南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (8): 1706-1717.doi: 10.12122/j.issn.1673-4254.2025.08.15
• • 上一篇
房尚萍1,2, 刘佳梦1,2, 岳星辰1,2, 李欢1,2, 利琬柠1,2, 汤晓宇1,2, 包鹏举3( )
)
收稿日期:2025-03-27
出版日期:2025-08-20
发布日期:2025-09-05
通讯作者:
包鹏举
E-mail:273574156@qq.com
作者简介:房尚萍、刘佳梦共同为第一作者基金资助:
Shangping FANG1,2, Jiameng LIU1,2, Xingchen YUE1,2, Huan LI1,2, Wanning Li1,2, Xiaoyu TANG1,2, Pengju BAO3( )
)
Received:2025-03-27
Online:2025-08-20
Published:2025-09-05
Contact:
Pengju BAO
E-mail:273574156@qq.com
摘要:
目的 利用美国国立癌症研究院监测、流行病学和结果(SEER)数据库和癌症基因组图谱(TCGA)数据库分析胃印戒细胞癌在不同种族间预后情况的差异。 方法 对 2000~2020 年美国 SEER 数据库和TCGA数据库进行回顾性分析,研究对象为胃印戒细胞癌患者,按种族分为白人、黑人、亚洲人或太平洋岛民、美洲印第安人/阿拉斯加土著队列。通过基线人口学分析、KM 生存曲线、列线图等方法,评估胃印戒细胞癌的预后与治疗情况。 结果 本研究共纳入2058例患者,其中黑人占8.6%,白人占 72.4%,亚洲人或太平洋岛民占 16.6%,美洲印第安人/阿拉斯加土著占1.0%,其他种族占1.4%。肿瘤分级因种族而异,不同种族的患病率和生存率差异显著,白人队列的差异最为突出,且所有差异均具有统计学意义(P<0.05)。此外,患者的管理和预后结果也呈现出种族差异。 结论 胃印戒细胞癌的肿瘤分级和预后存在种族差异,这一特性具有潜在的临床特异性,这为优化临床诊疗策略及改善患者预后提供参考依据。
房尚萍, 刘佳梦, 岳星辰, 李欢, 利琬柠, 汤晓宇, 包鹏举. 胃印戒细胞癌的分级与预后存在种族差异:一项SEER数据库和TCGA数据库的研究[J]. 南方医科大学学报, 2025, 45(8): 1706-1717.
Shangping FANG, Jiameng LIU, Xingchen YUE, Huan LI, Wanning Li, Xiaoyu TANG, Pengju BAO. Racial differences in treatment and prognosis of gastric signet ring cell carcinoma: analysis based on SEER and TCGA databases[J]. Journal of Southern Medical University, 2025, 45(8): 1706-1717.
| Characteristic | Cohort | P | ||
|---|---|---|---|---|
| Overall (2057) | Training cohort (1440) | Internal test cohort (617) | ||
| Gender | 0.718 | |||
| Female | 1026 (49.9%) | 722 (50.1%) | 304 (49.3%) | |
| Male | 1031 (50.1%) | 718 (49.9%) | 313 (50.7%) | |
| Race recode | 0.964 | |||
| American indian/alaska native | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Asian or pacific islander | 343 (16.7%) | 236 (16.4%) | 107 (17.3%) | |
| Black | 179 (8.7%) | 128 (8.9%) | 51 (8.3%) | |
| Unknown | 22 (1.1%) | 15 (1.0%) | 7 (1.1%) | |
| White | 1492 (72.5%) | 1047 (72.7%) | 445 (72.1%) | |
| Age recode with <1 year | ||||
| 20-24 years | 15 (0.7%) | 11 (0.8%) | 4 (0.6%) | |
| 25-29 years | 21 (1.0%) | 18 (1.3%) | 3 (0.5%) | |
| 30-34 years | 50 (2.4%) | 36 (2.5%) | 14 (2.3%) | |
| 35-39 years | 85 (4.1%) | 58 (4.0%) | 27 (4.4%) | |
| 40-44 years | 106 (5.2%) | 69 (4.8%) | 37 (6.0%) | |
| 45-49 years | 159 (7.7%) | 114 (7.9%) | 45 (7.3%) | |
| 50-54 years | 181 (8.8%) | 134 (9.3%) | 47 (7.6%) | |
| 55-59 years | 232 (11.3%) | 162 (11.3%) | 70 (11.3%) | |
| 60-64 years | 260 (12.6%) | 172 (11.9%) | 88 (14.3%) | |
| 65-69 years | 254 (12.3%) | 176 (12.2%) | 78 (12.6%) | |
| 70-74 years | 224 (10.9%) | 159 (11.0%) | 65 (10.5%) | |
| 75-79 years | 176 (8.6%) | 123 (8.5%) | 53 (8.6%) | |
| 80-84 years | 151 (7.3%) | 106 (7.4%) | 45 (7.3%) | |
| 85+ years | 143 (7.0%) | 102 (7.1%) | 41 (6.6%) | |
| Year of diagnosis | 0.683 | |||
| 2016 | 1036 (50.4%) | 721 (50.1%) | 315 (51.1%) | |
| 2017 | 1021 (49.6%) | 719 (49.9%) | 302 (48.9%) | |
| Primary site (Mean±SD) | 164.28±3.29 | 164.23±3.26 | 164.39±3.35 | 0.315 |
| Grade Recode (thru 2017) | 0.899 | |||
| Moderately differentiated; Grade II | 35 (1.7%) | 25 (1.7%) | 10 (1.6%) | |
| Poorly differentiated; Grade III | 1594 (77.5%) | 1116 (77.5%) | 478 (77.5%) | |
| Undifferentiated; anaplastic; Grade IV | 48 (2.3%) | 31 (2.2%) | 17 (2.8%) | |
| Unknown | 379 (18.4%) | 267 (18.5%) | 112 (18.2%) | |
| Well differentiated; Grade I | 1 (0.0%) | 1 (0.1%) | 0 (0.0%) | |
| TNM/TCS v0204+Schema (thru 2017) | 0.963 | |||
| Esophagus/GEJunction | 408 (19.8%) | 286 (19.9%) | 122 (19.8%) | |
| Stomach | 1649 (80.2%) | 1154 (80.1%) | 495 (80.2%) | |
| Radiation recode | 0.823 | |||
| Beam radiation | 377 (18.3%) | 260 (18.1%) | 117 (19.0%) | |
| None/Unknown | 1626 (79.0%) | 1144 (79.4%) | 482 (78.1%) | |
| Radiation, NOS method or source not specified | 9 (0.4%) | 5 (0.3%) | 4 (0.6%) | |
| Recommended, unknown if administered | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Refused (1988+) | 24 (1.2%) | 17 (1.2%) | 7 (1.1%) | |
| Chemotherapy recode (yes, no/unk) | 0.621 | |||
| No/Unknown | 760 (36.9%) | 537 (37.3%) | 223 (36.1%) | |
| Yes | 1297 (63.1%) | 903 (62.7%) | 394 (63.9%) | |
| Surgery recode | 0.634 | |||
| Not recommended | 955 (57.8%) | 667 (57.7%) | 286 (57.5%) | |
| Surgery performed | 639 (38.7%) | 446 (38.6%) | 191 (38.5%) | |
| Recommended but not performed, patient refused | 56 (3.5%) | 42 (3.7%) | 18 (3.8%) | |
| Tumor size summary (2016+) (mm, Mean±SD) | 570±474 | 563±475 | 587±471 | 0.275 |
表1 基线人口学特征表
Tab.1 Baseline demographic characteristics of the patients included [n (%)]
| Characteristic | Cohort | P | ||
|---|---|---|---|---|
| Overall (2057) | Training cohort (1440) | Internal test cohort (617) | ||
| Gender | 0.718 | |||
| Female | 1026 (49.9%) | 722 (50.1%) | 304 (49.3%) | |
| Male | 1031 (50.1%) | 718 (49.9%) | 313 (50.7%) | |
| Race recode | 0.964 | |||
| American indian/alaska native | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Asian or pacific islander | 343 (16.7%) | 236 (16.4%) | 107 (17.3%) | |
| Black | 179 (8.7%) | 128 (8.9%) | 51 (8.3%) | |
| Unknown | 22 (1.1%) | 15 (1.0%) | 7 (1.1%) | |
| White | 1492 (72.5%) | 1047 (72.7%) | 445 (72.1%) | |
| Age recode with <1 year | ||||
| 20-24 years | 15 (0.7%) | 11 (0.8%) | 4 (0.6%) | |
| 25-29 years | 21 (1.0%) | 18 (1.3%) | 3 (0.5%) | |
| 30-34 years | 50 (2.4%) | 36 (2.5%) | 14 (2.3%) | |
| 35-39 years | 85 (4.1%) | 58 (4.0%) | 27 (4.4%) | |
| 40-44 years | 106 (5.2%) | 69 (4.8%) | 37 (6.0%) | |
| 45-49 years | 159 (7.7%) | 114 (7.9%) | 45 (7.3%) | |
| 50-54 years | 181 (8.8%) | 134 (9.3%) | 47 (7.6%) | |
| 55-59 years | 232 (11.3%) | 162 (11.3%) | 70 (11.3%) | |
| 60-64 years | 260 (12.6%) | 172 (11.9%) | 88 (14.3%) | |
| 65-69 years | 254 (12.3%) | 176 (12.2%) | 78 (12.6%) | |
| 70-74 years | 224 (10.9%) | 159 (11.0%) | 65 (10.5%) | |
| 75-79 years | 176 (8.6%) | 123 (8.5%) | 53 (8.6%) | |
| 80-84 years | 151 (7.3%) | 106 (7.4%) | 45 (7.3%) | |
| 85+ years | 143 (7.0%) | 102 (7.1%) | 41 (6.6%) | |
| Year of diagnosis | 0.683 | |||
| 2016 | 1036 (50.4%) | 721 (50.1%) | 315 (51.1%) | |
| 2017 | 1021 (49.6%) | 719 (49.9%) | 302 (48.9%) | |
| Primary site (Mean±SD) | 164.28±3.29 | 164.23±3.26 | 164.39±3.35 | 0.315 |
| Grade Recode (thru 2017) | 0.899 | |||
| Moderately differentiated; Grade II | 35 (1.7%) | 25 (1.7%) | 10 (1.6%) | |
| Poorly differentiated; Grade III | 1594 (77.5%) | 1116 (77.5%) | 478 (77.5%) | |
| Undifferentiated; anaplastic; Grade IV | 48 (2.3%) | 31 (2.2%) | 17 (2.8%) | |
| Unknown | 379 (18.4%) | 267 (18.5%) | 112 (18.2%) | |
| Well differentiated; Grade I | 1 (0.0%) | 1 (0.1%) | 0 (0.0%) | |
| TNM/TCS v0204+Schema (thru 2017) | 0.963 | |||
| Esophagus/GEJunction | 408 (19.8%) | 286 (19.9%) | 122 (19.8%) | |
| Stomach | 1649 (80.2%) | 1154 (80.1%) | 495 (80.2%) | |
| Radiation recode | 0.823 | |||
| Beam radiation | 377 (18.3%) | 260 (18.1%) | 117 (19.0%) | |
| None/Unknown | 1626 (79.0%) | 1144 (79.4%) | 482 (78.1%) | |
| Radiation, NOS method or source not specified | 9 (0.4%) | 5 (0.3%) | 4 (0.6%) | |
| Recommended, unknown if administered | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Refused (1988+) | 24 (1.2%) | 17 (1.2%) | 7 (1.1%) | |
| Chemotherapy recode (yes, no/unk) | 0.621 | |||
| No/Unknown | 760 (36.9%) | 537 (37.3%) | 223 (36.1%) | |
| Yes | 1297 (63.1%) | 903 (62.7%) | 394 (63.9%) | |
| Surgery recode | 0.634 | |||
| Not recommended | 955 (57.8%) | 667 (57.7%) | 286 (57.5%) | |
| Surgery performed | 639 (38.7%) | 446 (38.6%) | 191 (38.5%) | |
| Recommended but not performed, patient refused | 56 (3.5%) | 42 (3.7%) | 18 (3.8%) | |
| Tumor size summary (2016+) (mm, Mean±SD) | 570±474 | 563±475 | 587±471 | 0.275 |
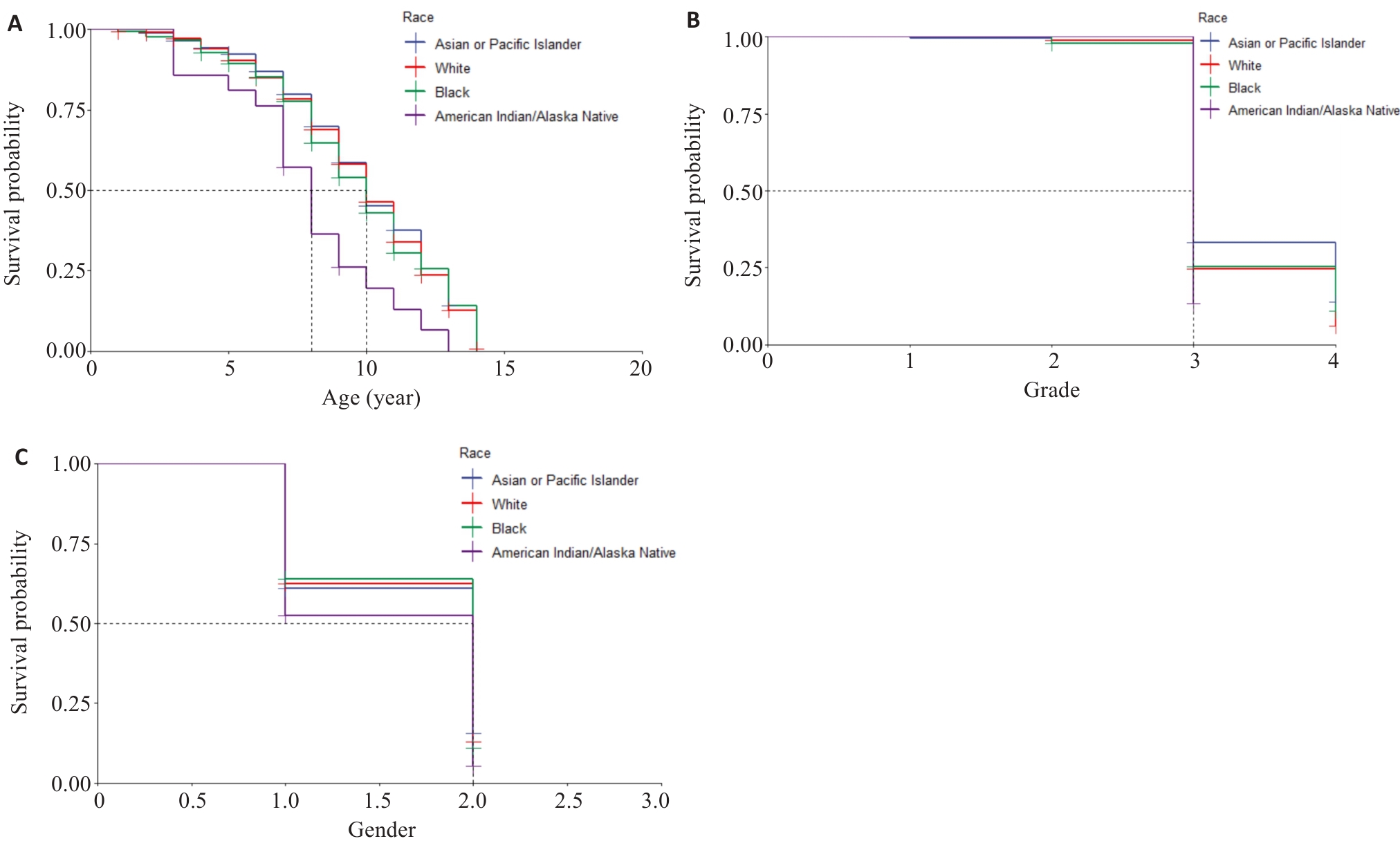
图2 KM生存曲线
Fig.2 Kaplan-Meier survival curves. A: Survival rates of the patients of different ages in different races. B: Survival rates of patients with different tumor grades in different races. C: Survival rates of the patients of different genders in different races (1 and 2 represents male and female, respectively).
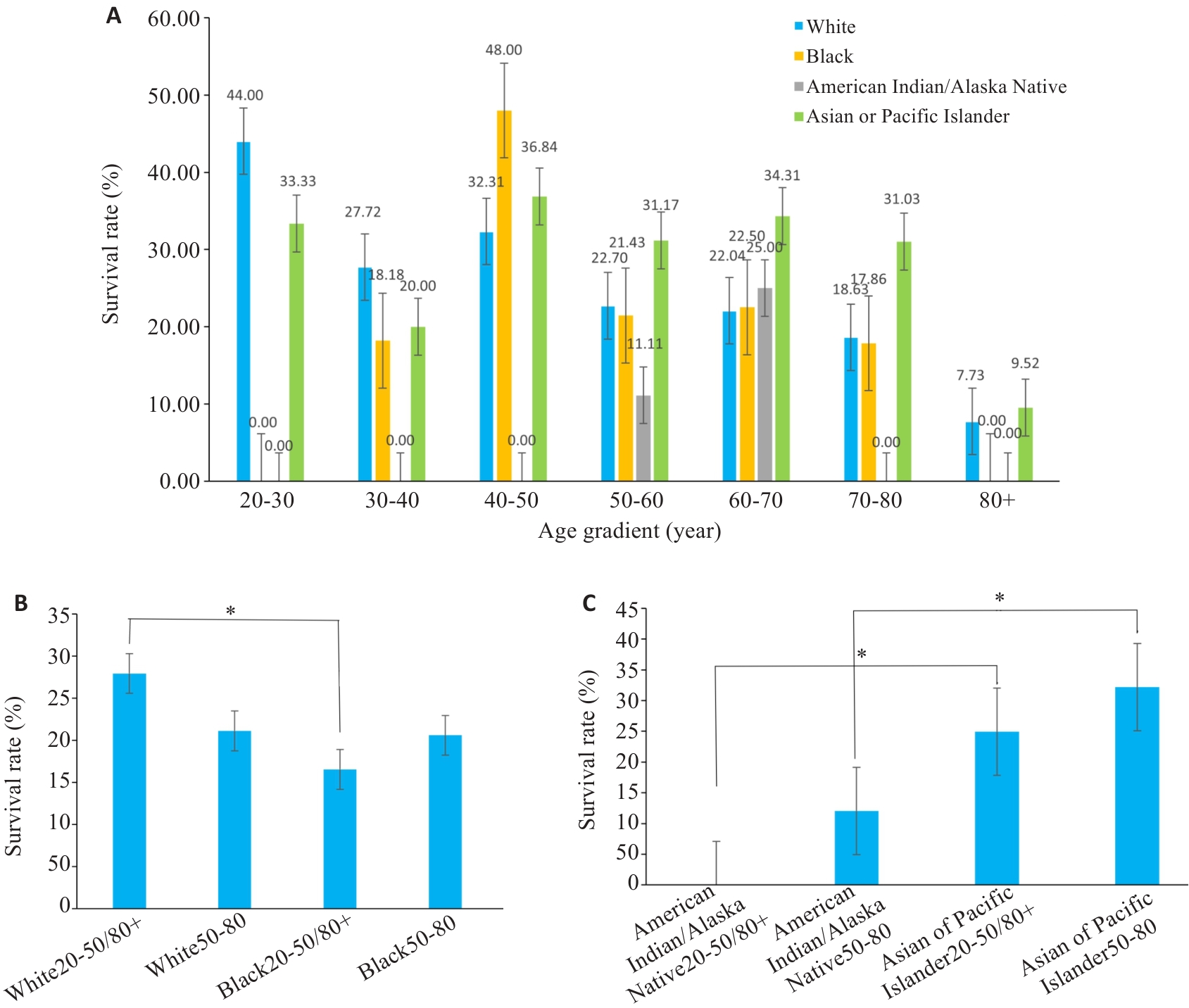
图3 分组柱状图
Fig.3 Bar charts of subgroup analysis. A: Survival rates for different races at different ages. B, C: Statistical analyses of the survival rates. *P<0.05.
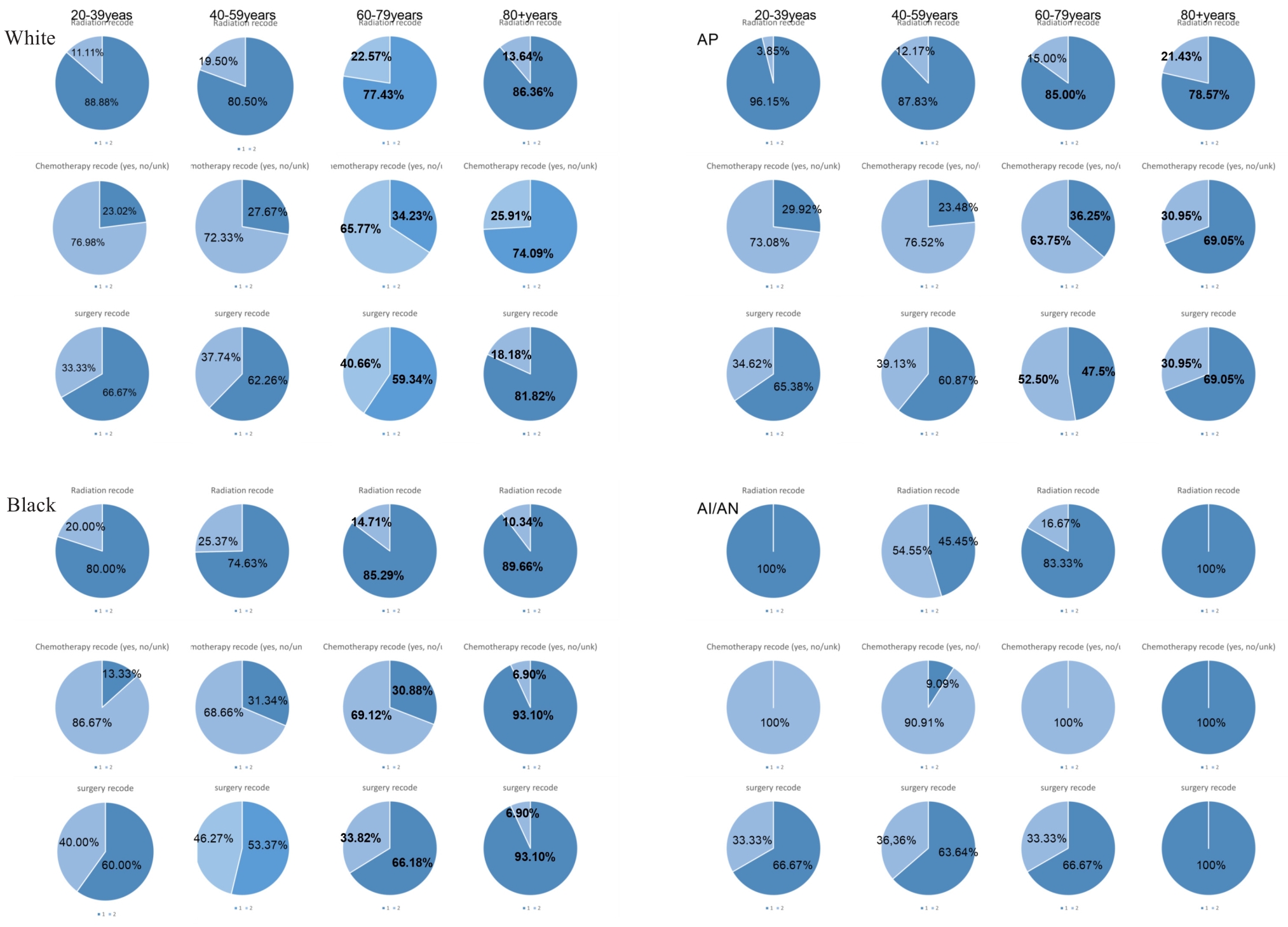
图4 不同年龄段不同种族治疗方式饼状图
Fig.4 Pie charts of treatment modalities by age and ethnicity. AP: Asian or Pacific Islander; AI/AN: American Indian/Alaska Native. Superscripts Radiation recode is radiation, Chemotherapy recode is chemotherapy, surgery recode is surgery; subscripts 1 represents not receiving the treatment modality, 2 means received the treatment modality.
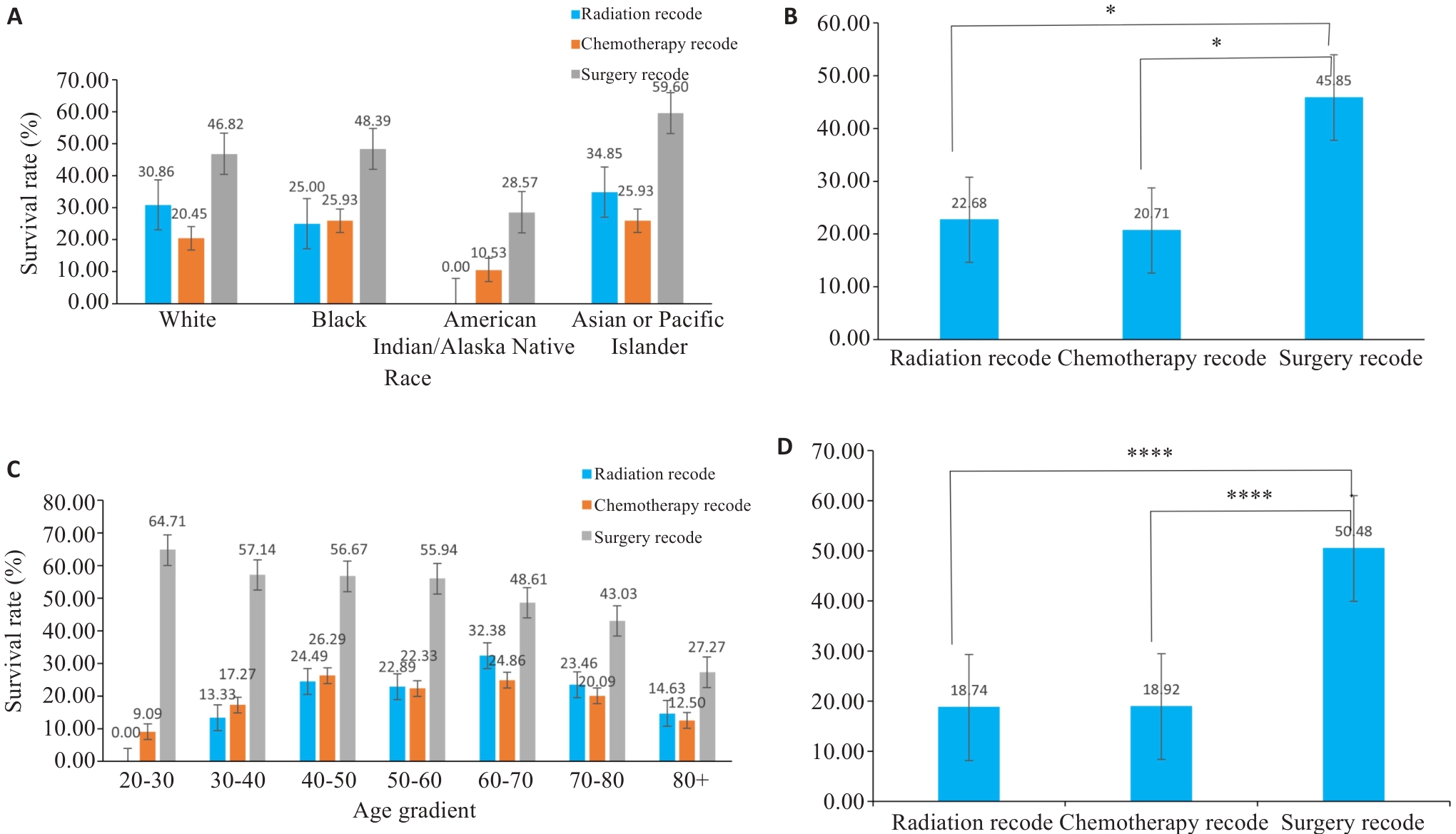
图5 分组柱状图
Fig.5 Bar graphs of subgroup analysis. A, C: Survival rates of patients of different races using various treatment methods. B, D: Corresponding statistical analyses for A and C. *P<0.05, ****P<0.0001.
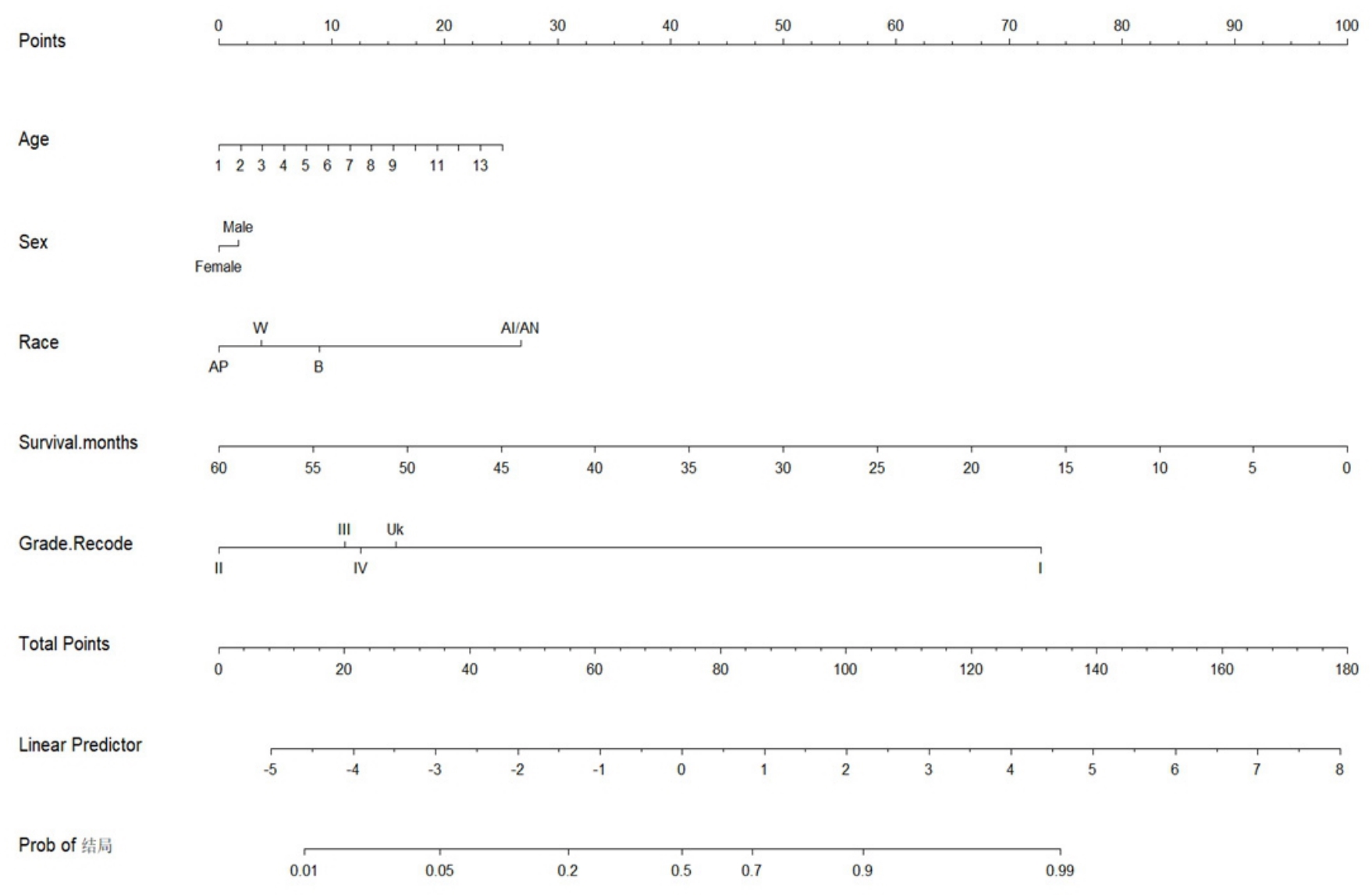
图6 列线图
Fig.6 Nomogram analysis. In the row of Age, the number 1 represents patients aged 20-24 years, 2 represents patients aged 25-29 years, and so forth; the number 14 represents patients above 85 years. In the row of Race, W represents Caucasians, B represents Blacks, AI/AN represents American Indians/Alaskan Natives, and Ap represents Asians and Pacific Islanders.
| Variable | OR | 95% CI (profile likelihood) | P |
|---|---|---|---|
| Age[50-54] | 0.2301 | 0.06367 to 0.7122 | 0.0159 |
| Age[80-84] | 0.4881 | 0.1247 to 1.695 | 0.2762 |
| Age[55-59] | 0.2203 | 0.06110 to 0.6788 | 0.0128 |
| Age[60-64] | 0.2239 | 0.06378 to 0.6660 | 0.0114 |
| Age[75-79] | 0.2472 | 0.06738 to 0.7818 | 0.0239 |
| Age[70-74] | 0.2988 | 0.08325 to 0.9184 | 0.0461 |
| Age[65-69] | 0.2962 | 0.08392 to 0.8893 | 0.041 |
| Age[ | 0.1224 | 0.03227 to 0.4025 | 0.001 |
| Age[ | 0.1179 | 0.03002 to 0.4066 | 0.0012 |
| Age[ | 0.03687 | 0.006592 to 0.2072 | 0.0002 |
| Age[ | 0.1643 | 0.04528 to 0.5101 | 0.0031 |
| Age[ | 0.208 | 0.04599 to 0.8911 | 0.0359 |
| Age[ | 0.09731 | 0.01383 to 0.8520 | 0.0254 |
| Gender | 0.8876 | 0.6470 to 1.217 | 0.4589 |
| Race | 0.825 | 0.6946 to 0.9818 | 0.0291 |
| Grade | 1.089 | 0.8700 to 1.370 | 0.4621 |
| Survival months | 0.8865 | 0.8768 to 0.8957 | <0.0001 |
| Tumor size summary (2016+) | 1 | 0.0755 |
表3 logistic回归分析
Tab.3 Logistic regression analysis
| Variable | OR | 95% CI (profile likelihood) | P |
|---|---|---|---|
| Age[50-54] | 0.2301 | 0.06367 to 0.7122 | 0.0159 |
| Age[80-84] | 0.4881 | 0.1247 to 1.695 | 0.2762 |
| Age[55-59] | 0.2203 | 0.06110 to 0.6788 | 0.0128 |
| Age[60-64] | 0.2239 | 0.06378 to 0.6660 | 0.0114 |
| Age[75-79] | 0.2472 | 0.06738 to 0.7818 | 0.0239 |
| Age[70-74] | 0.2988 | 0.08325 to 0.9184 | 0.0461 |
| Age[65-69] | 0.2962 | 0.08392 to 0.8893 | 0.041 |
| Age[ | 0.1224 | 0.03227 to 0.4025 | 0.001 |
| Age[ | 0.1179 | 0.03002 to 0.4066 | 0.0012 |
| Age[ | 0.03687 | 0.006592 to 0.2072 | 0.0002 |
| Age[ | 0.1643 | 0.04528 to 0.5101 | 0.0031 |
| Age[ | 0.208 | 0.04599 to 0.8911 | 0.0359 |
| Age[ | 0.09731 | 0.01383 to 0.8520 | 0.0254 |
| Gender | 0.8876 | 0.6470 to 1.217 | 0.4589 |
| Race | 0.825 | 0.6946 to 0.9818 | 0.0291 |
| Grade | 1.089 | 0.8700 to 1.370 | 0.4621 |
| Survival months | 0.8865 | 0.8768 to 0.8957 | <0.0001 |
| Tumor size summary (2016+) | 1 | 0.0755 |
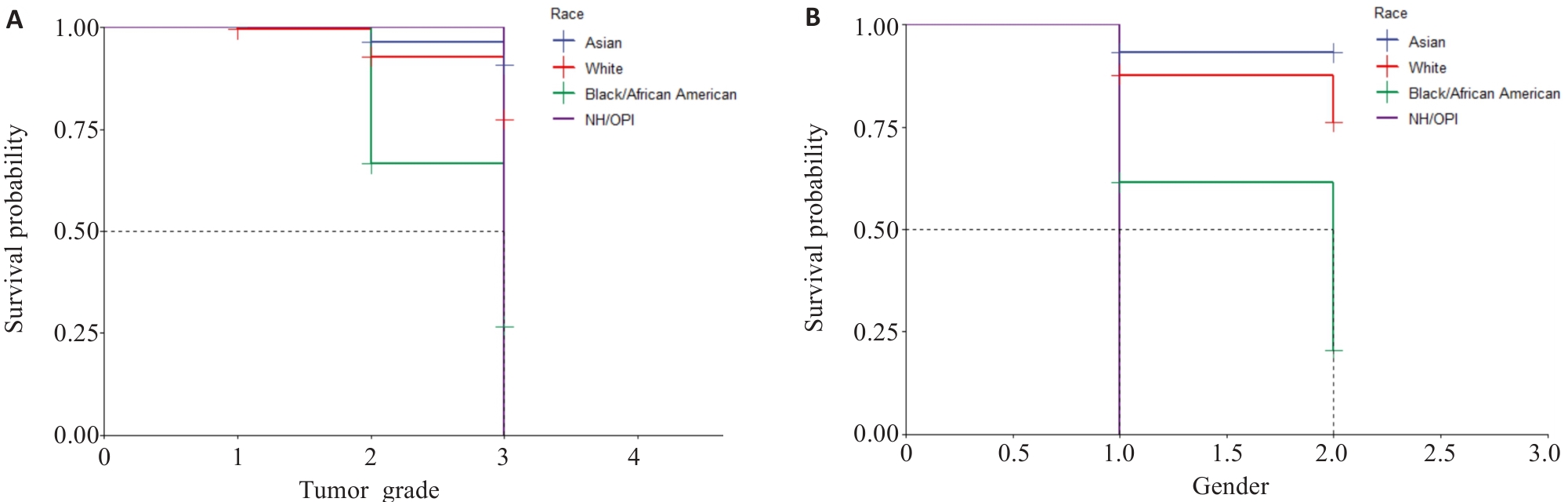
图9 KM生存曲线
Fig.9 Kaplan-Meier survival curves. A: Survival of patients with different tumor grades in different races (1, 2, and 3 represent tumor grades I, II, and III, respectively). B: Survival curves of patients of different genders in different races (1 and 2 represents male and female, respectively. BOAA: Black or African American; NHOOPI: Native Hawallan or other pacific islanders).
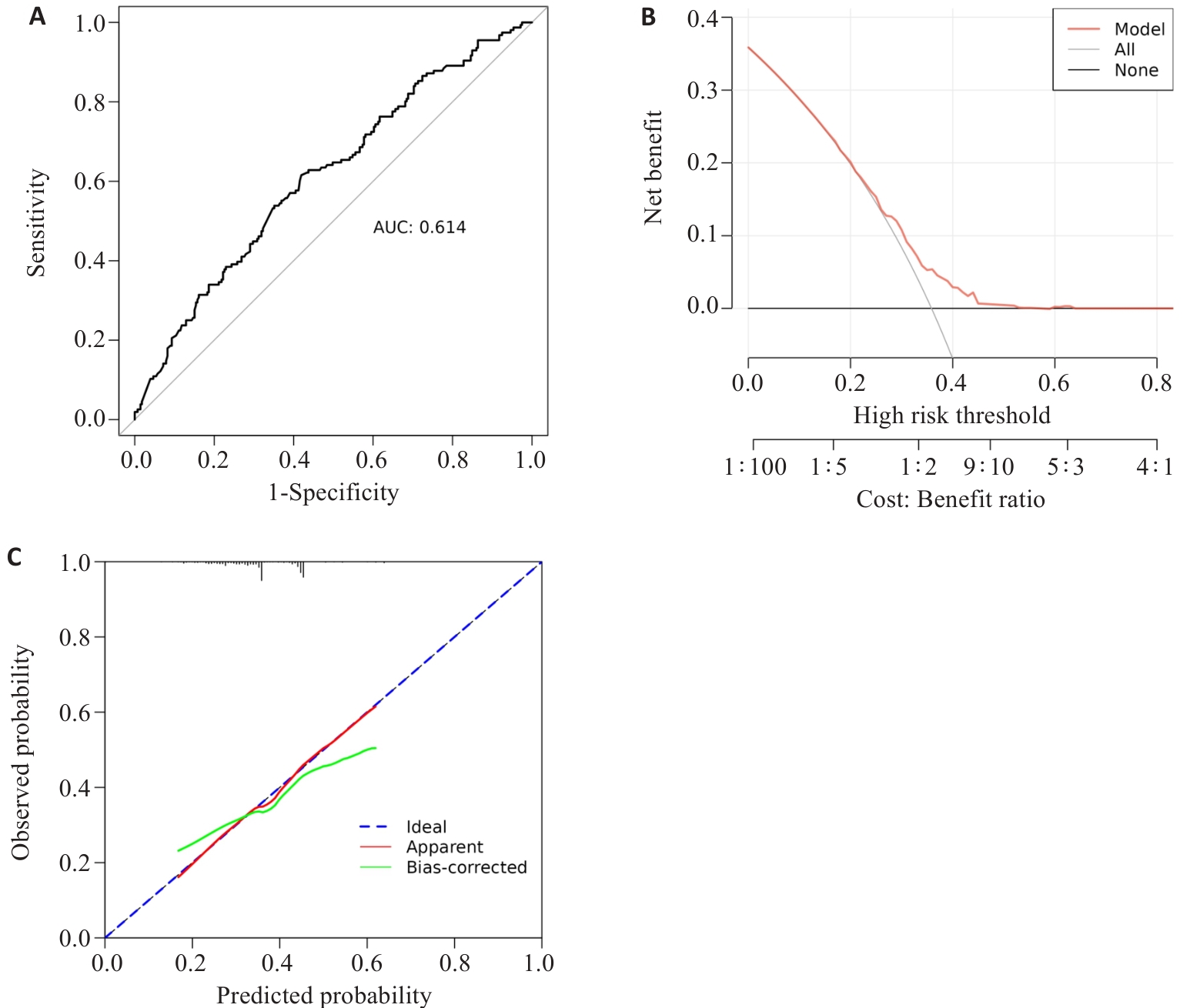
图10 ROC曲线、calibration校准曲线、DCA曲线对外部验证集
Fig.10 10 ROC curve, calibration curve, DCA curve for the external validation set. A: ROC curve; B: DCA curve; C: calibration curve.
| [1] | Costa TM, Alves F, Miranda H, et al. Pure signet ring cell carcinoma of the breast: a rare entity[J]. BMJ Case Rep, 2024, 17(11): e252263. doi:10.1136/bcr-2022-252263 |
| [2] | Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classi-fication of tumours of the digestive system[J]. Histopathology, 2020, 76(2): 182-8. doi:10.1111/his.13975 |
| [3] | Machlowska J, Pucułek M, Sitarz M, et al. State of the art for gastric signet ring cell carcinoma: from classification, prognosis, and genomic characteristics to specified treatments[J]. Cancer Manag Res, 2019, 11: 2151-61. doi:10.2147/cmar.s188622 |
| [4] | Kumar S, Seshadri RA, Trivadi SG, et al. Perioperative versus postoperative chemotherapy for gastric cancer: a propensity score matched analysis[J]. Asia Pac J Clin Oncol, 2020, 16(5): e252-6. doi:10.1111/ajco.13401 |
| [5] | Yang MG, Yang YL, Chen J, et al. A case report of primary signet ring cell carcinoma of the lung: imaging study and literature review[J]. Transl Lung Cancer Res, 2021, 10(9): 3840-9. doi:10.21037/tlcr-21-654 |
| [6] | Han YH, Yang WM, Ma Q, et al. Case Report: Systemic treatment for breast and vulvar metastases from resected rectal signet ring cell carcinoma[J]. Front Oncol, 2023, 13: 1213888. doi:10.3389/fonc.2023.1213888 |
| [7] | An Y, Zhou JL, Lin GL, et al. Clinicopathological and molecular characteristics of colorectal signet ring cell carcinoma: a review[J]. Pathol Oncol Res, 2021, 27: 1609859. doi:10.3389/pore.2021.1609859 |
| [8] | Chen L, Liu X, Gao LG, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: a 10-year retrospective study in China[J]. PLoS One, 2017, 12(5): e0176637. doi:10.1371/journal.pone.0176637 |
| [9] | Zhang L, Wu LY, Li JY, et al. Gastrointestinal metastatic signet ring cell breast cancer in young females: a case report[J]. Gland Surg, 2022, 11(5): 943-52. doi:10.21037/gs-22-242 |
| [10] | Kasapoğlu E, Kandil B, Gökyer A, et al. Primary signet ring cell carcinoma of the breast: a case report and literature review[J]. J Cancer Res Ther, 2024, 20(5): 1615-7. doi:10.4103/jcrt.jcrt_1963_22 |
| [11] | Luo S, Tian XX, Xu T, et al. Primary signet-ring cell carcinoma of the prostate involving the pelvis: a case report[J]. Front Oncol, 2024, 14: 1444541. doi:10.3389/fonc.2024.1444541 |
| [12] | Gupta M, Budhwar A, Prasad N, et al. Primary signet ring cell carcinoma of prostate: a rare case report and review of literature[J]. J Cancer Res Ther, 2023, 19(5): 1075-8. doi:10.4103/jcrt.jcrt_827_21 |
| [13] | Tan Y, Huang YH, Xue JW, et al. Clinicopathological features and prognostic significance of pulmonary adenocarcinoma with signet ring cell components: meta-analysis and SEER analysis[J]. Clin Exp Med, 2023, 23(8): 4341-54. doi:10.1007/s10238-023-01200-3 |
| [14] | 田华开. 基于SEER数据库比较研究胃印戒细胞癌与不同分化程度胃腺癌临床病理特点及预后[D]. 南昌: 南昌大学, 2021. |
| [15] | 陈碧钰. 基于SEER数据库的回顾性研究: 放疗联合手术治疗进展期胃印戒细胞癌的疗效分析[D]. 福州: 福建医科大学, 2021. |
| [16] | 王 震. 基于SEER数据库的胃印戒细胞癌临床病理特征及预后分析[D]. 郑州: 郑州大学, 2020. |
| [17] | 赵金匣, 康慧慧, 张 垚, 等. 胃癌患者根治性切除术前血脂水平与预后的相关性[J]. 临床荟萃, 2024, 39(10): 889-95. doi:10.3969/j.issn.1004-583X.2024.10.004 |
| [18] | 邢智远, 张凤娟, 谭晓杰, 等. 脐上或脐下观察孔在腹腔镜远端胃癌根治性切除术中的选择[J]. 腹腔镜外科杂志, 2024, 29(11): 825-9. |
| [19] | 常 宁. 胃印戒细胞癌手术方式及预后的临床研究[D]. 郑州: 郑州大学, 2018. doi:10.19347/j.cnki.2096-1413.201818004 |
| [20] | Gaba AG, Cao L, Renfrew RJ, et al. Impact of racial disparities on treatment of early triple negative breast cancer among American indians/Alaska natives and non-hispanic whites[J]. Clin Breast Cancer, 2025, 25(6): 534-43. doi:10.1016/j.clbc.2025.04.002 |
| [21] | Krishnamurthy S, Jazowski SA, Roberson ML, et al. Racial and ethnic disparities in receipt of ERBB2-targeted therapy for breast cancer, 2010-2020[J]. JAMA Netw Open, 2025, 8(5): e258086. doi:10.1001/jamanetworkopen.2025.8086 |
| [22] | Tsai MH, Shahsavari D, Chen J, et al. Racial/ethnic disparities in early-onset colorectal cancer outcomes[J]. J Racial Ethn Health Disparities. 2025, [Online ahead of print]. doi:10.1007/s40615-025-02450-5 |
| [23] | Thakkar Z, Khan MA, Wu Y, et al. Disaggregated colorectal cancer mortality among asian american subgroups between 2005-2020[J]. Cancer Epidemiol Biomarkers Prev, 2025, 34(7):1134-40. doi:10.1158/1055-9965.epi-24-1688 |
| [24] | Qiu HB, Cao SM, Xu RH. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020[J]. Cancer Commun (Lond), 2021, 41(10): 1037-48. doi:10.1002/cac2.12197 |
| [25] | Aitchison A, Hakkaart C, Whitehead M, et al. CDH1 gene mutation in early-onset, colorectal signet-ring cell carcinoma[J]. Pathol Res Pract, 2020, 216(5): 152912. doi:10.1016/j.prp.2020.152912 |
| [26] | 杨易成, 曹 佳. 早期胃印戒细胞癌研究现状与进展[J]. 临床军医杂志, 2023, 51(9): 896-900. |
| [27] | Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma[J]. Ann Surg Oncol, 2014, 21(5): 1678-85. doi:10.1245/s10434-013-3466-8 |
| [28] | Sargent C, Larchanché S. The construction of “cultural difference” and its therapeutic significance in immigrant mental health services in France[J]. Cult Med Psychiatry, 2009, 33(1): 2-20. doi:10.1007/s11013-008-9115-1 |
| [29] | 田 帆. 区域均衡发展视角下中国县域医疗卫生资源的空间配置及其优化研究[D]. 成都: 四川大学, 2023. |
| [30] | 于宏博, 何晓丹, 张书芸, 等. 基层医疗卫生机构优质卫生资源配置区域差异与公平性分析[J]. 中国卫生经济, 2025, 44(2): 59-63. |
| [31] | 孙 瑜, 吴 爽, 曹志辉. 我国优质医疗资源配置公平性的区域差异及时空演进研究[J]. 中国医院, 2024, 28(12): 29-35. doi:10.19660/j.issn.1671-0592.2024.12.06 |
| [32] | Saunders PA, Clark L, Matthews T, et al. Exploring empathy and patient-centered communication behaviors of third-year medical students during a clinical skills examination[J]. Patient Educ Couns, 2025, 137: 108786. doi:10.1016/j.pec.2025.108786 |
| [33] | Norberg BL, Austad B, Kristiansen E, et al. The dynamics of doctor-patient communication during remote consultations: qualitative study among Norwegian contract general practitioners[J]. J Med Internet Res, 2025, 27: e57679. doi:10.2196/57679 |
| [34] | 陈 剑. 论健康公平[J]. 中国农村卫生事业管理, 2022, 42(1): 2-9. |
| [35] | Prue G, Czamanski-Cohen J, Kassianos AP, et al. Models of care and associated targeted implementation strategies for cancer survivorship support in Europe: a scoping review protocol[J]. BMJ Open, 2025, 15(2): e085456. doi:10.1136/bmjopen-2024-085456 |
| [36] | El-Deiry WS, Bresson C, Wunder F, et al. Worldwide innovative network (WIN) consortium in personalized cancer medicine: bringing next-generation precision oncology to patients[J]. Oncotarget, 2025, 16: 140-62. |
| [37] | Srivastava R. Advancing precision oncology with AI-powered genomic analysis[J]. Front Pharmacol, 2025, 16: 1591696. doi:10.3389/fphar.2025.1591696 |
| [38] | Roman Y. Bridging the United States population diversity gaps in clinical research: roadmap to precision health and reducing health disparities[J]. Pers Med, 2025,[Online ahead of print]. doi:10.1080/17410541.2025.2504329 |
| [39] | Bhatte S, Frederick J, Serrano S, et al. Bridging the vitamin A and deworming coverage gap among underserved populations in India through government and civil society organization partnerships[J]. Arch Public Health, 2024, 82(1): 75. doi:10.1186/s13690-024-01302-8 |
| [40] | 孙梅华, 刁青花, 王秀珍. 胃癌患者实施人文关怀护理的探讨: 评《癌症·瘀毒论》[J]. 中国实验方剂学杂志, 2024, 30(17): 202. |
| [41] | 苏喜凰. 将人文关怀融入手术患者的各阶段[N]. 甘肃科技报, 2024-07-19(008). |
| [42] | Che WQ, Li YJ, Tsang CK, et al. How to use the surveillance, epidemiology, and end results (SEER) data: research design and methodology[J]. Mil Med Res, 2023, 10(1): 50. doi:10.1186/s40779-023-00488-2 |
| [43] | Fung BM, Patel M, Patel N, et al. Signet ring cell gastric carcinoma: clinical epidemiology and outcomes in a predominantly Latino County hospital population[J]. Dig Dis Sci, 2021, 66(4): 1240-8. doi:10.1007/s10620-020-06341-z |
| [44] | 陈鑫明, 黄 坤, 赵平武, 等. 手术治疗对胃印戒细胞癌预后的影响: 基于SEER数据库分析[J]. 中国普外基础与临床杂志, 2023, 30(12): 1472-7. |
| [45] | 齐忆虹. 医疗卫生服务公平中的责任机制探讨[J]. 健康教育与健康促进, 2023, 18(1): 98-102. |
| [46] | 芦鹏飞, 李 响, 王丽娜, 等. 基于空间可达性的特定医疗与综合医疗资源分配公平差异研究[J]. 测绘科学技术学报, 2024, 41(4): 418-24. |
| [1] | 马振岩, 阿鑫, 赵蕾, 张洪博, 刘科, 赵依晴, 钱赓. 急性ST段抬高型心肌梗死经皮冠状动脉介入术后左心室不良重构的新型风险预测模型:基于心脏磁共振的多中心前瞻性研究[J]. 南方医科大学学报, 2025, 45(4): 669-683. |
| [2] | 潘甚豪, 李炎坤, 伍哲维, 毛玉玲, 王春艳. 子宫内膜异位症患者新鲜胚胎移植临床妊娠率预测模型的建立与验证[J]. 南方医科大学学报, 2024, 44(7): 1407-1415. |
| [3] | 刘云泽, 李宬润, 郭俊唐, 刘 阳. 基于临床-影像组学列线图模型鉴别局灶性机化性肺炎与肺腺癌[J]. 南方医科大学学报, 2024, 44(2): 397-404. |
| [4] | 张浩轩, 陆 进, 蒋成义, 方美芳. 基于人工智能技术的鼻咽癌风险预测模型的构建与评价[J]. 南方医科大学学报, 2023, 43(2): 271-279. |
| [5] | 张奔龙, 鲁意迅, 李 力, 高云鹤, 梁文全, 郗洪庆, 王鑫鑫, 张珂诚, 陈 凛. 基于单中心490例胃神经内分泌肿瘤建立的列线图具有良好的预后预测性能[J]. 南方医科大学学报, 2023, 43(2): 183-190. |
| [6] | 孔德贤, 宋丽萍, 向 阳. PET/CT代谢参数与血液炎症标志物对一线化疗的非小细胞肺癌患者预后列线图的构建及临床意义[J]. 南方医科大学学报, 2023, 43(12): 2139-2144. |
| [7] | 刘昭君, 周晓莉. 基于系统性炎症指标构建的列线图可预测心力衰竭患者的不良结局[J]. 南方医科大学学报, 2022, 42(8): 1149-1158. |
| [8] | 罗 钞, 王高明, 胡力文, 强 勇, 郑 超, 申 翼. 食管癌患者术后预测模型的构建和验证:基于SEER数据库[J]. 南方医科大学学报, 2022, 42(6): 794-804. |
| [9] | 孟令飞, 朱学研, 杨立明, 李忻阳, 程思宇, 郭师正, 庄小花, 邹洪斌, 崔文鹏. 腹膜透析相关腹膜炎患者治疗失败预测模型的构建和验证:一项多中心临床研究[J]. 南方医科大学学报, 2022, 42(4): 546-553. |
| [10] | 赵晨玲, 董 婷, 孙伦燕, 胡慧冰, 王 琼, 田丽伟, 江张胜. Wilson病脂代谢异常患者发生肝纤维化的列线图预测模型的建立与验证[J]. 南方医科大学学报, 2022, 42(11): 1720-1725. |
| [11] | 张海捷, 殷 夫, 陈梦林, 漆安琪, 杨丽洋, 崔维维, 杨姗姗, 文 戈. 基于术前CT影像组学列线图可预测Ⅰ~Ⅲ期肾透明性细胞癌术后复发[J]. 南方医科大学学报, 2021, 41(9): 1358-1365. |
| [12] | 张力苹, 刘喜娟, 林 卉, 王嘉丽, 张启周. 晚期胃癌生存预后因素及列线图预测模型的建立与验证[J]. 南方医科大学学报, 2021, 41(4): 621-627. |
| [13] | 成东亮, 冯红梅, 文 戈, 刘健萍, 洪居陆, 高明勇. 基于磁共振脂肪定量技术IDEAL-IQ的列线图模型在预测早期骨量丢失中的价值[J]. 南方医科大学学报, 2021, 41(11): 1707-1711. |
| [14] | 徐 伟, 程 瑶, 涂 兵. 乙型肝炎肝硬化患者行脾切除术后门静脉血栓形成的列线图预测模型的建立与验证[J]. 南方医科大学学报, 2020, 40(09): 1265-1272. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||