南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (3): 554-565.doi: 10.12122/j.issn.1673-4254.2025.03.13
• • 上一篇
罗嘉纯( ), Sodnomjamts Batzaya, 高雪锋, 陈晶宇, 余政颖, 熊莎莎, 曹虹(
), Sodnomjamts Batzaya, 高雪锋, 陈晶宇, 余政颖, 熊莎莎, 曹虹( )
)
收稿日期:2024-11-19
出版日期:2025-03-20
发布日期:2025-03-28
通讯作者:
曹虹
E-mail:q929188753@163.com;gzhcao@smu.edu.cn
作者简介:罗嘉纯,硕士,E-mail: q929188753@163.com
基金资助:
Jiachun LUO( ), Sodnomjamts Batzaya, Xuefeng GAO, Jingyu CHEN, Zhengying YU, Shasha XIONG, Hong CAO(
), Sodnomjamts Batzaya, Xuefeng GAO, Jingyu CHEN, Zhengying YU, Shasha XIONG, Hong CAO( )
)
Received:2024-11-19
Online:2025-03-20
Published:2025-03-28
Contact:
Hong CAO
E-mail:q929188753@163.com;gzhcao@smu.edu.cn
Supported by:摘要:
目的 探究Akkermansia muciniphila(A. muciniphila)灌胃对HIV-1的包膜病毒蛋白gp120诱导HIV相关神经认知功能障碍(HAND)动物模型中肠道菌群紊乱及肠-脑相互作用障碍(DGBIs)的改善作用。 方法 用16S rRNA基因测序技术检测6、9、12月龄野生型(WT型)小鼠与gp120转基因(gp120tg)小鼠肠道微生物组。将12月龄小鼠分为WT+PBS组、WT+A. muciniphila组、gp120+PBS组、gp120+A. muciniphila组,持续6周用A. mucinophila(2×108 CFU/小鼠)灌胃WT+A. muciniphila组及gp120+A. muciniphila组小鼠,1次/d。使用电子天平称量小鼠体质量。免疫组化法观察各组小鼠结肠内糖基化黏蛋白表达水平、嗜酸性粒细胞浸润情况、嗜酸性粒细胞激活标志物主要碱性蛋白(MBP)表达水平。ELISA法检测12月龄小鼠血清内脂多糖表达情况及结肠内IL-1β表达水平。qPCR法检测各组小鼠结肠内Occludin、ZO-1、IL-10、TNF-α、INF-γ表达水平。通过Morris水迷宫试验探究各组小鼠学习及空间记忆能力,通过免疫荧光法观察各组小鼠脑神经元损伤情况。 结果 与WT型小鼠相比,gp120tg小鼠肠道菌群辛普森多样性降低(P<0.001),其中12月龄gp120tg小鼠Akkermansia属丰富度下降(P<0.05);与12月龄WT型小鼠相比,12月龄gp120tg小鼠血清内脂多糖含量明显升高(P<0.01),小鼠结肠内糖基化黏蛋白表达明显下调(P<0.01),灌胃减小结肠内糖基化黏蛋白的差异且灌胃前后各组小鼠体质量差异无统计学意义(P>0.05);12月龄gp120tg小鼠结肠内紧密连接蛋白Occludin、ZO-1表达水平降低(P<0.001),并于灌胃后增加(P<0.001);12月龄gp120tg小鼠结肠内嗜酸性粒细胞存在明显的浸润激活及促炎细胞因子TNF-α(P<0.001)、INF-y(P<0.001)、IL-1β(P<0.05)表达水平的上调,而抑炎细胞因子IL-10表达水平降低(P<0.001),灌胃可减小其差异;12月龄gp120tg小鼠存在明显的认知损伤且海马及皮层中神经元数量减少(P<0.05),灌胃后小鼠认知损伤得到明显改善且海马及皮层中神经元数量增加(P<0.05)。 结论 gp120tg小鼠菌群丰富度及多样性显著低于WT型小鼠,12月龄gp120tg小鼠Akkermansia属丰度明显低于WT型小鼠,二者DGBIs相关指标表达情况存在明显差异。A. muciniphila灌胃显著减少12月龄gp120tg小鼠肠屏障损伤、降低结肠炎症反应及嗜酸性粒细胞激活水平、减少小鼠认知损伤及脑神经元损伤。
罗嘉纯, Sodnomjamts Batzaya, 高雪锋, 陈晶宇, 余政颖, 熊莎莎, 曹虹. Akkermansia muciniphila改善gp120转基因小鼠的肠-脑相互作用障碍[J]. 南方医科大学学报, 2025, 45(3): 554-565.
Jiachun LUO, Sodnomjamts Batzaya, Xuefeng GAO, Jingyu CHEN, Zhengying YU, Shasha XIONG, Hong CAO. Akkermansia muciniphila gavage improves gut-brain interaction disorders in gp120 transgenic mice[J]. Journal of Southern Medical University, 2025, 45(3): 554-565.
| Gene | Primer sequences (5'-3')-F | Primer sequences (3'-5')-R |
|---|---|---|
| Occludin | TTTCCTGCGGTGACTTCTCC | GGGGAACGTGGCCGATATAA |
| ZO-1 | CTCAAGTTCCTGAAGCCCGT | GCAAAAGACCAACCGTCAGG |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| INF-γ | ATGAACGCTACACACTGCATC | CCATCCTTTTGCCAGTTCCTC |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| GAPDH | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG |
表1 qPCR引物序列
Tab.1 Primers used for qPCR
| Gene | Primer sequences (5'-3')-F | Primer sequences (3'-5')-R |
|---|---|---|
| Occludin | TTTCCTGCGGTGACTTCTCC | GGGGAACGTGGCCGATATAA |
| ZO-1 | CTCAAGTTCCTGAAGCCCGT | GCAAAAGACCAACCGTCAGG |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| INF-γ | ATGAACGCTACACACTGCATC | CCATCCTTTTGCCAGTTCCTC |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| GAPDH | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG |
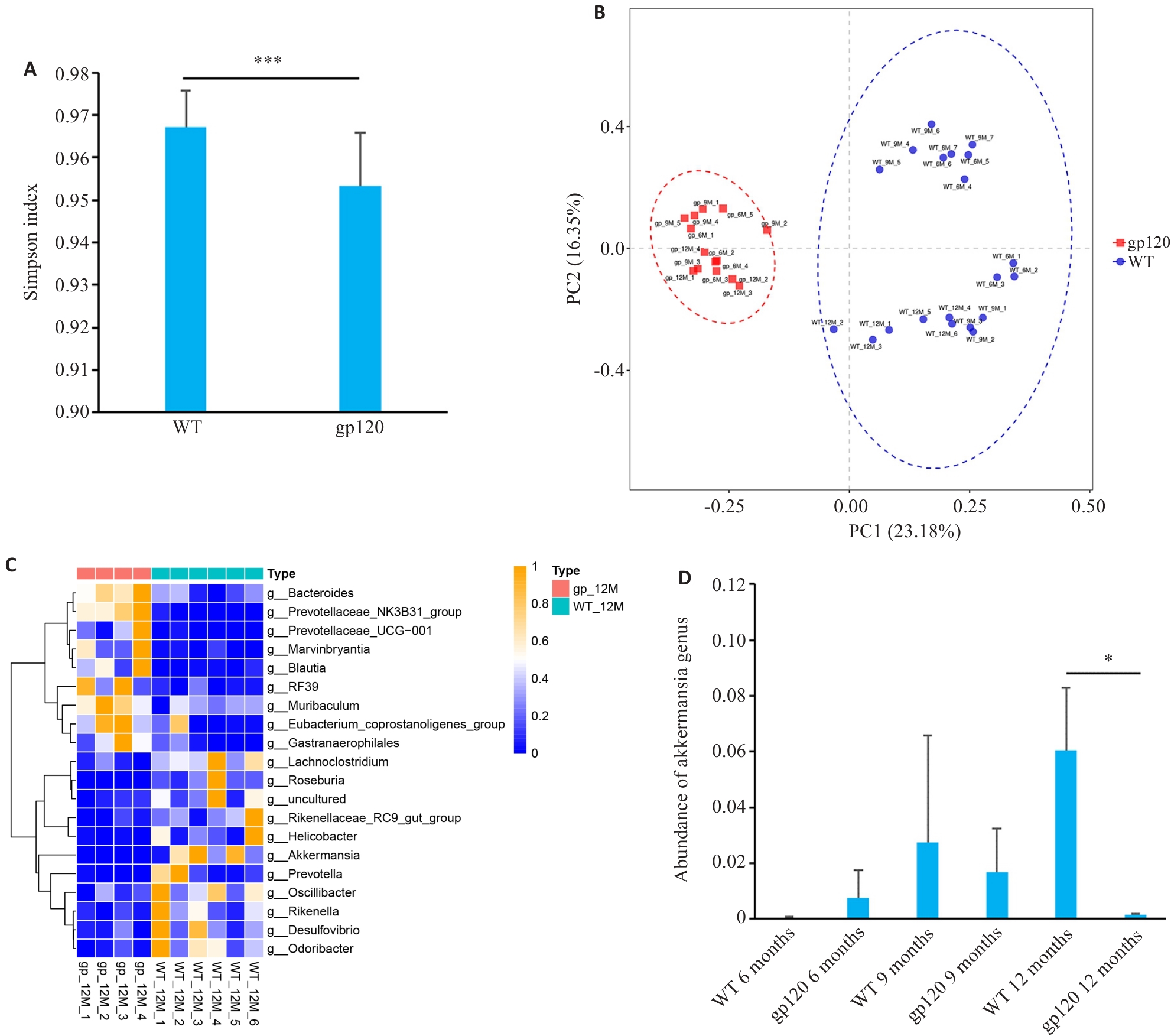
图1 gp120tg小鼠与WT型小鼠肠道菌群多样性及菌属丰度差异分析
Fig.1 Analysis of diversity and abundance of intestinal microbiota in gp120tg mice and WT mice. A: Simpson's diversity index of the microbiome in gp120tg mice (n=14) and WT mice (n=20). B: Principal coordinate analysis (PCoA) using unweighted UniFrac distance in gp120tg mice (n=14) and WT mice (n=20). C: Heatmap for comparison of microbiota abundance at the genus level between 12-month-old gp120tg mice (n=4) and WT mice (n=6). D: Abundance of Akkermansia genus in different groups of mice (n=3-4). *P<0.05, ***P < 0.001.
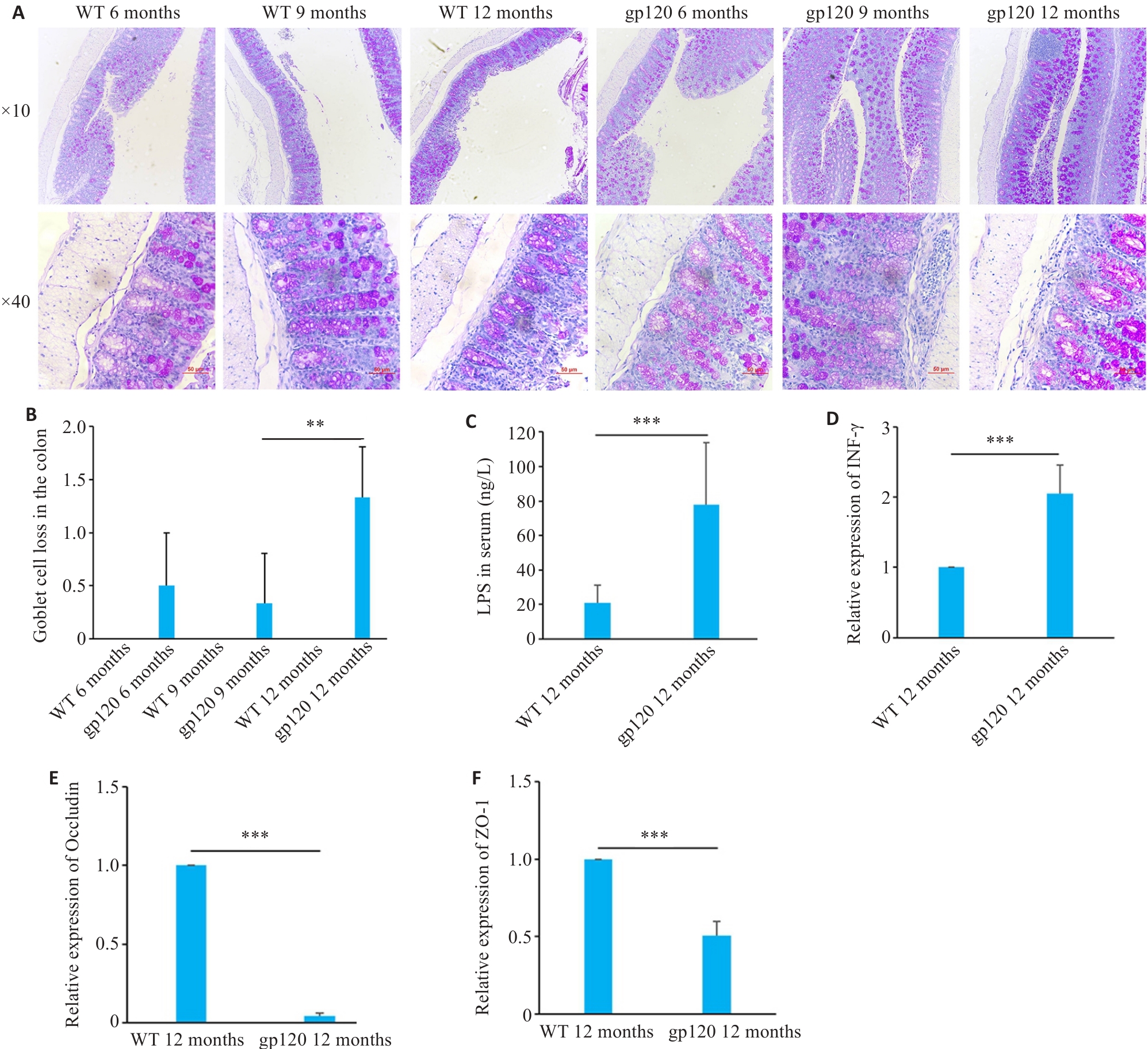
图2 gp120tg小鼠与WT型小鼠肠屏障相关指标表达情况
Fig.2 Expression of intestinal barrier-related indexes in gp120tg mice and WT mice. A: PAS staining of mouse colon tissues. B: Quantification of goblet cell loss of mouse colon tissues (n=3-4). C: ELISA validation of LPS levels in mouse serum (n=5). D-F: Quantification of INF-γ, occludin and ZO-1 expression levels in the mouse colon (n=3). **P<0.01, ***P<0.001.
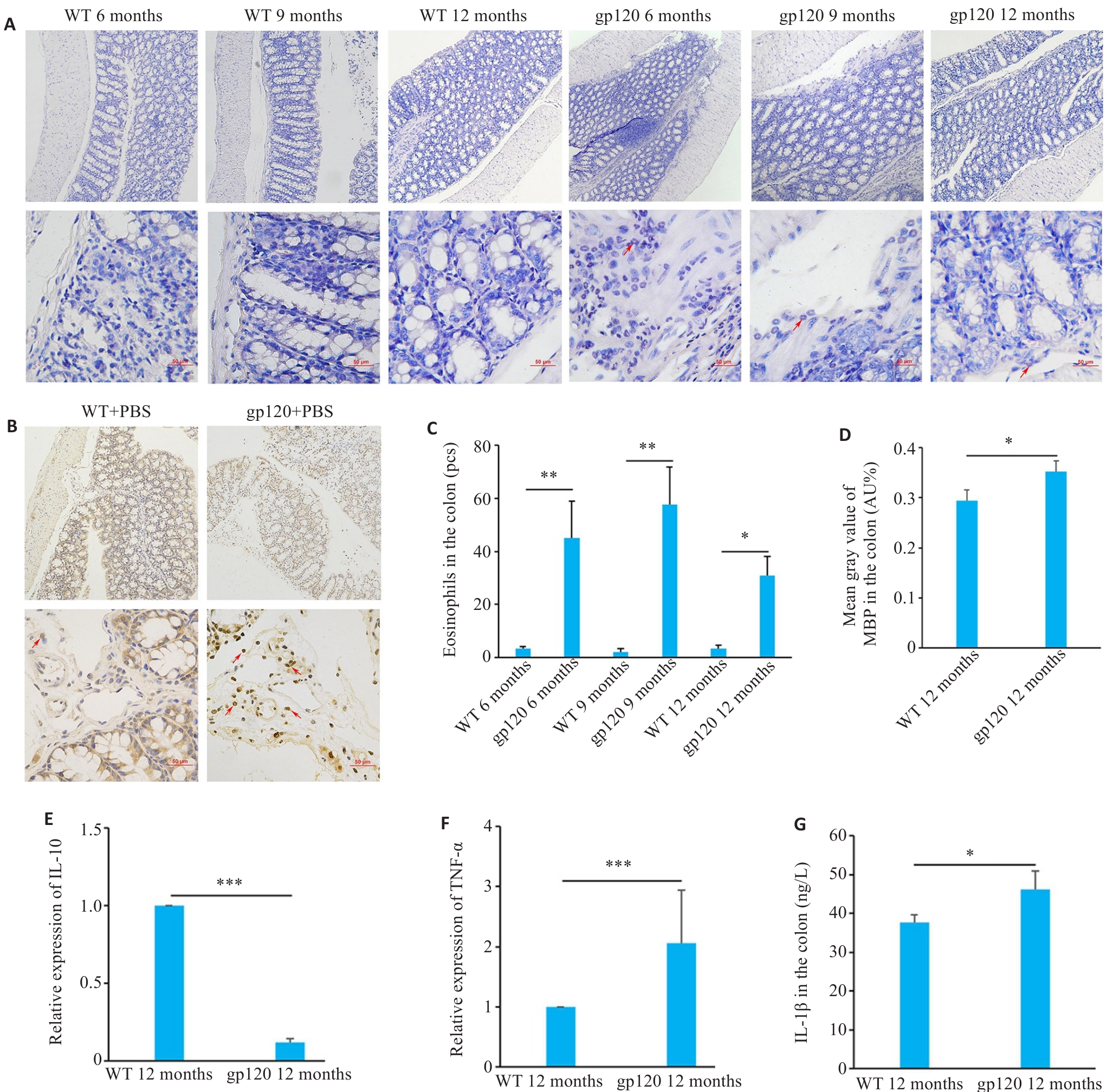
图3 gp120tg小鼠与WT型小鼠炎症及免疫反应相关指标表达情况
Fig.3 Expression of inflammation- and immune response-related indexes in gp120tg mice and WT mice. A: Hematoxylin-chromotrope staining of mouse colon tissues (scale bar=50 μm). B: MBP staining of the mouse colon tissues (MBP-positive areas are brown or reddish-brown; scale bar=50 μm). C: Quantification of eosinophils in the mouse colon tissues (n=3 or 4). D: Mean gray value of MBP in the colon (n=3). E, F: Quantification of IL-10 and TNF-α expression levels in the mouse colon (n=3). G: ELISA validation of IL-1β levels in the mouse colon (n=4). *P<0.05, **P<0.01, ***P<0.001.
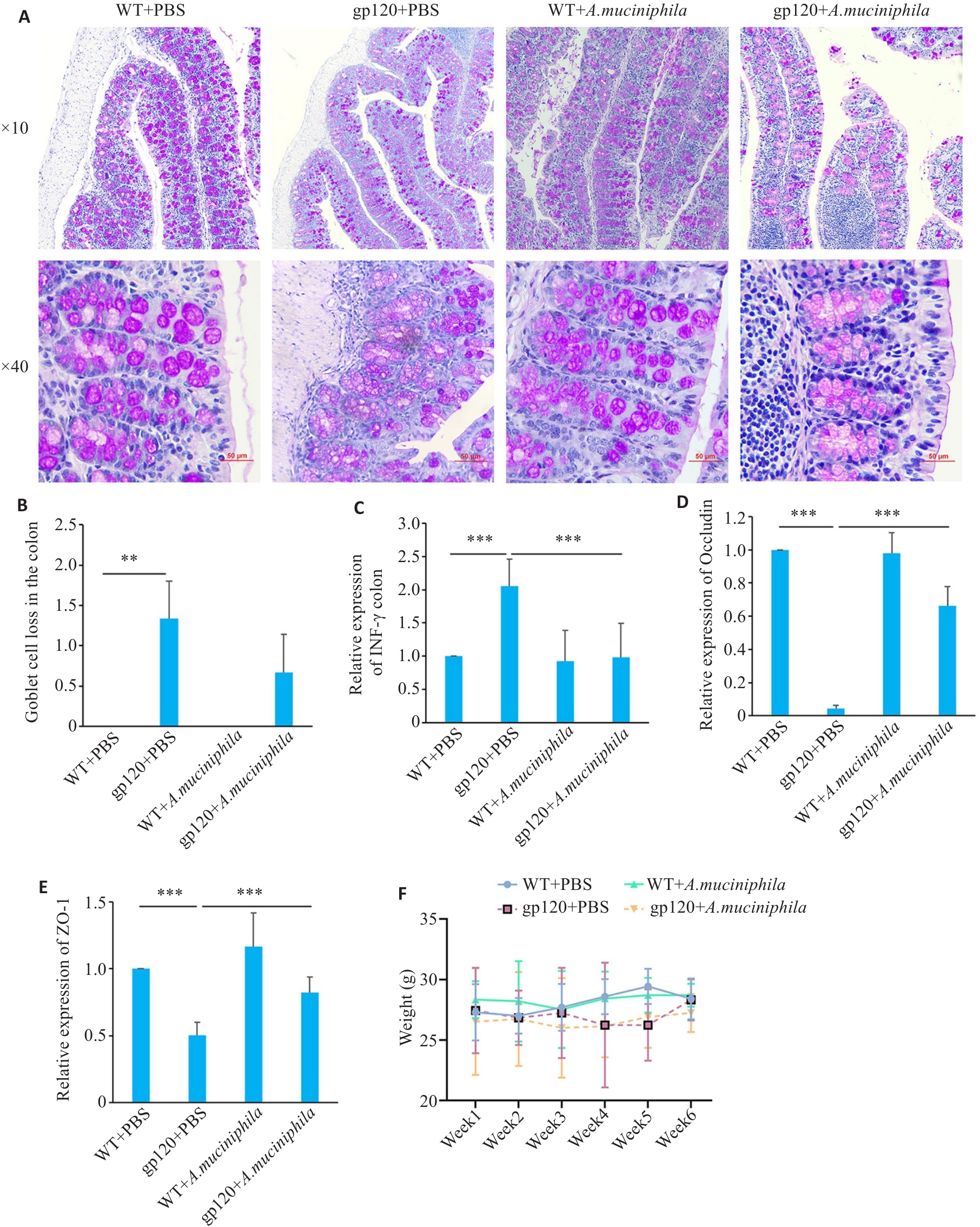
图4 A.muciniphila灌胃对12月龄gp120tg小鼠与WT型小鼠肠屏障相关指标表达情况的影响
Fig.4 Effects of oral gavage of A.muciniphila on expression of gut barrier-related indexes in the colon of 12-month-old gp120tg mice and WT mice. A: PAS staining of mouse colon tissues (scale bar=50 μm). B: Quantification of goblet cell loss in mouse colon tissues (n=3 or 4). C-E: Quantification of INF-γ, occludin and ZO-1 expression levels in mouse colon (n=3). Mice were gavaged once a day with A. muciniphila (2×108 CFU per mouse) for 6 weeks. F: Changes of body weight of the mice during oral gavage of A.muciniphila. **P<0.01, ***P< 0.001.
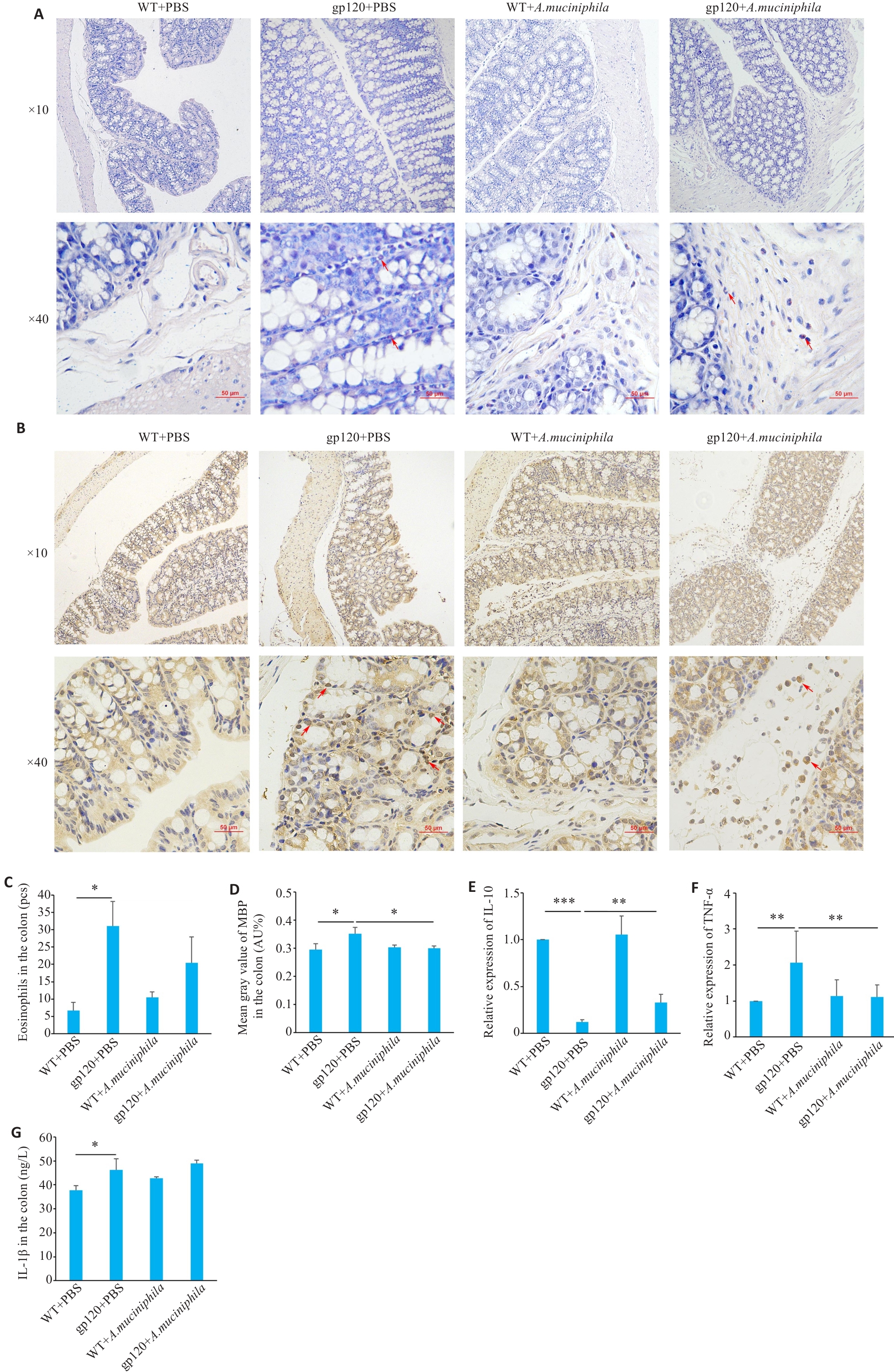
图5 A.muciniphila灌胃对12月龄gp120tg小鼠与WT型小鼠炎症及免疫反应相关指标表达情况的影响
Fig.5 Effects of oral gavage of A.muciniphila onexpression of inflammation- and immune response-related indexes in 12-month-old gp120tg mice and WT mice. A: Hematoxylin-chromotrope staining of mouse colon tissues. B: MBP staining in the mouse colon tissues. C: Quantification of eosinophils in the mouse colon tissues (n=3 or 4). D: Mean gray value of MBP in the colon (n=3). E, F: Quantification of IL-10 and TNF-α expression levels in mouse colon (n=3). G: ELISA validation of IL-1β levels in mouse colon (n=3 or 4). *P<0.05, **P<0.01, ***P<0.001.
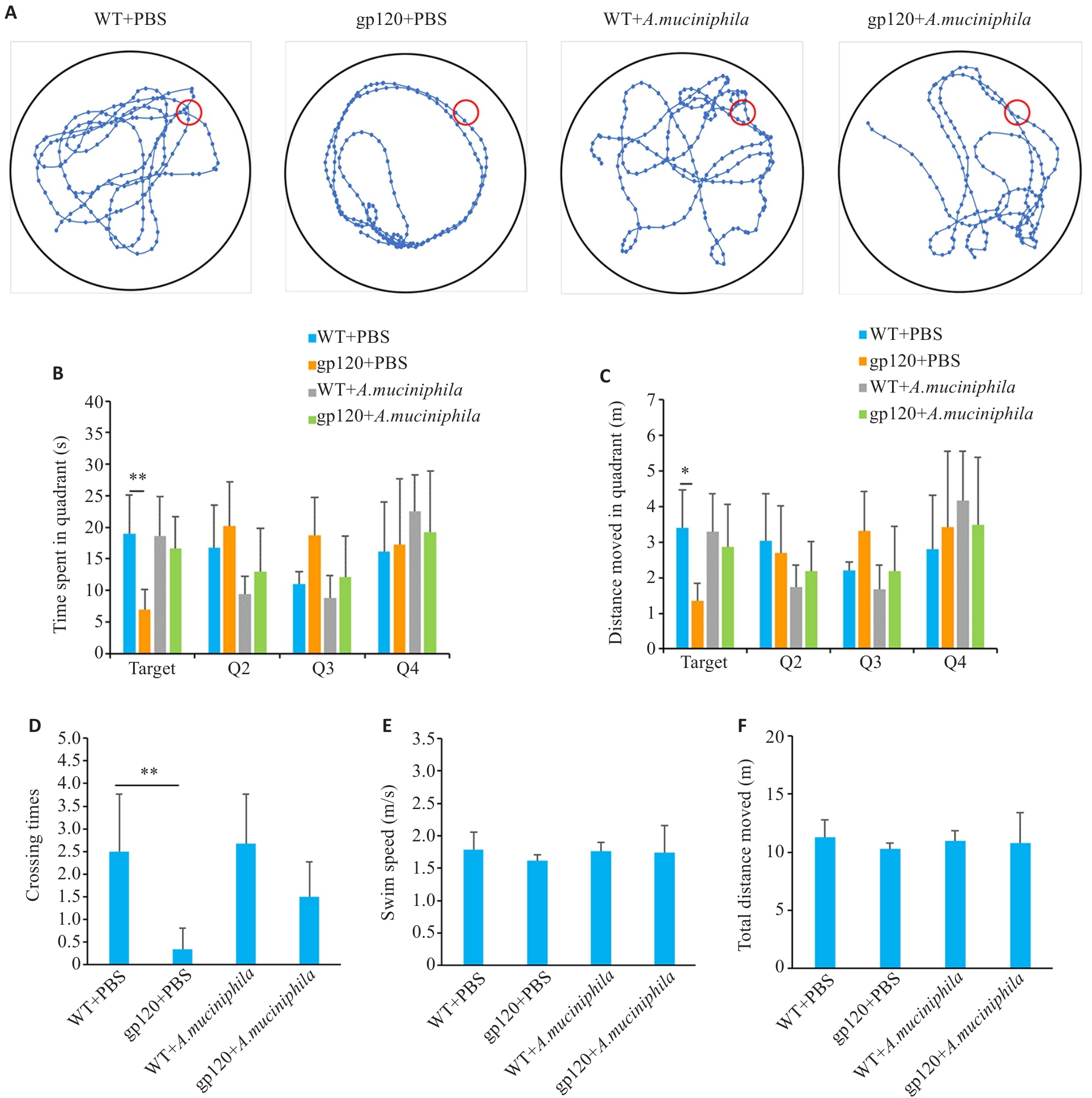
图6 A.muciniphila灌胃对12月龄gp120tg小鼠与WT型小鼠学习及空间记忆能力的影响
Fig.6 Effects of oral gavage of A.muciniphila on learning and spatial memory abilities of 12-month-old gp120tg mice and WT mice. A: Movement trajectory diagrams of the mice in the spatial exploration experiments (n=6). B: Movement time in each quadrant in the spatial exploration experiments (n=6). C: Movement distance in each quadrant in the spatial exploration experiments (n=6). D: Crossing times of the platform in the spatial exploration experiments (n=6). E: Swimming speed of the mice in the spatial exploration experiments (n=6). F: Total distance moved of the mice in the spatial exploration experiments (n=6). *P<0.05, **P<0.01.
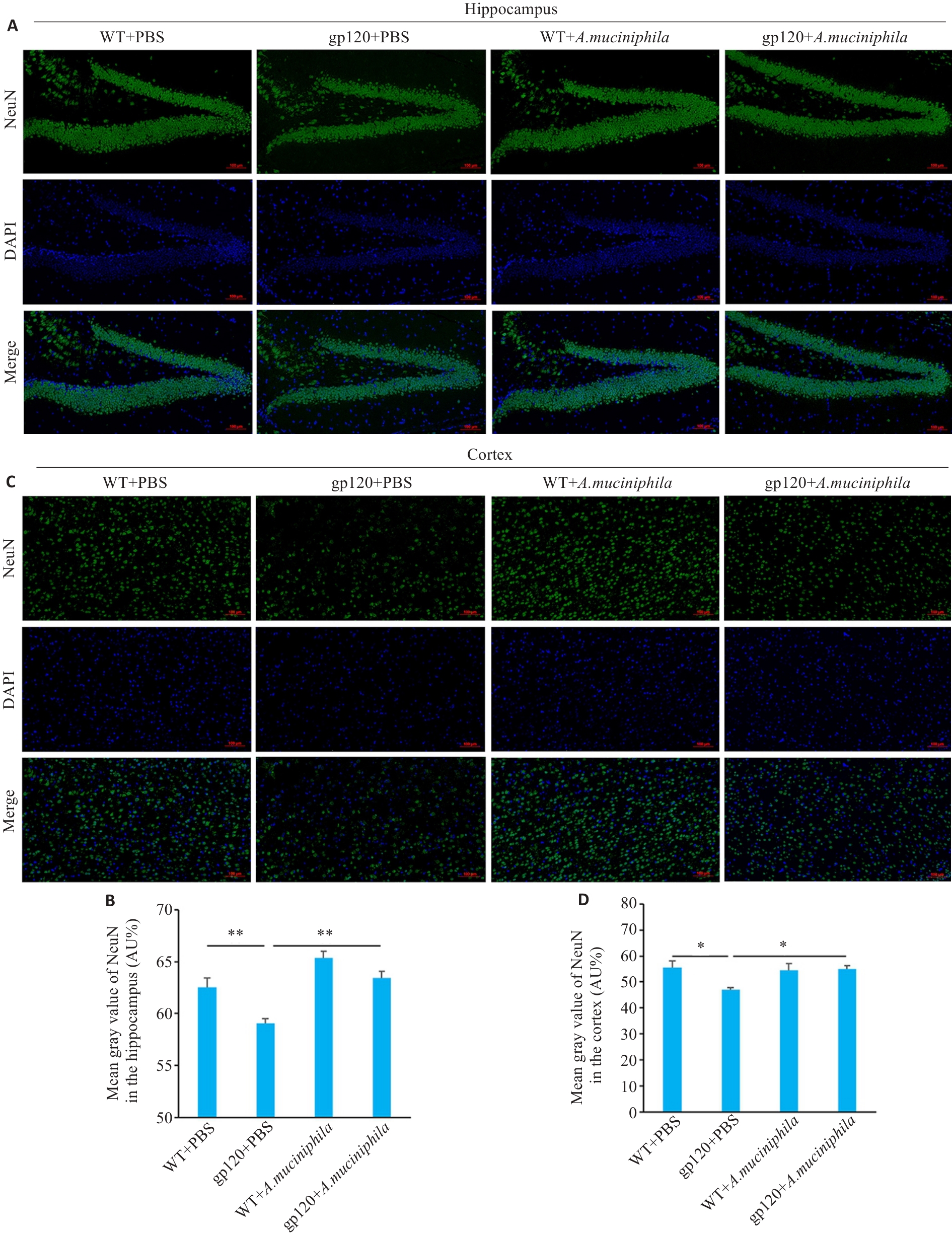
图7 A.muciniphila灌胃对12月龄gp120tg小鼠与WT型小鼠神经元的影响
Fig.7 Effects of A.muciniphila gavage on neuronal damage in 12-month-old gp120tg mice and WT mice. A: Immunofluorescence showing the distribution of NeuN in the mouse hippocampus (scale bar=100 μm). B: Mean gray value of NeuN in the mouse hippocampus (n=3). C: Immunofluorescence showing the distribution of NeuN in the mouse cortex (scale bar=100 μm). D: Mean gray value of NeuN in the mouse cortex (n=3). *P<0.05, **P<0.01.
| 1 | Namagga JK, Rukundo GZ, Niyonzima V, et al. Depression and HIV associated neurocognitive disorders among HIV infected adults in rural southwestern Uganda: a cross-sectional quantitative study[J]. BMC Psychiatry, 2021, 21(1): 350. |
| 2 | Ji J, Zhang Y, Ma Y, et al. People who living with HIV/AIDS also have a high prevalence of anxiety disorders: a systematic review and meta-analysis[J]. Front Psychiatry, 2024, 15: 1259290. |
| 3 | Myszka DG, Sweet RW, Hensley P, et al. Energetics of the HIV gp120-CD4 binding reaction[J]. Proc Natl Acad Sci USA, 2000, 97(16): 9026-31. |
| 4 | He XL, Yang WJ, Zeng ZJ, et al. NLRP3-dependent pyroptosis is required for HIV-1 gp120-induced neuropathology[J]. Cell Mol Immunol, 2020, 17: 283-99. |
| 5 | Tan SY, Li WJ, Yang C, et al. gp120-derived amyloidogenic peptides form amyloid fibrils that increase HIV-1 infectivity[J]. Cell Mol Immunol, 2024, 21: 479-94. |
| 6 | Ellis RJ, Marquine MJ, Kaul M, et al. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management[J]. Nat Rev Neurol, 2023, 19: 668-87. |
| 7 | Maung R, Hoefer MM, Sanchez AB, et al. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120[J]. J Immunol, 2014, 193(4): 1895-910. |
| 8 | Toggas SM, Masliah E, Rockenstein EM, et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice[J]. Nature, 1994, 367(6459): 188-93. |
| 9 | Bonnechère B, Amin N, van Duijn C. What are the key gut microbiota involved in neurological diseases? A systematic review[J]. Int J Mol Sci, 2022, 23(22): 13665. |
| 10 | Yang H, Li S, Le W. Intestinal permeability, dysbiosis, inflammation and enteric Glia cells: the intestinal etiology of Parkinson's disease[J]. Aging Dis, 2022, 13(5): 1381-90. |
| 11 | Rocafort M, Noguera-Julian M, Rivera J, et al. Evolution of the gut microbiome following acute HIV-1 infection[J]. Microbiome, 2019, 7(1): 73. |
| 12 | Guo XY, Guo YT, Wang ZR, et al. Severe intestinal barrier damage in HIV-infected immunological non-responders[J]. Heliyon, 2023, 9(10): e20790. |
| 13 | Van Oudenhove L, Crowell MD, Drossman DA, et al. Biopsychosocial aspects of functional gastrointestinal disorders[J]. Gastroenterology, 2016: S0016-5085(16)00218-3. |
| 14 | Mohamed AA, Oduor C, Kinyanjui D. HIV-associated neurocognitive disorders at Moi teaching and referral hospital, Eldoret, Kenya[J]. BMC Neurol, 2020, 20(1): 280. |
| 15 | Vujkovic-Cvijin I, Sortino O, Verheij E, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases[J]. Nat Commun, 2020, 11: 2448. |
| 16 | Vázquez-Castellanos JF, Serrano-Villar S, Jiménez-Hernández N, et al. Interplay between gut microbiota metabolism and inflammation in HIV infection[J]. ISME J, 2018, 12: 1964-76. |
| 17 | Ouyang J, Lin J, Isnard S, et al. The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation[J]. Front Immunol, 2020, 11: 645. |
| 18 | Zheng M, Han R, Yuan Y, et al. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives[J]. Front Immunol, 2022, 13: 1089600. |
| 19 | Xu R, Zhang Y, Chen S, et al. The role of the probiotic Akkermansia muciniphila in brain functions: insights underpinning therapeutic potential[J]. Crit Rev Microbiol, 2023, 49(2): 151-76. |
| 20 | Mo C, Lou X, Xue J, et al. The influence of Akkermansia muciniphila on intestinal barrier function[J]. Gut Pathog, 2024, 16(1): 41. |
| 21 | Erben U, Loddenkemper C, Doerfel K, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models[J]. Int J Clin Exp Pathol, 2014, 7(8): 4557-76. |
| 22 | Barbaro MR, Cremon C, Marasco G, et al. Molecular mechanisms underlying loss of vascular and epithelial integrity in irritable bowel syndrome[J]. Gastroenterology, 2024, 167(6): 1152-66. |
| 23 | Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia[J]. Gut, 2014, 63(2): 262-71. |
| 24 | Vanuytsel T, Bercik P, Boeckxstaens G. Understanding neuroimmune interactions in disorders of gut-brain interaction: from functional to immune-mediated disorders[J]. Gut, 2023, 72(4): 787-98. |
| 25 | Lechuga S, Braga-Neto MB, Naydenov NG, et al. Understanding disruption of the gut barrier during inflammation: Should we abandon traditional epithelial cell lines and switch to intestinal organoids?[J]. Front Immunol, 2023, 14: 1108289. |
| 26 | Awad K, Barmeyer C, Bojarski C, et al. Epithelial barrier dysfunction in diarrhea-predominant irritable bowel syndrome (IBS-D) via downregulation of claudin-1[J]. Cells, 2023, 12(24): 2846. |
| 27 | Han X, Lee A, Huang S, et al. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids[J]. Gut Microbes, 2019, 10(1): 59-76. |
| 28 | Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers[J]. J Clin Invest, 1989, 83(2): 724-7. |
| 29 | Pabst O, Cerovic V. Interferon-γ sensing by epithelial cells tames gut inflammation[J]. Nat Immunol, 2024, 25: 9-10. |
| 30 | Smolinska S, Winiarska E, Globinska A, et al. Histamine: a mediator of intestinal disorders-a review[J]. Metabolites, 2022, 12(10): 895. |
| 31 | Salvo-Romero E, Rodiño-Janeiro BK, Albert-Bayo M, et al. Eosinophils in the gastrointestinal tract: key contributors to neuro-immune crosstalk and potential implications in disorders of brain-gut interaction[J]. Cells, 2022, 11(10): 1644. |
| 32 | Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein[J]. Am J Physiol Gastrointest Liver Physiol, 2005, 289(5): G890-7. |
| 33 | Qin TT, Fang F, Song MT, et al. Umbelliferone reverses depression-like behavior in chronic unpredictable mild stress-induced rats by attenuating neuronal apoptosis via regulating ROCK/Akt pathway[J]. Behav Brain Res, 2017, 317: 147-56. |
| 34 | Sakon JJ, Suzuki WA. Neural evidence for recognition of naturalistic videos in monkey hippocampus[J]. Hippocampus, 2021, 31(8): 916-32. |
| 35 | Qiao CM, Zhou Y, Quan W, et al. Fecal microbiota transplantation from aged mice render recipient mice resistant to MPTP-induced nigrostriatal degeneration via a neurogenesis-dependent but inflammation-independent manner[J]. Neurotherapeutics, 2023, 20(5): 1405-26. |
| 36 | Qiao CM, Huang WY, Zhou Y, et al. Akkermansia muciniphila is beneficial to a mouse model of Parkinson’s disease, via alleviated neuroinflammation and promoted neurogenesis, with involvement of SCFAs[J]. Brain Sci, 2024, 14(3): 238. |
| [1] | 黄鹏伟, 陈洁, 邹金虎, 高雪锋, 曹虹. 槲皮素促进应激颗粒G3BP1解聚改善HIV-1 gp120诱导的星形胶质细胞神经毒性[J]. 南方医科大学学报, 2025, 45(2): 304-312. |
| [2] | 刘莹, 李柏睿, 李永财, 常禄博, 王娇, 杨琳, 颜永刚, 屈凯, 刘继平, 张岗, 沈霞. 加味逍遥丸通过神经递质调节、抗炎抗氧化及肠道菌群调控改善大鼠的抑郁样行为[J]. 南方医科大学学报, 2025, 45(2): 347-358. |
| [3] | 刘佳进, 缪长宏, 徐健康, 余伟杰, 陈继鑫, 唐好知, 刘爱峰. 肠道菌群与色素沉着绒毛结节性滑膜炎之间的因果关系:基于孟德尔随机化分析[J]. 南方医科大学学报, 2024, 44(7): 1397-1406. |
| [4] | 李新翼, 刘玉杰, 邓克崇, 胡义奎. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-410. |
| [5] | 朱继伟, 卢曼路, 焦倩倩, 孙运良, 刘 璐, 丁红红, 于 燕, 潘 磊. 基于16S rRNA测序分析阻塞性睡眠呼吸暂停患者肠道靶标菌群的变化[J]. 南方医科大学学报, 2024, 44(1): 146-155. |
| [6] | 王 敏, 张 茜, 徐桂铃, 黄淑榆, 赵文驱, 梁健鹏, 黄俊文, 蔡绍曦, 赵海金. 健康人群和慢性阻塞性肺疾病患者的血清维生素D与血嗜酸性粒细胞计数的关系[J]. 南方医科大学学报, 2023, 43(5): 727-732. |
| [7] | 杨金水, 万月华, 张江林, 朱 剑. 45例嗜酸性筋膜炎的临床特征、超声诊断及治疗随访的单中心回顾性研究[J]. 南方医科大学学报, 2023, 43(1): 145-152. |
| [8] | 库尔班乃木·卡合曼, 赵健锋, 穆凯代斯·艾合买提, 王汉铭, 朱稷蔚, 潘文涛, 卡思木江·阿西木江. 屎肠球菌QH06能减轻溃疡性结肠炎大鼠的结肠黏膜损伤[J]. 南方医科大学学报, 2022, 42(7): 976-987. |
| [9] | 王展强, 徐开宇, 周宏伟. 卒中患者肠道宏病毒组的组成和菌群特征[J]. 南方医科大学学报, 2021, 41(6): 862-869. |
| [10] | 肖 瑶, 牛 玥, 毛明慧, 林 涵, 汪佰丽, 乌恩奇, 赵焕虎, 李树春. 2型糖尿病与肠道核心菌群的相关性[J]. 南方医科大学学报, 2021, 41(3): 358-369. |
| [11] | 林琼希, 伦静娴, 张吉敏, 何肖龙, 龚泽龙, 高雪峰, 曹 虹. 学龄期儿童肉类食品摄入模式对其肠道菌群结构的影响[J]. 南方医科大学学报, 2021, 41(12): 1801-1808. |
| [12] | 张星星, 蔡文文, 廖生武, 何璇昱, 杨秋玉, 白 杨, 阮伟清. 亚健康状态人群肠道菌群结构明显失调:基于某医院150例护理人员分析[J]. 南方医科大学学报, 2021, 41(12): 1870-1876. |
| [13] | 杨少杰, 邓小燕, 张铁松, 肖 仪, 彭 亮, 李 莉, 何肖龙, 魏 轶, 刘利群, 曹 虹, 龙北国, 黄胜和. SBi4211通过抑制S100B/RAGE表达减轻gp120诱导的中枢神经损伤[J]. 南方医科大学学报, 2020, 40(12): 1693-1702. |
| [14] | 黄嘉裕, 王利平, 吴小琴, 陈焕钧, 付秀丽, 陈少华, 刘 涛. 基于高通量测序技术分析慢性鼻窦炎患者的肠道菌群结构特点[J]. 南方医科大学学报, 2020, 40(09): 1319-1324. |
| [15] | 胡彤彤,龚泽龙,万 宇,李煜彬,高雪锋,伦静娴,黄胜和,曹 虹. α7nAChR基因敲除HIV-1gp120转基因小鼠模型的构建[J]. 南方医科大学学报, 2020, 40(08): 1184-1191. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||