南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (12): 2335-2346.doi: 10.12122/j.issn.1673-4254.2024.12.09
• • 上一篇
牛民主1( ), 殷丽霞2, 段婷3, 黄菊4, 李静2, 耿志军4, 胡建国2, 宋传旺1(
), 殷丽霞2, 段婷3, 黄菊4, 李静2, 耿志军4, 胡建国2, 宋传旺1( )
)
收稿日期:2024-09-27
出版日期:2024-12-20
发布日期:2024-12-26
通讯作者:
宋传旺
E-mail:nmz8033@163.com;bbmcscw@foxmail.com
作者简介:牛民主,在读硕士研究生,E-mail: nmz8033@163.com
基金资助:
Minzhu NIU1( ), Lixia YIN2, Ting DUAN3, Ju HUANG4, Jing LI2, Zhijun GENG4, Jianguo HU2, Chuanwang SONG1(
), Lixia YIN2, Ting DUAN3, Ju HUANG4, Jing LI2, Zhijun GENG4, Jianguo HU2, Chuanwang SONG1( )
)
Received:2024-09-27
Online:2024-12-20
Published:2024-12-26
Contact:
Chuanwang SONG
E-mail:nmz8033@163.com;bbmcscw@foxmail.com
Supported by:摘要:
目的 探讨川续断皂苷VI(AVI)对小鼠克罗恩病(CD)样结肠炎的肠上皮细胞凋亡和肠屏障的影响及其作用机制。 方法 将30只雄性C57BL/6小鼠随机分成对照组(WT组)、2,4,6-三硝基苯磺酸诱导模型组(TNBS组)、AVI药物治疗组(AVI组,150 mg/kg),每组10只。通过监测小鼠体质量、测量结肠长度、疾病活动度(DAI)评分、HE染色、AB-PAS染色、组织炎症评分、ELISA和RT-qPCR实验,验证AVI对小鼠结肠炎的缓解作用。采用TNF-α诱导Caco-2细胞建立体外凋亡模型,分为Control组、TNF-α组、AVI组(250 μmol/L)。CCK-8实验检测AVI对Caco-2细胞活力的影响。采用免疫荧光、TUNEL实验和Western blotting检测AVI对小鼠肠上皮细胞和Caco-2细胞屏障损伤与凋亡的改善情况。利用网络药理学预测AVI干预CD的分子机制可能与PI3K/AKT/NF-κB通路有关,Western blotting检测体内外通路的蛋白表达,以及经PI3K/AKT通路激活剂(Recilisib)和AKT1 siRNA转染干预细胞后,通过TUNEL实验和Western blotting验证其对细胞凋亡的调控作用。 结果 AVI能明显地缓解小鼠体质量降低、结肠缩短、DAI与组织炎症评分的增加、肠绒毛和杯状细胞的损伤,以及炎症因子的高表达(P<0.05)。AVI浓度在0~250 μmol/L时对Caco-2细胞活力无影响。体内外实验证实,AVI可阻断紧密连接蛋白的缺失,减少肠上皮细胞的凋亡(P<0.05)。KEGG富集通路分析发现AVI干预CD可能与抑制PI3K/AKT/NF-κB通路活化有关,在体内外模型证实AVI干预后p-PI3K、p-AKT和p-p65的表达降低(P<0.05)。此外,Recilisib干预后可逆转AVI对通路的抑制作用和抗凋亡作用(P<0.05),AKT1 siRNA转染细胞后证实PI3K/AKT通路可介导下游NF-κB信号的活化(P<0.05)。 结论 AVI可通过拮抗肠上皮细胞的凋亡和减轻肠屏障损伤,达到改善TNBS诱导的CD小鼠结肠炎的目的,其机制可能与AVI负向调控PI3K/AKT/NF-κB有关。
牛民主, 殷丽霞, 段婷, 黄菊, 李静, 耿志军, 胡建国, 宋传旺. 川续断皂苷VI通过抑制PI3K/AKT/NF-κB通路拮抗肠上皮细胞凋亡缓解TNBS诱导的小鼠克罗恩病样结肠炎[J]. 南方医科大学学报, 2024, 44(12): 2335-2346.
Minzhu NIU, Lixia YIN, Ting DUAN, Ju HUANG, Jing LI, Zhijun GENG, Jianguo HU, Chuanwang SONG. Asperosaponin VI alleviates TNBS-induced Crohn's disease-like colitis in mice by reducing intestinal epithelial cell apoptosis via inhibiting the PI3K/AKT/NF-κB signaling pathway[J]. Journal of Southern Medical University, 2024, 44(12): 2335-2346.
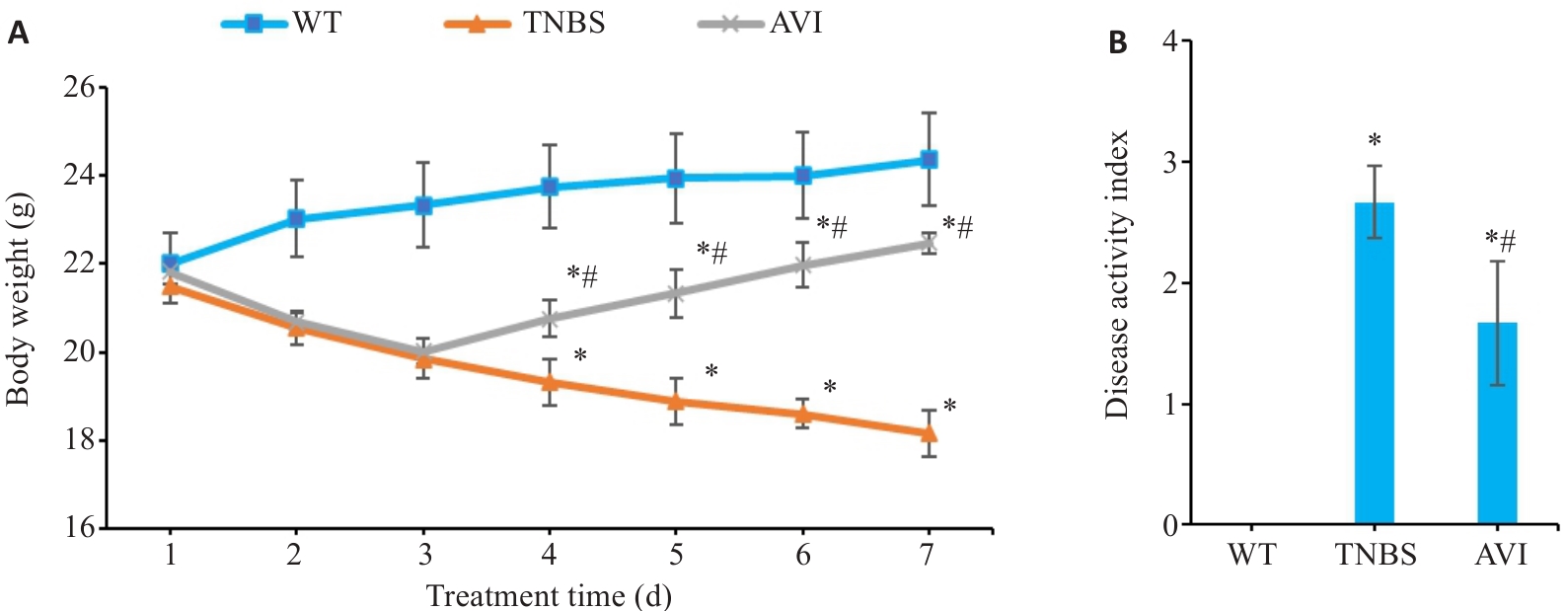
图1 AVI干预对TNBS模型小鼠疾病状态的影响
Fig.1 Effect of AVI intervention on body weight and disease activity in mice with TNBS-induced CD-like colitis. A: Changes of body weight. B: Changes of DAI scores. WT: Wild type group; TNBS: TNBS-induced model group; AVI: AVI treatment group. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
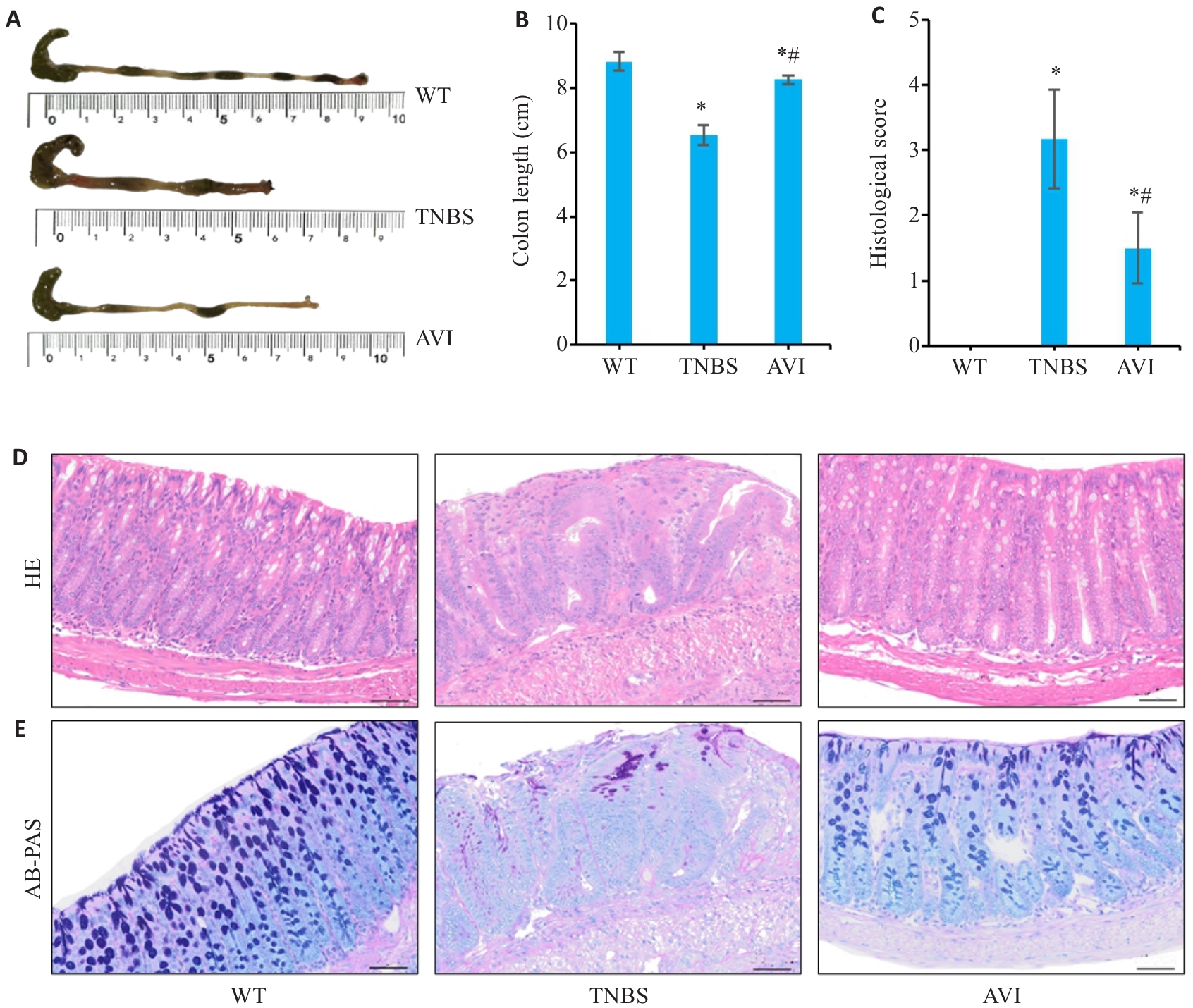
图2 AVI干预对TNBS模型小鼠肠道炎症的影响
Fig.2 Effect of AVI on intestinal inflammation in the mouse models. A, B: Comparison of colon lengths among the groups. C: Histopathological score of the colon. D: HE staining of colon tissue in different groups. E: AB-PAS staining of colon tissue in different groups. *P<0.05 vs WT group. #P<0.05 vs TNBS group. Scale bar=50 μm.
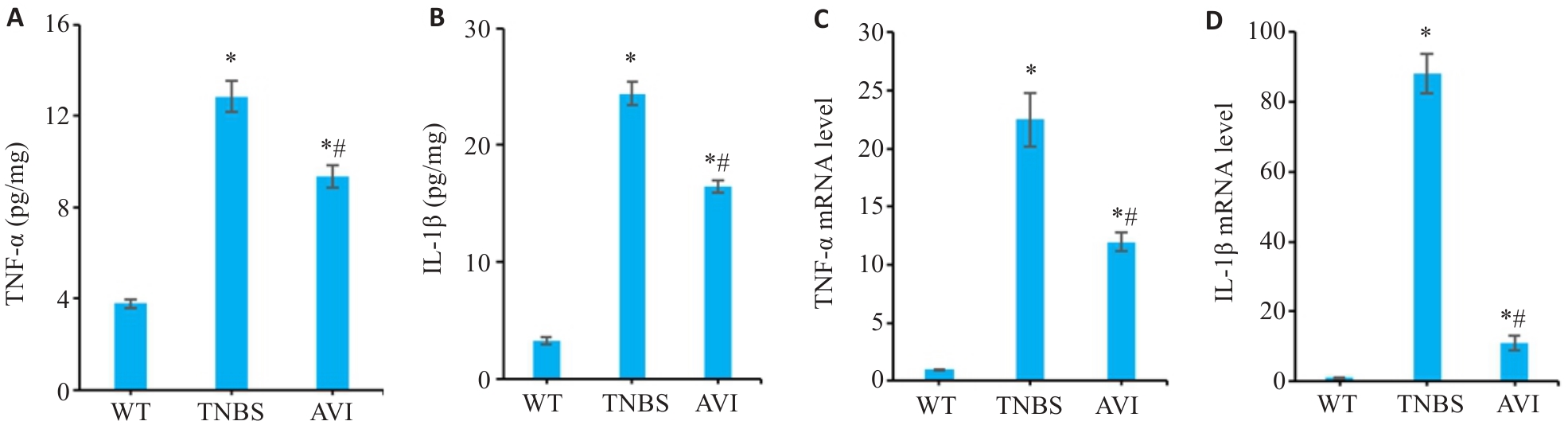
图3 AVI干预对TNBS模型小鼠肠道炎症因子的影响
Fig.3 Effect of AVI on intestinal inflammatory factors in the mouse models. A, B: ELISA results of TNF‑α and IL-1β in the intestinal mucosa of the mice. C, D: TNF-α and IL-1β mRNA expression in the intestinal mucosa of the mice detected by RT-qPCR. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
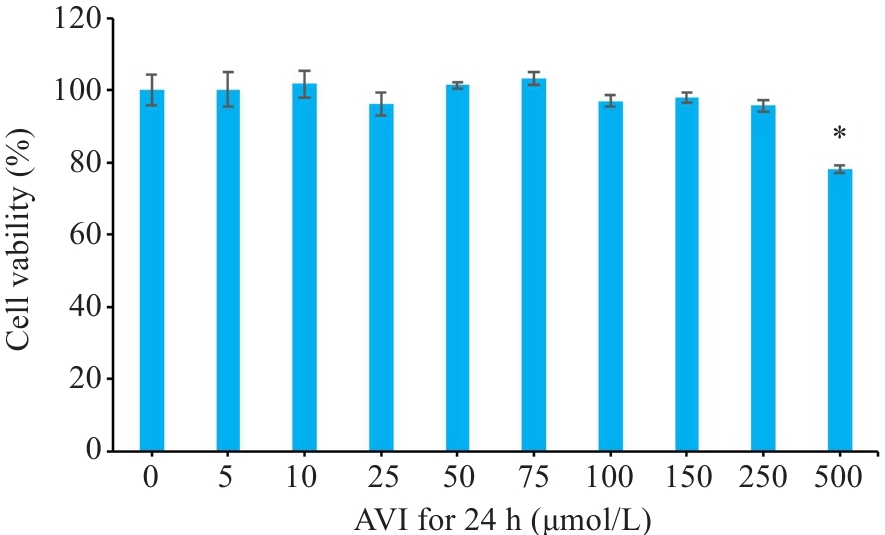
图4 不同浓度的AVI作用24 h对Caco-2细胞活力的影响
Fig.4 Changes in viability of Caco-2 cells after treatment with different concentrations of AVI for 24 h. *P<0.05 vs 0 μmol/L.
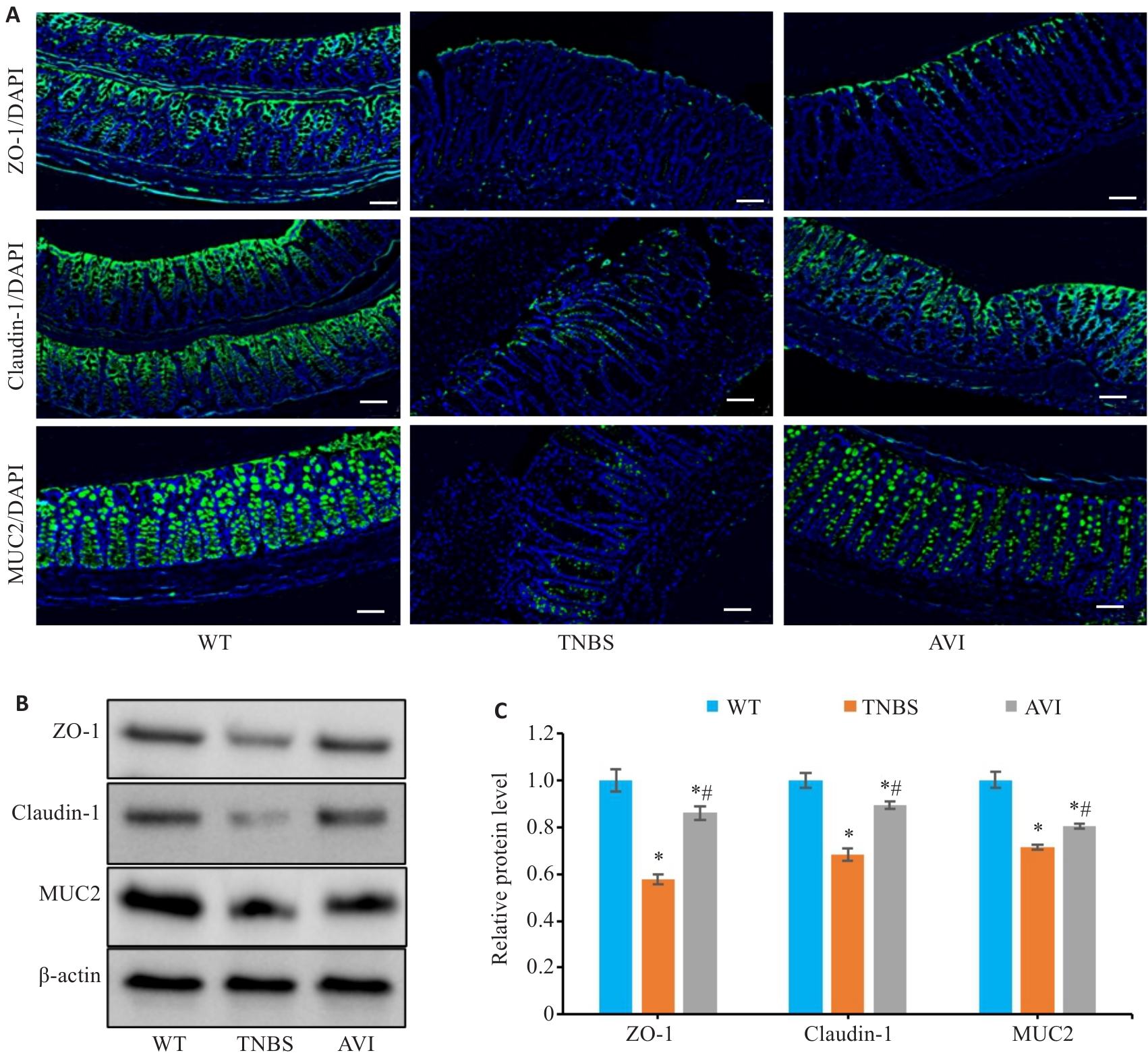
图5 AVI干预对TNBS模型小鼠结肠炎肠屏障的影响
Fig.5 Effect of AVI on intestinal barrier function in mice with TNBS-induced colitis. A: Immunofluorescence staining for detecting ZO-1, claudin-1 and MUC2 in the mouse colon (Scale bar=50 μm). B, C: Relative expression levels of ZO-1, Claudin-1 and MUC2 proteins in intestinal mucosa detected by Western blotting. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
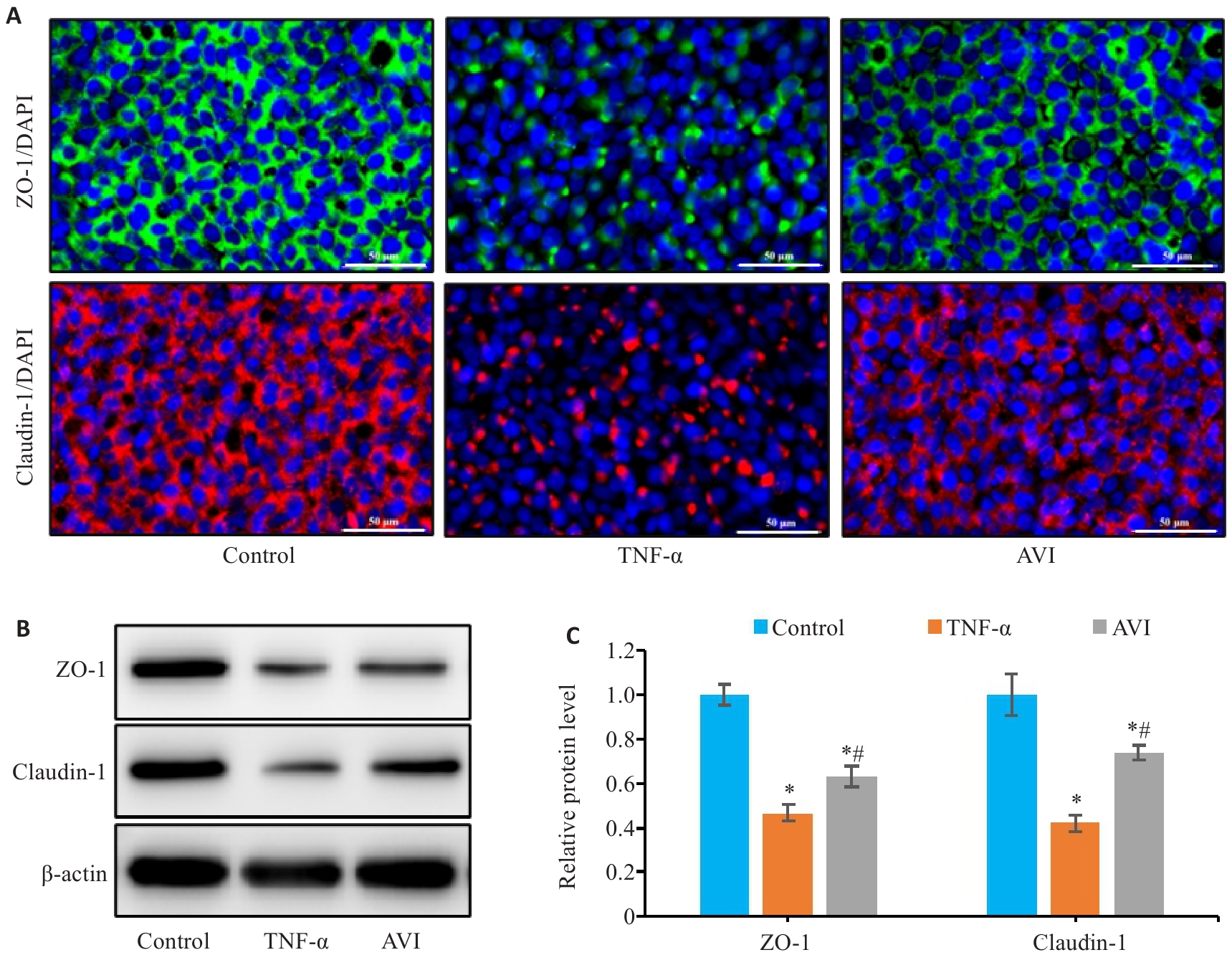
图6 AVI干预对TNF-α诱导的Caco-2细胞屏障损伤的影响
Fig.6 Effect of AVI on the barrier damage of Caco-2 cells induced by TNF-α. A: Immunofluorescence staining for detecting expressions of ZO-1 and claudin-1 in Caco-2 cells (Scale bar=50 μm). B, C: Relative expression levels of ZO-1 and claudin-1 proteins in Caco-2 cells detected by Western blotting. *P<0.05 vs Control group. #P<0.05 vs TNF-α group.
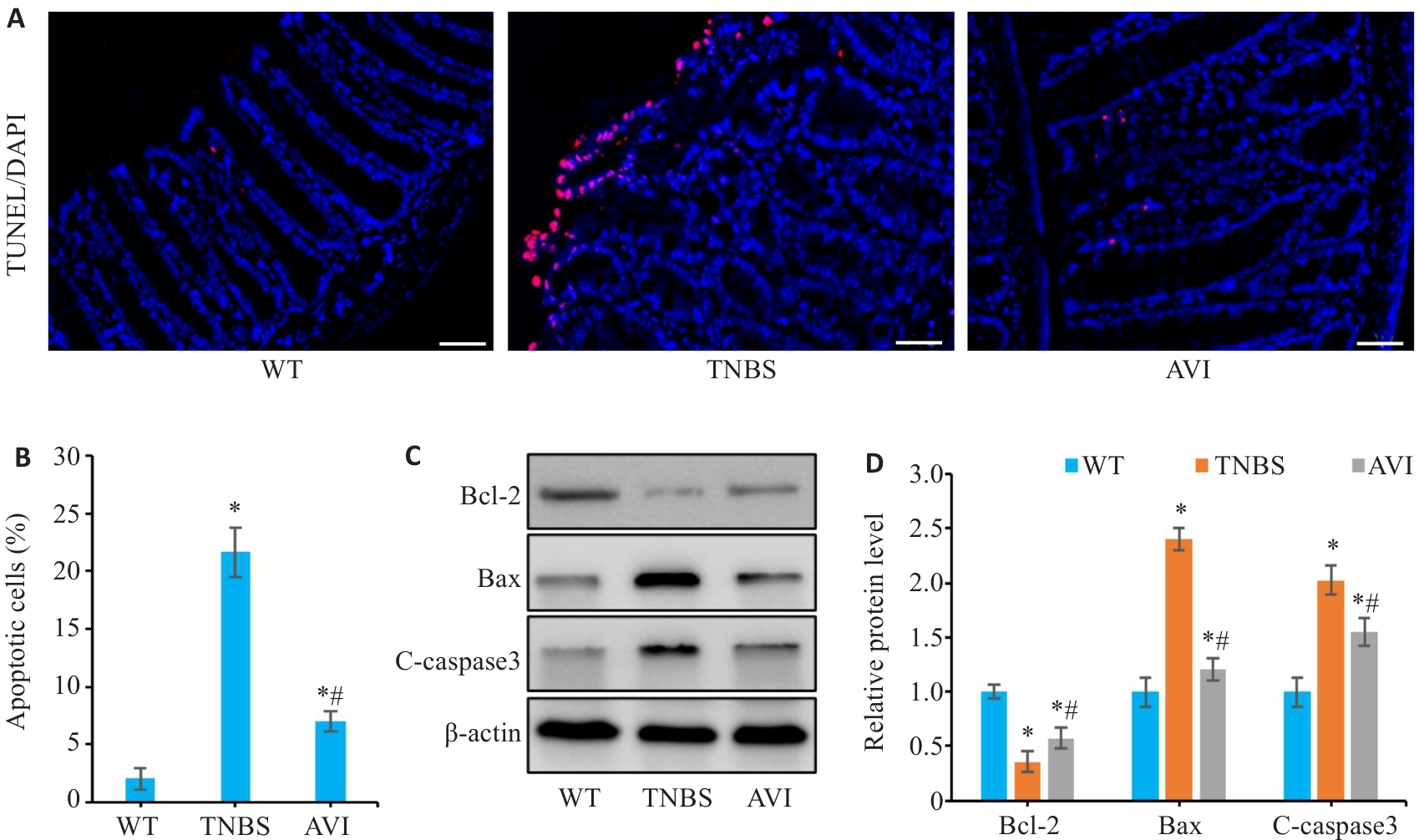
图7 AVI干预对TNBS模型小鼠肠上皮细胞凋亡的影响
Fig.7 Effect of AVI on intestinal epithelial cell apoptosis in mice with TNBS-induced colitis. A: TUNEL staining of the colon tissue. B: Apoptosis rate of the intestinal epithelial cells (Scale bar=50 μm). C, D: Western blotting for detecting relative expression levels of Bcl-2, Bax and C-caspase3 in colonic mucosa. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
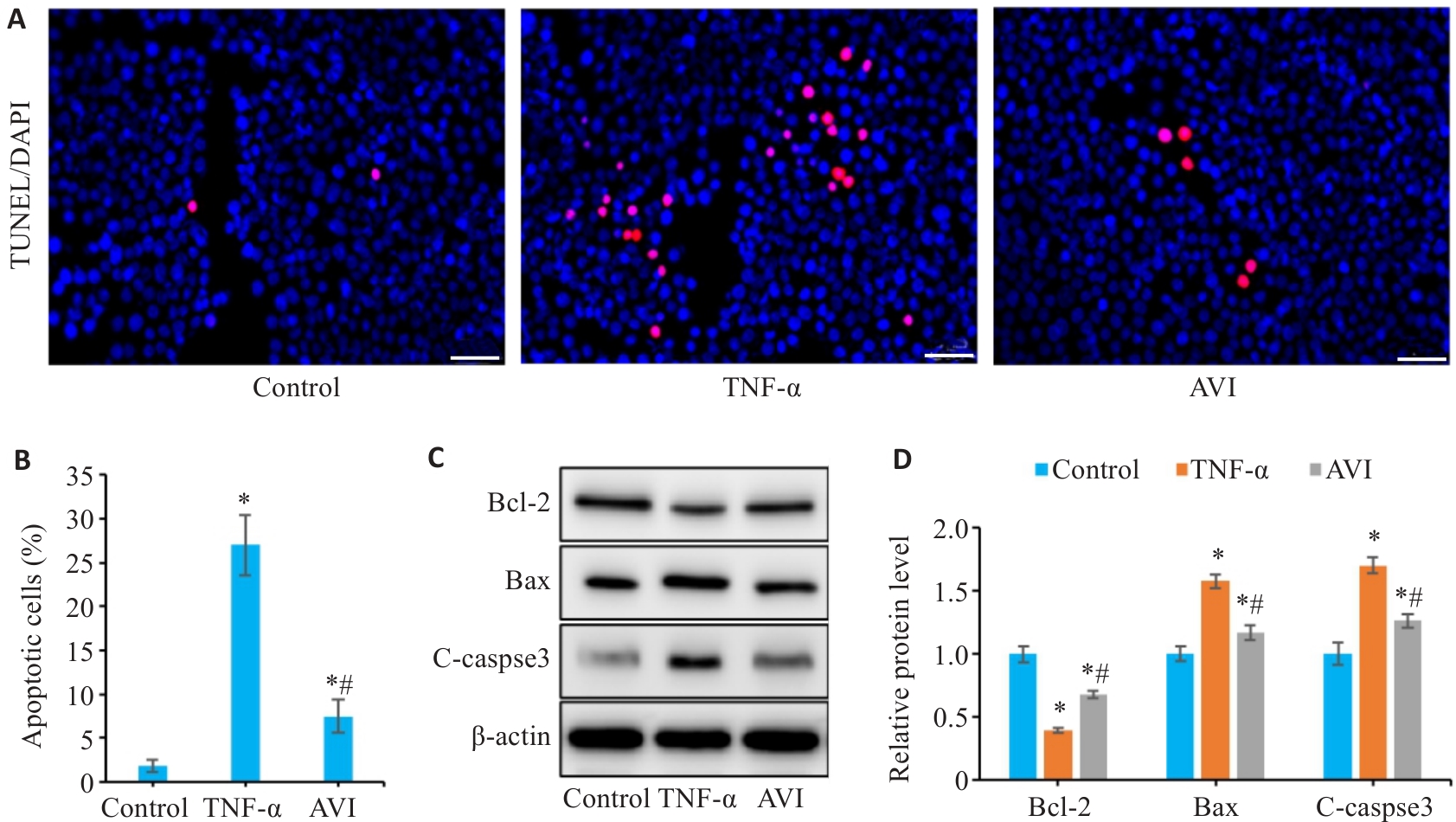
图8 AVI干预对TNF-α诱导的凋亡模型中Caco-2细胞凋亡的影响
Fig.8 Effect of AVI on apoptosis in TNF-α-induced Caco-2 cells. A: TUNEL staining of Caco-2 cells (Scale bar=50 μm). B: Apoptosis rate of Caco-2 cells. C, D: Relative expression levels of Bcl-2, Bax and C-caspase-3 in Caco-2 cells detected by Western blotting.*P<0.05 vs Control group. #P<0.05 vs TNF-α group.
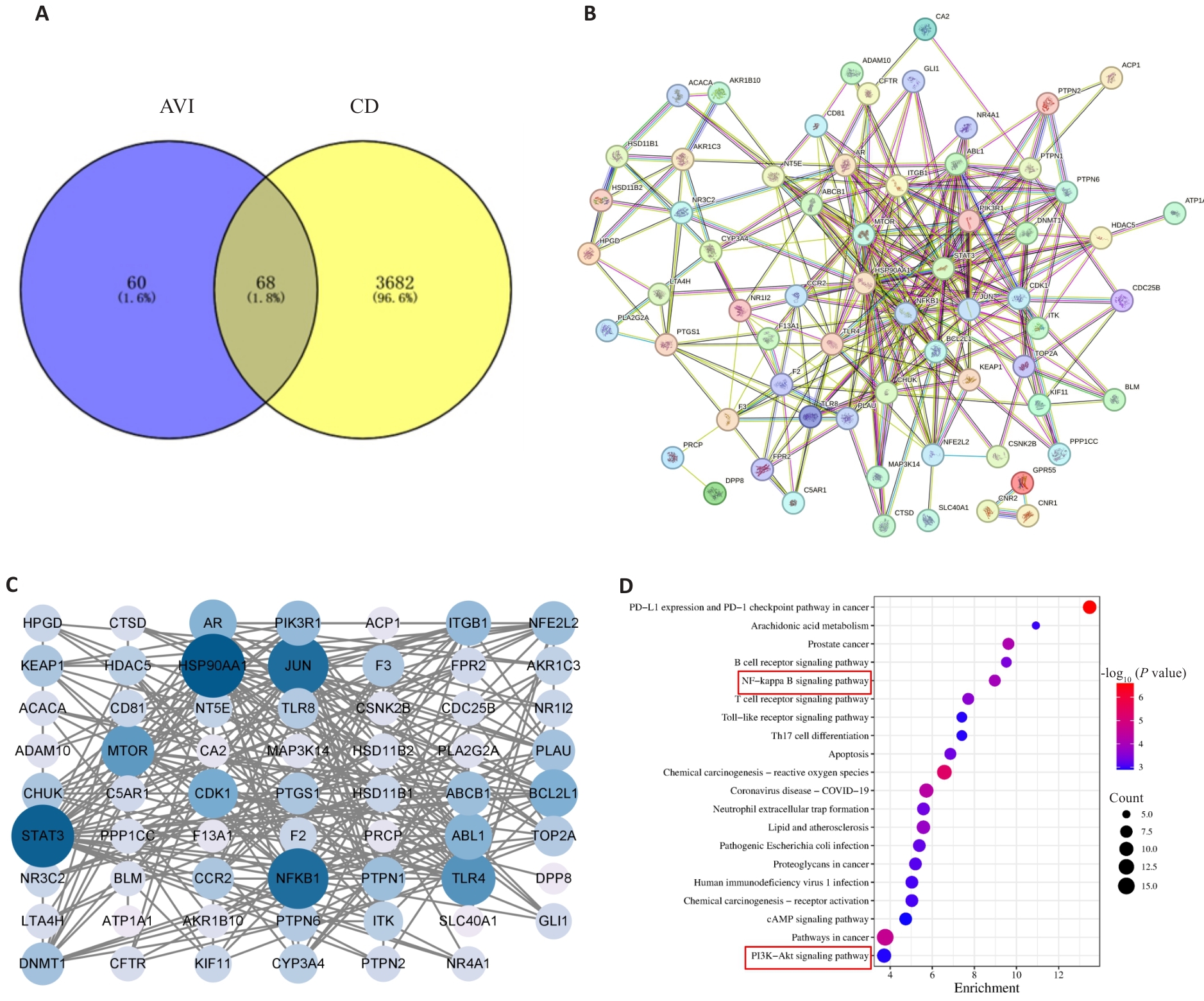
图9 网络药理学分析结果
Fig.9 Network pharmacology analysis results. A: Venn diagram of the intersection between CD genes and AVI genes. B, C: PPI network diagram. D: Results of KEGG pathway enrichment analysis for the intersection genes between AVI and CD.
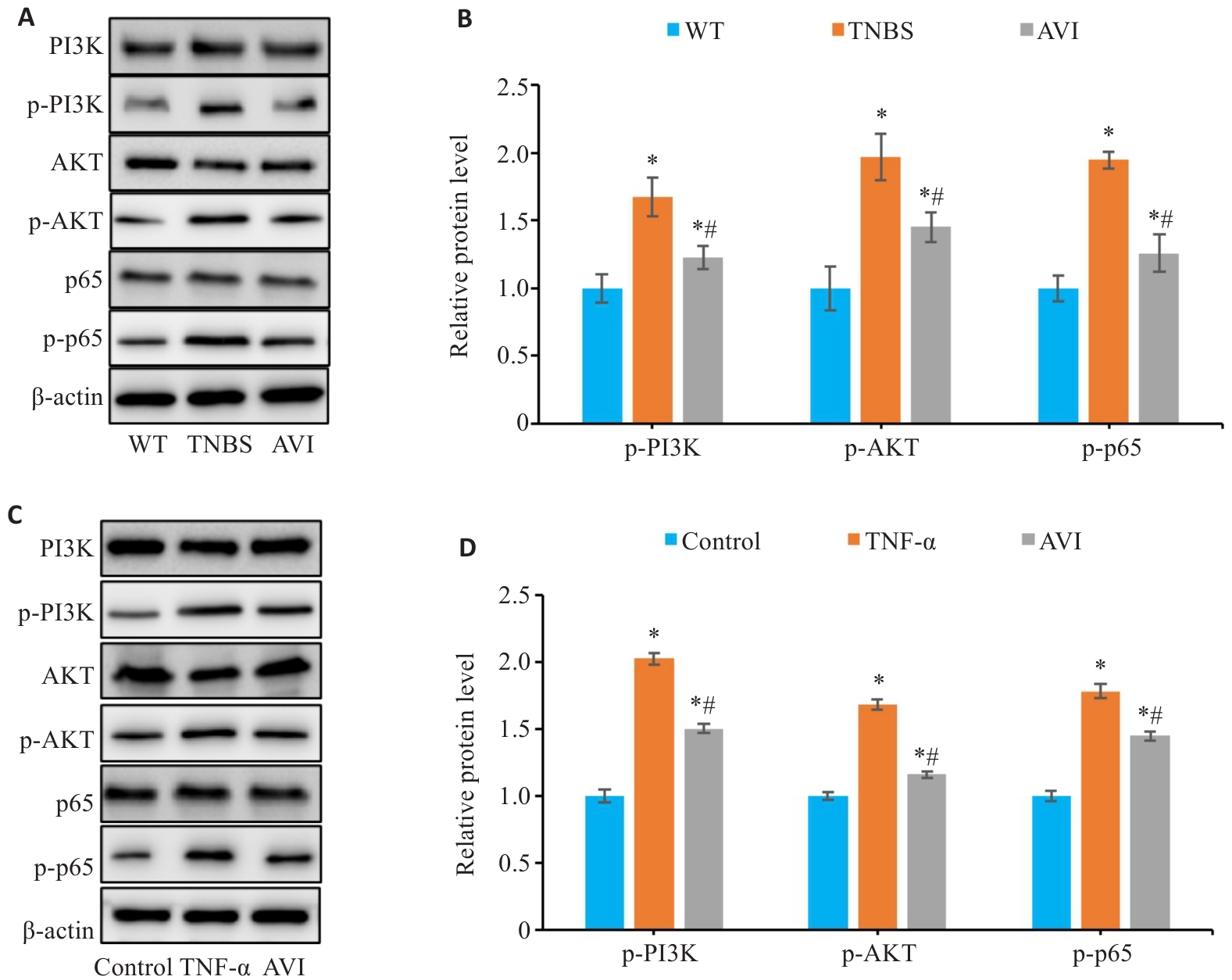
图10 AVI干预对体内和体外PI3K/AKT/NF-κB通路的影响
Fig.10 Effect of AVI on the PI3K/AKT/NF-κB pathway in the mouse models and in Caco-2 cells. A, B: Relative expression levels of PI3K, p-PI3K, AKT, p-AKT, p65 and p-p65 proteins in mouse colon tissue detected by Western blotting (*P<0.05 vs WT group; #P<0.05 vs TNBS group). C, D: Relative expression levels of PI3K, p-PI3K, AKT, p-AKT, p65 and p-p65 proteins in Caco-2 cells detected by Western blotting (*P<0.05 vs Control group. #P<0.05 vs TNF-α group).
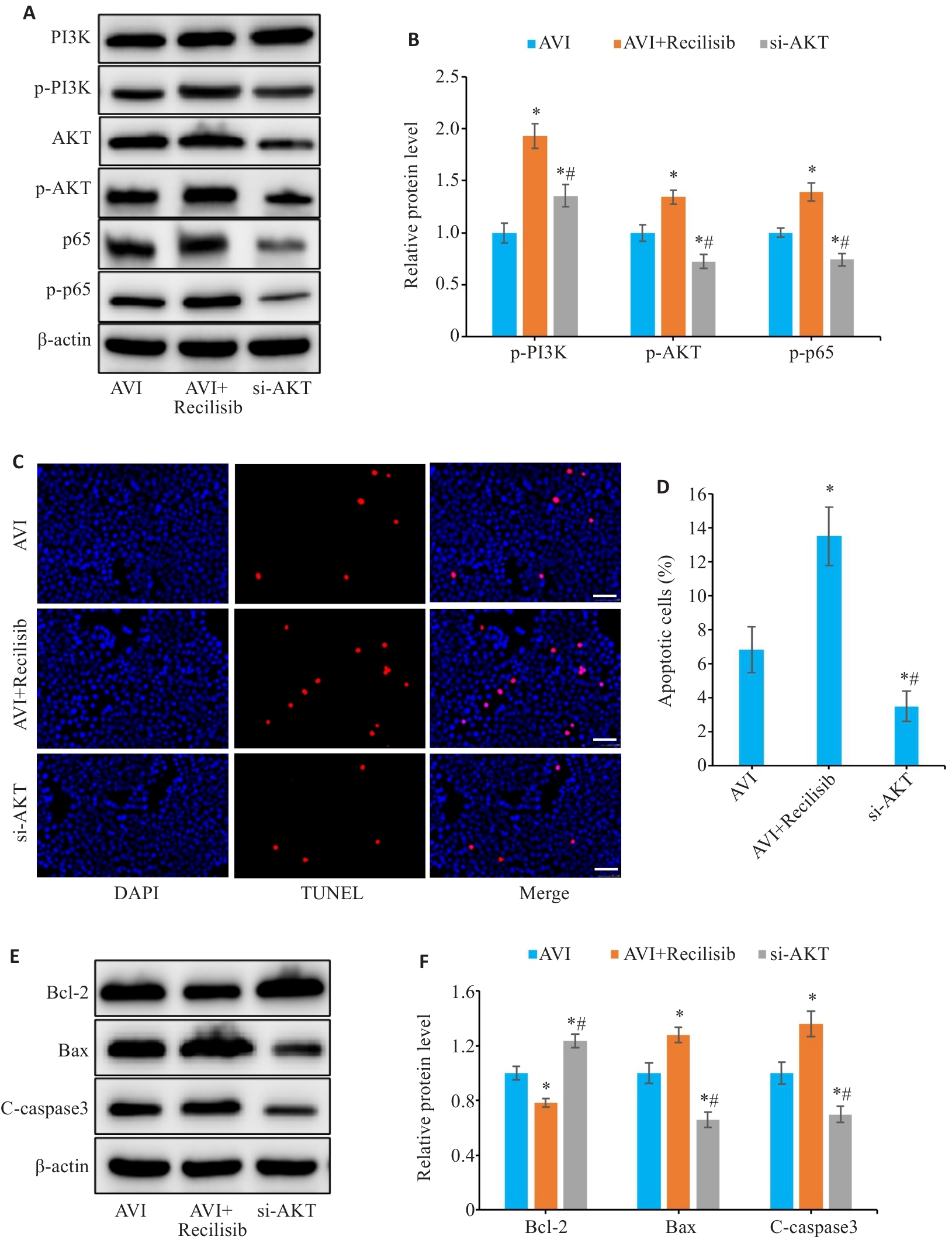
图11 Recilisib对AVI治疗肠上皮细胞凋亡及AKT1 siRNA对体外凋亡模型的影响
Fig.11 Effect of Recilisib and AKT1 siRNA on apoptosis of AVI-treated Caco-2 cells. A, B: Relative expression levels of PI3K, p-PI3K, AKT, p-AKT, p65 and p-p65 proteins detected by Western blotting. C: TUNEL staining of Caco-2 cells (Scale bar=50 μm). D: Apoptosis rate of Caco-2 cells. E, F: Relative expression levels of Bcl-2, Bax, and C-caspase3 proteins detected by Western blotting. *P<0.05 vs AVI group, #P<0.05 vs Recilisib group.
| 1 | Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management[J]. Mayo Clin Proc, 2017, 92(7): 1088-103. |
| 2 | Torres J, Mehandru S, Colombel JF, et al. Crohn's disease[J]. Lancet, 2017, 389(10080): 1741-55. |
| 3 | Keyashian K, Dehghan M, Sceats L, et al. Comparative incidence of inflammatory bowel disease in different age groups in the United States[J]. Inflamm Bowel Dis, 2019, 25(12): 1983-9. |
| 4 | Hutfless S, Jasper RA, Chen PH, et al. Burden of Crohn's disease in the United States Medicaid population, 2010-2019[J]. Clin Gastroenterol Hepatol, 2024, 22(5): 1087-97.e6. |
| 5 | Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management[J]. J Crohns Colitis, 2017, 11(1): 3-25. |
| 6 | Lin K, Zheng WY, Guo MY, et al. The intestinal microbial metabolite acetyl l-carnitine improves gut inflammation and immune homeostasis via CADM2[J]. Biochim Biophys Acta Mol Basis Dis, 2024, 1870(4): 167089. |
| 7 | Vesci L, Tundo G, Soldi S, et al. A novel Lactobacillus brevis fermented with a vegetable substrate (AL0035) counteracts TNBS-induced colitis by modulating the gut microbiota composition and intestinal barrier[J]. Nutrients, 2024, 16(7): 937. |
| 8 | Kuo WT, Shen L, Zuo L, et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression[J]. Gastroenterology, 2019, 157(5): 1323-37. |
| 9 | Petagna L, Antonelli A, Ganini C, et al. Pathophysiology of Crohn's disease inflammation and recurrence[J]. Biol Direct, 2020, 15(1): 23. |
| 10 | Zhang J, Cen L, Zhang XF, et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT[J]. Redox Biol, 2022, 56: 102469. |
| 11 | Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment[J]. J Crohns Colitis, 2020, 14(1): 4-22. |
| 12 | Yuan S, Wang Q, Li J, et al. Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment[J]. Chin Med, 2022, 17(1): 74. |
| 13 | Li CM, Tian JW, Li GS, et al. Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways[J]. Eur J Pharmacol, 2010, 649(1/2/3): 100-7. |
| 14 | Jiang X, Yi SN, Liu Q, et al. Asperosaponin VI ameliorates the CMS-induced depressive-like behaviors by inducing a neuroprotective microglial phenotype in hippocampus via PPAR‑γ pathway[J]. J Neuroinflammation, 2022, 19(1): 115. |
| 15 | Wei LL, Luo H, Jin Y, et al. Asperosaponin VI protects alcohol-induced hepatic steatosis and injury via regulating lipid metabolism and ER stress[J]. Phytomedicine, 2023, 121: 155080. |
| 16 | Xuan LL, Yang S, Ren LL, et al. Akebia saponin D attenuates allergic airway inflammation through AMPK activation[J]. J Nat Med, 2024, 78(2): 393-402. |
| 17 | Couto M, Andrade N, Magro F, et al. Taurocholate uptake by Caco-2 cells is inhibited by pro-inflammatory cytokines and butyrate[J]. Cytokine, 2023, 169: 156307. |
| 18 | Lu JJ, Shi XJ, Fu Q, et al. New mechanistic understanding of osteoclast differentiation and bone resorption mediated by P2X7 receptors and PI3K-Akt-GSK3β signaling[J]. Cell Mol Biol Lett, 2024, 29(1): 100. |
| 19 | Wang ZY, Chen JY, Babicheva A, et al. Endothelial upregulation of mechanosensitive channel Piezo1 in pulmonary hypertension[J]. Am J Physiol Cell Physiol, 2021, 321(6): C1010-C1027. |
| 20 | George B, Gui B, Raguraman R, et al. AKT1 transcriptomic landscape in breast cancer cells[J]. Cells, 2022, 11(15): 2290. |
| 21 | Dai YX, Lu QL, Li PY, et al. Xianglian Pill attenuates ulcerative colitis through TLR4/MyD88/NF‑κB signaling pathway[J]. J Ethnopharmacol, 2023, 300: 115690. |
| 22 | Li CL, Liu MG, Deng L, et al. Oxyberberine ameliorates TNBS-induced colitis in rats through suppressing inflammation and oxidative stress via Keap1/Nrf2/NF‑κB signaling pathways[J]. Phytomedicine, 2023, 116: 154899. |
| 23 | Dolinger M, Torres J, Vermeire S. Crohn's disease[J]. Lancet, 2024, 403(10432): 1177-91. |
| 24 | Zhang ZN, Zuo LG, Song X, et al. Arjunolic acid protects the intestinal epithelial barrier, ameliorating Crohn's disease-like colitis by restoring gut microbiota composition and inactivating TLR4 signalling[J]. Phytomedicine, 2024, 123: 155223. |
| 25 | 苏 超, 包 阔, 李佳威, 等. 人参皂苷衍生物AD-1通过抑制NLRP3炎症小体活化改善TNBS诱导的小鼠急性IBD[J]. 中国免疫学杂志, 2023, 39(11): 2305-10, 2317. |
| 26 | Yang XR, Liang J, Shu Y, et al. Asperosaponin VI facilitates the regeneration of skeletal muscle injury by suppressing GSK-3β-mediated cell apoptosis[J]. J Cell Biochem, 2024, 125(1): 115-26. |
| 27 | Jang DI, Lee AH, Shin HY, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics[J]. Int J Mol Sci, 2021, 22(5): 2719. |
| 28 | Lenti MV, Santacroce G, Broglio G, et al. Recent advances in intestinal fibrosis[J]. Mol Aspects Med, 2024, 96: 101251. |
| 29 | Kim JT, Napier DL, Kim J, et al. Ketogenesis alleviates TNF‑α-induced apoptosis and inflammatory responses in intestinal cells[J]. Free Radic Biol Med, 2021, 172: 90-100. |
| 30 | Liu LQ, Yan MJ, Yang R, et al. Adiponectin attenuates lipopolysaccharide-induced apoptosis by regulating the Cx43/PI3K/AKT pathway[J]. Front Pharmacol, 2021, 12: 644225. |
| 31 | Lin CY, Tsai PH, Kandaswami CC, et al. Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells[J]. Mol Cancer, 2011, 10: 87. |
| 32 | Chalabi-Dchar M, Cassant-Sourdy S, Duluc C, et al. Loss of somatostatin receptor subtype 2 promotes growth of KRAS-induced pancreatic tumors in mice by activating PI3K signaling and overexpression of CXCL16[J]. Gastroenterology, 2015, 148(7): 1452-65. |
| 33 | Guo Q, Jin YZ, Chen XY, et al. NF‑κB in biology and targeted therapy: new insights and translational implications[J]. Signal Transduct Target Ther, 2024, 9(1): 53. |
| 34 | 刘兴隆, 张培旭, 熊珮宇, 等. 基于PI3K/Akt/NF-κB通路探讨人参败毒散、榆瑞灌肠液内外合治干预溃疡性结肠炎大鼠肠黏膜损伤的作用机制[J]. 中国实验方剂学杂志, 2023, 29(19): 42-51. |
| 35 | 李 多, 彭 昭, 张泽天, 等. 复方黄柏液通过PI3K/Akt/NF-κB信号通路逆转大鼠溃疡性结肠炎[J]. 中国老年学杂志, 2023, 43(9): 2241-4. |
| [1] | 刘硕, 李静, 吴兴旺. Swertiamarin通过抑制肠上皮细胞细胞凋亡改善TNBS诱导的实验性结肠炎[J]. 南方医科大学学报, 2024, 44(8): 1545-1552. |
| [2] | 从小凡, 陈腾, 李硕, 王媛媛, 周龙云, 李小龙, 张配, 孙小锦, 赵素容. 双氢青蒿素通过促进活性氧的产生增强鼻咽癌细胞对顺铂诱导凋亡的敏感性[J]. 南方医科大学学报, 2024, 44(8): 1553-1560. |
| [3] | 肖林雨, 段婷, 夏勇生, 陈悦, 孙洋, 许轶博, 徐磊, 闫兴洲, 胡建国. 蒙花苷通过抑制TLR4/NF-κB通路抑制小鼠脊髓损伤后小胶质细胞活化介导的神经炎症和神经元凋亡[J]. 南方医科大学学报, 2024, 44(8): 1589-1598. |
| [4] | 陶怀祥, 骆金光, 闻志远, 虞亘明, 苏萧, 王鑫玮, 关翰, 陈志军. STING高表达通过调控TLR4/NF-κB/NLRP3通路和影响炎症与凋亡水平促进小鼠肾脏缺血再灌注损伤[J]. 南方医科大学学报, 2024, 44(7): 1345-1354. |
| [5] | 郑孟冬, 刘妍, 刘娇娇, 康巧珍, 王婷. 蛋白4.1R对肝细胞HL-7702增殖、凋亡以及糖酵解的影响[J]. 南方医科大学学报, 2024, 44(7): 1355-1360. |
| [6] | 王元国, 张鹏. 铁死亡抑制基因在食管癌中的高表达分析[J]. 南方医科大学学报, 2024, 44(7): 1389-1396. |
| [7] | 任志军, 刁建新, 王奕婷. 芎归汤通过抑制氧化应激诱导的心肌凋亡减轻小鼠心梗后心衰引起的心肌损伤[J]. 南方医科大学学报, 2024, 44(7): 1416-1424. |
| [8] | 陈桂玲, 廖晓凤, 孙鹏涛, 岑欢, 舒盛春, 李碧晶, 黎金华. 澳洲茄碱通过调控Bcl-2/Bax/caspase-3信号通路促进非小细胞肺癌发生凋亡[J]. 南方医科大学学报, 2024, 44(6): 1109-1116. |
| [9] | 鲁玲君, 杨小迪, 张华平, 梁媛, 石秀兰, 周鑫. 重组日本血吸虫半胱氨酸蛋白酶抑制剂对急性肝损伤小鼠的保护作用及机制[J]. 南方医科大学学报, 2024, 44(6): 1126-1134. |
| [10] | 杨泽, 张秀森, 张旭东, 柳颖, 张嘉诚, 原翔. 基于YTHDF2介导凋亡相关因子降解途径研究牙龈卟啉单胞菌协助食管癌免疫逃逸[J]. 南方医科大学学报, 2024, 44(6): 1159-1165. |
| [11] | 梁国新, 唐红悦, 郭畅, 张明明. miR-224-5p调控PI3K/Akt/FoxO1轴抑制氧化应激减轻缺氧/复氧诱导的心肌细胞损伤[J]. 南方医科大学学报, 2024, 44(6): 1173-1181. |
| [12] | 席 进, 张 敏, 张永玉, 张 晨, 张雨路, 王 锐, 申 林, 李 静, 宋 雪. 上调KLF11可改善结肠炎模型小鼠的肠道炎症:基于抑制JAK2/STAT3信号通路[J]. 南方医科大学学报, 2024, 44(4): 765-772. |
| [13] | 张笑颜, 王 谢, 王 杰, 邵 楠, 蔡 标, 谢道俊. 黄蒲通窍胶囊改善Wilson病铜负荷大鼠的认知损害:基于抑制内质网应激介导的凋亡途径[J]. 南方医科大学学报, 2024, 44(3): 447-454. |
| [14] | 陈国栋, 罗素新. 秋水仙碱通过激活AMPK减轻小鼠心肌缺血再灌注损伤[J]. 南方医科大学学报, 2024, 44(2): 226-235. |
| [15] | 吴广阳, 宋添力, 唐 浪, 王一民, 刘 绪, 黄 胜. 竹节参总皂苷缓解CCl4诱导的大鼠急性肝损伤:基于调控PI3K/Akt/NF-κB信号通路[J]. 南方医科大学学报, 2024, 44(2): 244-251. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||