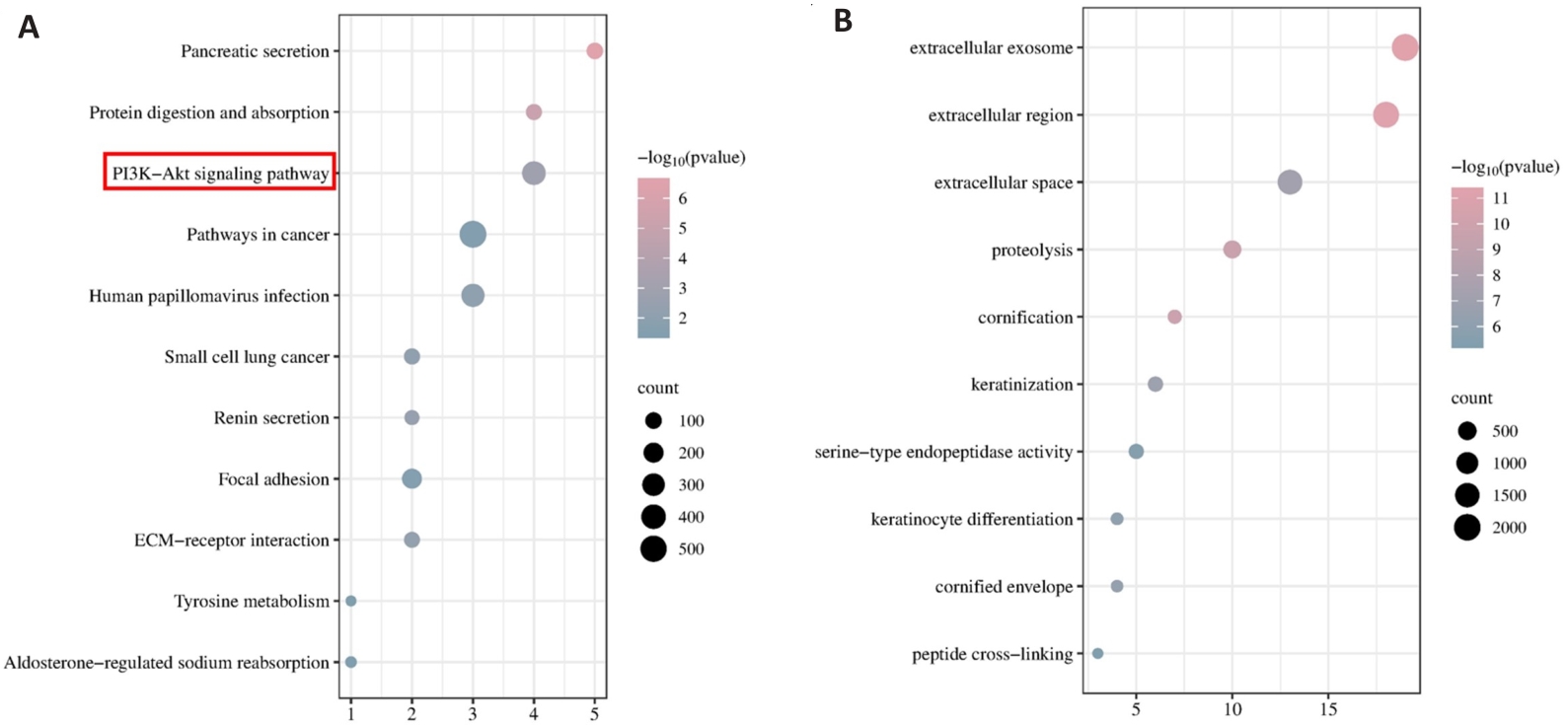
图3 TSR2的KEGG和GO富集分析
Fig.3 KEGG and GO enrichment analysis of TSR2. A: KEGG analysis of TSR2 in gastric cancer. B: GO analysis of TSR2 in gastric cancer.
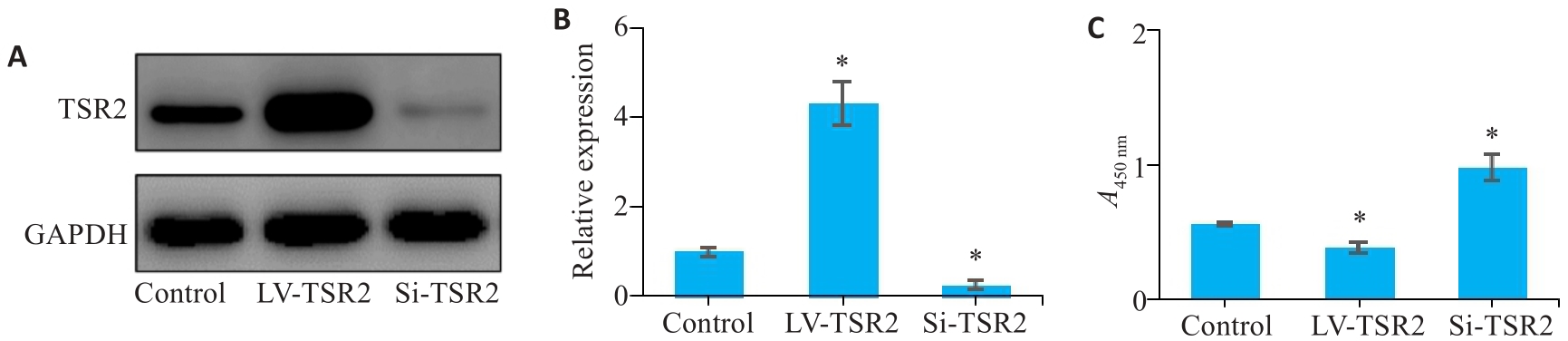
图4 TSR2对MGC-803细胞增殖能力的影响
Fig.4 Effect of TSR2 overexpression and knockdown on proliferation of MGC-803 cells. A, B: Lentivirus-mediated TSR2 overexpression and knockdown in MGC803 cells. C: TSR2 overexpression inhibits proliferation of gastric cancer cells (n=3). LV: Overexpression; Si: SiRNA. *P<0.05 vs Control.
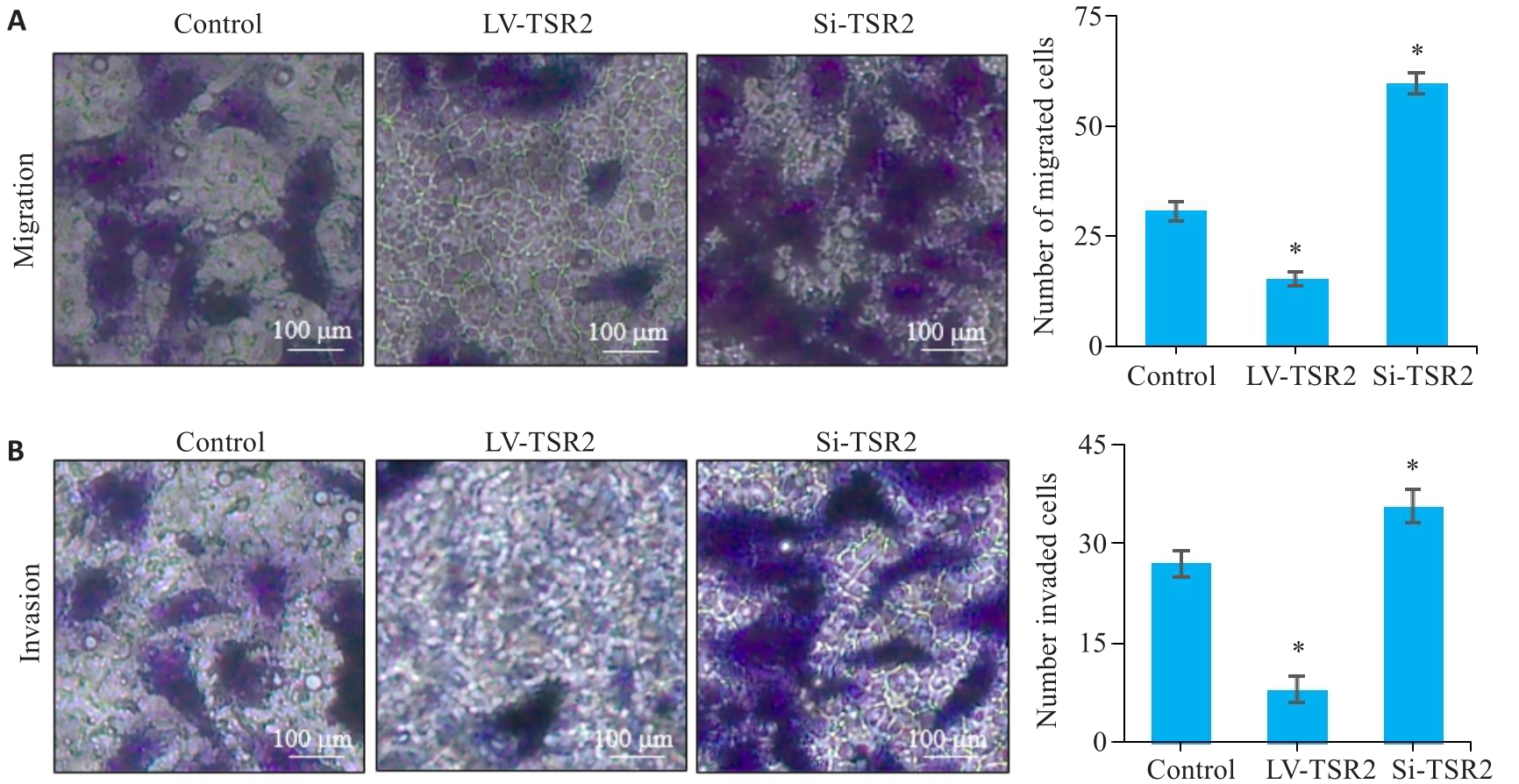
图5 TSR2对MGC-803细胞迁移和侵袭能力的影响
Fig.5 Effects of TSR2 overexpression and knockdown on migration (A) and invasion (B) of MGC-803 cells detected by Transwell assay (n=3). *P<0.05 vs Control.
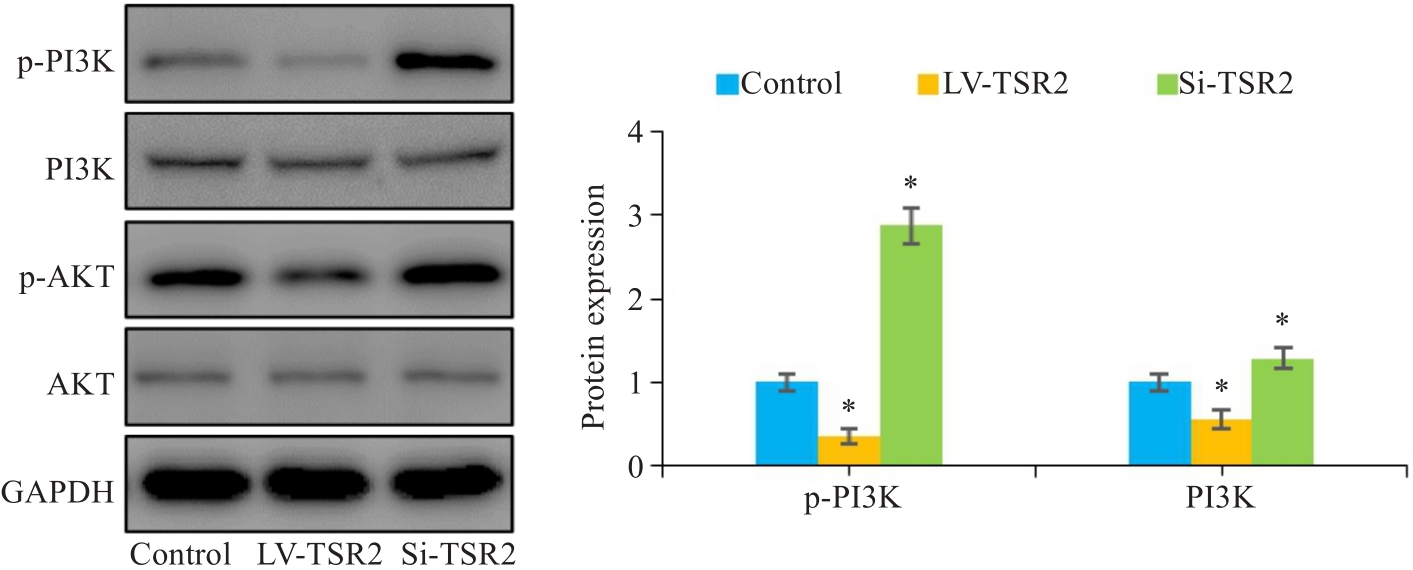
图6 TSR2调控MGC-803细胞中的PI3K/AKT信号通路
Fig.6 Western blotting for assessing the effect of TSR2 overexpression and knockdown on expression of p-PI3K and p-AKT in MGC803 cells (n=3). *P<0.05 vs Control.
| 1 | Mülder DT, Hahn AI, Huang RJ, et al. Prevalence of gastric precursor lesions in countries with differential gastric cancer burden: a systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2024: S1542-S3565(24)00227-1. DOI: 10.1016/j.cgh.2024.02.023 |
| 2 | Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492 |
| 3 | Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives[J]. World J Gastroenterol, 2016, 22(8): 2403-14. DOI: 10.3748/wjg.v22.i8.2403 |
| 4 | Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer[J]. Lancet, 2020, 396(10251): 635-48. DOI: 10.1016/s0140-6736(20)31288-5 |
| 5 | 朱正纲. 胃癌外科综合治疗的若干进展与展望[J]. 外科理论与实践, 2023, 28(1): 1-6. |
| 6 | Song ZY, Wu YY, Yang JB, et al. Progress in the treatment of advanced gastric cancer[J]. Tumour Biol, 2017, 39(7): 101042831771462. DOI: 10.1177/1010428317714626 |
| 7 | Brisinda G, Chiarello MM, Crocco A, et al. Postoperative mortality and morbidity after D2 lymphadenectomy for gastric cancer: a retrospective cohort study[J]. World J Gastroenterol, 2022, 28(3): 381-98. DOI: 10.3748/wjg.v28.i3.381 |
| 8 | Feng F, Tian YZ, Xu GH, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer[J]. BMC Cancer, 2017, 17(1): 737. DOI: 10.1186/s12885-017-3738-y |
| 9 | de Manzoni G, Yang HK. Gastric cancer surgery[J]. Updat Surg, 2018, 70(2):155. DOI: 10.1007/s13304-018-0551-3 |
| 10 | 贾 祺, 咸小红, 李泱润, 等. MAGE-A家族在胃癌中作用的研究进展[J]. 中南大学学报: 医学版, 2023, 48(2): 260-7. |
| 11 | Zhao QF, Cao L, Guan LL, et al. Immunotherapy for gastric cancer: dilemmas and prospect[J]. Brief Funct Genomics, 2019, 18(2): 107-12. DOI: 10.1093/bfgp/ely019 |
| 12 | Zheng CH, Xu YC, Zhao G, et al. Outcomes of laparoscopic total gastrectomy combined with spleen-preserving hilar lymphadenectomy for locally advanced proximal gastric cancer: a nonrandomized clinical trial[J]. JAMA Netw Open, 2021, 4(12): e2139992. DOI: 10.1001/jamanetworkopen.2021.39992 |
| 13 | Lin XL, Han T, Xia Q, et al. CHPF promotes gastric cancer tumorigenesis through the activation of E2F1[J]. Cell Death Dis, 2021, 12(10): 876. DOI: 10.1038/s41419-021-04148-y |
| 14 | Feng YY, Zhao M, Wang LJ, et al. The heterogeneity of signaling pathways and drug responses in intrahepatic cholangiocarcinoma with distinct genetic mutations[J]. Cell Death Dis, 2024, 15(1): 34. DOI: 10.1038/s41419-023-06406-7 |
| 15 | He HJ, Bing H, Liu GJ. TSR2 Induces laryngeal cancer cell apoptosis through inhibiting NF‑κB signaling pathway[J]. Laryngoscope, 2018, 128(4): E130-4. DOI: 10.1002/lary.27035 |
| 16 | He HJ, Zhu D, Sun J, et al. The Novel protein TSR2 inhibits the transcriptional activity of nuclear factor‑κB and induces apoptosis[J]. Mol Biol, 2011, 45(3): 451-7. DOI: 10.1134/s0026893311020099 |
| 17 | Zhao QX, Rangan R, Weng SN, et al. Inhibition of ribosome biogenesis in the epidermis is sufficient to trigger organism-wide growth quiescence independently of nutritional status in C. elegans[J]. PLoS Biol, 2023, 21(8): e3002276. DOI: 10.1371/journal.pbio.3002276 |
| 18 | Bu DC, Luo HT, Huo PP, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis[J]. Nucleic Acids Res, 2021, 49(W1): W317-25. DOI: 10.1093/nar/gkab447 |
| 19 | Sun XZ, Chen PX, Chen X, et al. KIF4A enhanced cell proliferation and migration via Hippo signaling and predicted a poor prognosis in esophageal squamous cell carcinoma[J]. Thorac Cancer, 2021, 12(4): 512-24. DOI: 10.1111/1759-7714.13787 |
| 20 | Thrift AP, El-Serag HB. Burden of gastric cancer[J]. Clin Gastroenterol Hepatol, 2020, 18(3): 534-42. DOI: 10.1016/j.cgh.2019.07.045 |
| 21 | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors[J]. J Surg Oncol, 2013, 107(3): 230-6. DOI: 10.1002/jso.23262 |
| 22 | Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention[J]. Cancer Epidemiol Biomarkers Prev, 2014, 23(5): 700-13. DOI: 10.1158/1055-9965.epi-13-1057 |
| 23 | Maddineni G, Xie JJ, Brahmbhatt B, et al. Diet and carcinogenesis of gastric cancer[J]. Curr Opin Gastroenterol, 2022, 38(6): 588-91. DOI: 10.1097/mog.0000000000000875 |
| 24 | Yusefi AR, Bagheri Lankarani K, Bastani P, et al. Risk factors for gastric cancer: a systematic review[J]. Asian Pac J Cancer Prev, 2018, 19(3): 591-603. |
| 25 | Ansari S, Gantuya B, Tuan VP, et al. Diffuse gastric cancer: a summary of analogous contributing factors for its molecular pathogenicity[J]. Int J Mol Sci, 2018, 19(8): 2424. DOI: 10.3390/ijms19082424 |
| 26 | Guo ZJ, Guo L. Abnormal activation of RFC3, A YAP1/TEAD downstream target, promotes gastric cancer progression[J]. Int J Clin Oncol, 2024, 29(4): 442-55. DOI: 10.1007/s10147-024-02478-3 |
| 27 | Rong L, Li ZD, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomed Pharmacother, 2020, 122: 109726. DOI: 10.1016/j.biopha.2019.109726 |
| 28 | Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and metastasis[J]. Cell Metab, 2021, 33(7): 1307-21. DOI: 10.1016/j.cmet.2021.04.002 |
| 29 | Wan GQ, Liu YH, Zhu J, et al. SLFN5 suppresses cancer cell migration and invasion by inhibiting MT1-MMP expression via AKT/GSK-3β/β-catenin pathway[J]. Cell Signal, 2019, 59: 1-12. DOI: 10.1016/j.cellsig.2019.03.004 |
| 30 | Yang YM, Karbstein K. The chaperone Tsr2 regulates Rps26 release and reincorporation from mature ribosomes to enable a reversible, ribosome-mediated response to stress[J]. Sci Adv, 2022, 8(8): eabl4386. DOI: 10.1126/sciadv.abl4386 |
| 31 | Yang YM, Jung Y, Abegg D, et al. Chaperone-directed ribosome repair after oxidative damage[J]. Mol Cell, 2023, 83(9): 1527-37, e5. DOI: 10.1016/j.molcel.2023.03.030 |
| 32 | Fernández-Parejo N, Lorenzo-Martín LF, García-Pedrero JM, et al. VAV2 orchestrates the interplay between regenerative proliferation and ribogenesis in both keratinocytes and oral squamous cell carcinoma[J]. Sci Rep, 2024, 14(1): 4060. DOI: 10.1038/s41598-024-54808-0 |
| 33 | Zang Y, Ran X, Yuan J, et al. Genomic hallmarks and therapeutic targets of ribosome biogenesis in cancer[J]. Brief Bioinform, 2024, 25(2): bbae023. DOI: 10.1093/bib/bbae023 |
| 34 | An Y, Xia YC, Wang ZY, et al. Clinical significance of ribosome production factor 2 homolog in hepatocellular carcinoma[J]. Clin Res Hepatol Gastroenterol, 2024, 48(3): 102289. DOI: 10.1016/j.clinre.2024.102289 |
| 35 | Miricescu D, Totan A, Stanescu-Spinu II, et al. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects[J]. Int J Mol Sci, 2020, 22(1): 173. DOI: 10.3390/ijms22010173 |
| 36 | Sun TS, Bi FF, Liu ZN, et al. TMEM119 facilitates ovarian cancer cell proliferation, invasion, and migration via the PDGFRB/PI3K/AKT signaling pathway[J]. J Transl Med, 2021, 19(1): 111. DOI: 10.1186/s12967-021-02781-x |
| 37 | Fresno Vara JA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer[J]. Cancer Treat Rev, 2004, 30(2): 193-204. DOI: 10.1016/j.ctrv.2003.07.007 |
| 38 | Maiello D, Varone M, Vicidomini R, et al. Dyskerin downregulation can induce ER stress and promote autophagy via AKT-mTOR signaling deregulation[J]. Biomedicines, 2022, 10(5): 1092. DOI: 10.3390/biomedicines10051092 |
| [1] | 陈鑫源, 吴成挺, 李瑞迪, 潘雪芹, 张耀丹, 陶俊宇, 林才志. 双术汤通过P53/SLC7A11/GPX4通路诱导胃癌细胞铁死亡[J]. 南方医科大学学报, 2025, 45(7): 1363-1371. |
| [2] | 谢婷, 王云云, 郭婷, 袁春华. 雷氏大疣蛛多肽毒素组分通过激活促凋亡通路和协同作用抑制癌细胞增殖[J]. 南方医科大学学报, 2025, 45(7): 1460-1470. |
| [3] | 龚秀莹, 侯顺福, 赵苗苗, 王晓娜, 张致涵, 刘清华, 尹崇高, 李洪利. LncRNA SNHG15通过miR-30b-3p调控COX6B1轴促进肺腺癌细胞增殖、迁移和侵袭的分子机制[J]. 南方医科大学学报, 2025, 45(7): 1498-1505. |
| [4] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [5] | 李嘉豪, 冼瑞婷, 李荣. 下调ACADM介导的脂毒性抑制雌激素受体阳性乳腺癌细胞的侵袭与转移[J]. 南方医科大学学报, 2025, 45(6): 1163-1173. |
| [6] | 侯鑫睿, 张振东, 曹明远, 杜予心, 王小平. 红景天苷靶向miR-1343-3p-OGDHL/PDHB糖代谢轴抑制胃癌细胞的体内外增殖[J]. 南方医科大学学报, 2025, 45(6): 1226-1239. |
| [7] | 曾玉梅, 李继科, 黄仲曦, 周毅波. 绒毛样蛋白VILL通过与LMO7蛋白相互作用抑制鼻咽癌细胞的增殖[J]. 南方医科大学学报, 2025, 45(5): 954-961. |
| [8] | 陈悦, 肖林雨, 任侣, 宋雪, 李静, 胡建国. 水晶兰苷通过抑制PI3K/AKT信号通路减少神经元凋亡改善脊髓损伤后小鼠的运动功能[J]. 南方医科大学学报, 2025, 45(4): 774-784. |
| [9] | 储菲, 陈孝华, 宋博文, 杨晶晶, 左芦根. 苏荠宁黄酮通过抑制PI3K/AKT信号通路拮抗肠上皮细胞凋亡改善小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(4): 819-828. |
| [10] | 岳雅清, 牟召霞, 王希波, 刘艳. Aurora-A过表达通过激活NF-κBp65/ARPC4信号轴促进宫颈癌细胞的侵袭和转移[J]. 南方医科大学学报, 2025, 45(4): 837-843. |
| [11] | 张毅, 沈昱, 万志强, 陶嵩, 柳亚魁, 王栓虎. CDKN3高表达促进胃癌细胞的迁移和侵袭:基于调控p53/NF-κB信号通路和抑制胃癌细胞凋亡[J]. 南方医科大学学报, 2025, 45(4): 853-861. |
| [12] | 董妍妍, 张可敬, 储俊, 储全根. 抵当汤含药血清通过PI3K/Akt/mTOR信号通路增强高糖诱导的大鼠肾小球内皮细胞自噬[J]. 南方医科大学学报, 2025, 45(3): 461-469. |
| [13] | 庆顺杰, 沈智勇. 过表达己糖激酶2通过激活JAK/STAT途径促进结直肠癌细胞的增殖、迁移和侵袭并调节肿瘤免疫微环境[J]. 南方医科大学学报, 2025, 45(3): 542-553. |
| [14] | 黄晴晴, 张文静, 张小凤, 王炼, 宋雪, 耿志军, 左芦根, 王月月, 李静, 胡建国. 高表达MYO1B促进胃癌细胞增殖、迁移和侵袭并与患者的不良预后有关[J]. 南方医科大学学报, 2025, 45(3): 622-631. |
| [15] | 宋雪, 陈悦, 张敏, 张诺, 左芦根, 李静, 耿志军, 张小凤, 王月月, 王炼, 胡建国. GPSM2在胃癌组织中高表达并通过促进肿瘤细胞的增殖影响患者预后[J]. 南方医科大学学报, 2025, 45(2): 229-238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||