南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (11): 2082-2091.doi: 10.12122/j.issn.1673-4254.2024.11.04
• • 上一篇
刘冬播1( ), 陈泽文2, 王云2, 李昕鹏2, 赵彭玉2, 郑浩贤2
), 陈泽文2, 王云2, 李昕鹏2, 赵彭玉2, 郑浩贤2
收稿日期:2024-08-07
出版日期:2024-11-20
发布日期:2024-11-29
通讯作者:
刘冬播
E-mail:liudongbo@bbmu.edu.cn
作者简介:刘冬播,副教授,E-mail: liudongbo@bbmu.edu.cn
基金资助:
Dongbo LIU1( ), Zewen CHEN2, Yun WANG2, Xinpeng LI2, Pengyu ZHAO2, Haoxian ZHENG2
), Zewen CHEN2, Yun WANG2, Xinpeng LI2, Pengyu ZHAO2, Haoxian ZHENG2
Received:2024-08-07
Online:2024-11-20
Published:2024-11-29
Contact:
Dongbo LIU
E-mail:liudongbo@bbmu.edu.cn
摘要:
目的 探讨由慢性不可预知温和应激(CUMS)引起的抑郁症小鼠神经网络的可塑性变化与空间学习记忆调控之间的关系。 方法 采用随机数字表法将C57Thy1-YFP/GAD67-GFP小鼠分为对照组和CUMS组(n=15),对照组不做处理,CUMS组施加8周的应激以建立模型。利用糖水偏好实验、旷场实验和强迫游泳实验评估小鼠的抑郁水平,通过Morris水迷宫实验检测小鼠空间学习记忆能力的变化,采用膜片钳全细胞模式检测中央杏仁核(CeA)与边缘前皮质(PrL)脑区中不同亚群神经元动作电位发放模式的改变。 结果 与对照组相比,CUMS组小鼠的糖水偏好指数降低(P<0.01),其在旷场实验中的总运动距离、穿越格子数、进入中心区域次数、中心区域停留时间等各项指标均下降(P<0.01),在强迫游泳实验中的挣扎时间、游泳时间和爬行时间均降低(P<0.01),而静止时间增高(P<0.01),在定位实验中定位潜伏期和路径长度均增加(P<0.05),在空间探索实验中目标象限内路程占总路程百分比和游泳时间均降低(P<0.05),而目标区首次入口时间和对角象限内游泳时间均增长(P<0.01)。同时CUMS提高了CeA区谷氨酸能神经元和PrL区GABA能神经元的能障、绝对不应期和动作电位间距(P<0.05),降低了CeA区GABA能神经元和PrL区谷氨酸能神经元的能障、绝对不应期和动作电位间距(P<0.05)。 结论 CUMS诱导的抑郁状态可能引起CeA和PrL区中兴奋性和抑制性神经元网络的可塑性变化,进而损伤了空间学习记忆能力。
刘冬播, 陈泽文, 王云, 李昕鹏, 赵彭玉, 郑浩贤. 慢性不可预知温和应激诱导的抑郁小鼠中央杏仁核和边缘前皮质神经网络的可塑性变化[J]. 南方医科大学学报, 2024, 44(11): 2082-2091.
Dongbo LIU, Zewen CHEN, Yun WANG, Xinpeng LI, Pengyu ZHAO, Haoxian ZHENG. Neuronal plasticity changes in the central amygdala and prelimbic cortex network in mice with chronic unpredictable mild stress-induced depression[J]. Journal of Southern Medical University, 2024, 44(11): 2082-2091.
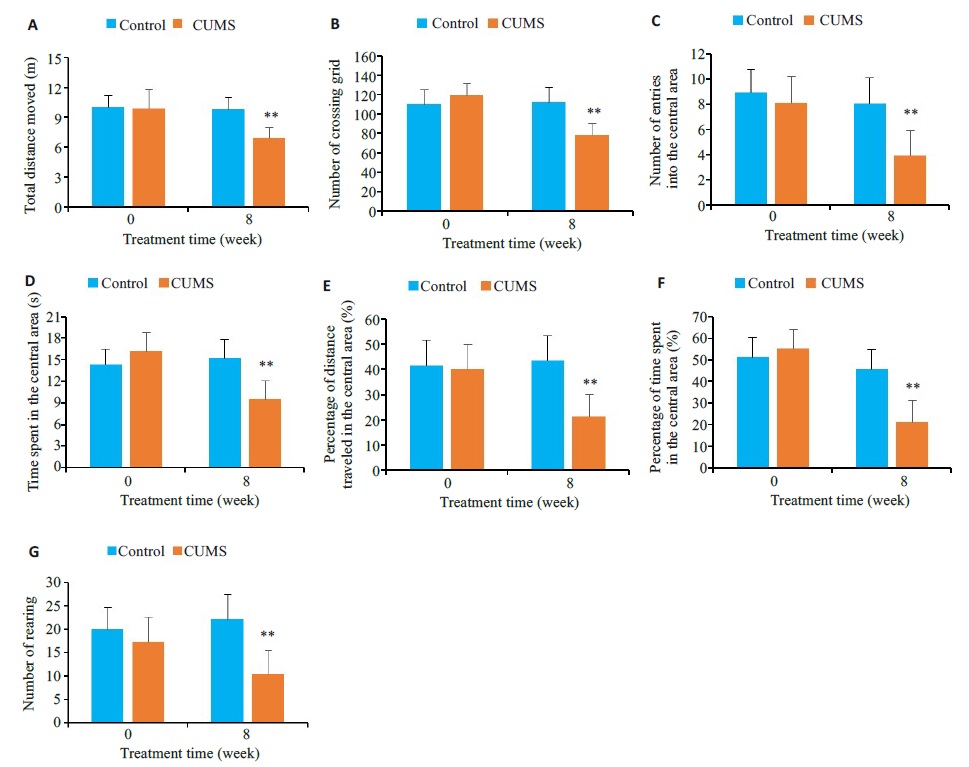
图2 CUMS对小鼠旷场活动行为的影响
Fig.2 Effects of CUMS performance of the mice in open field test.A: Total distance moved.B: Number of crossing grid.C: Number of entries into the central area.D: Time spent in the central area.EPercentage of distance traveled in the central area.F Percentage of time spent in the central area. G Number of rearing. **P<0.01 vs control group.
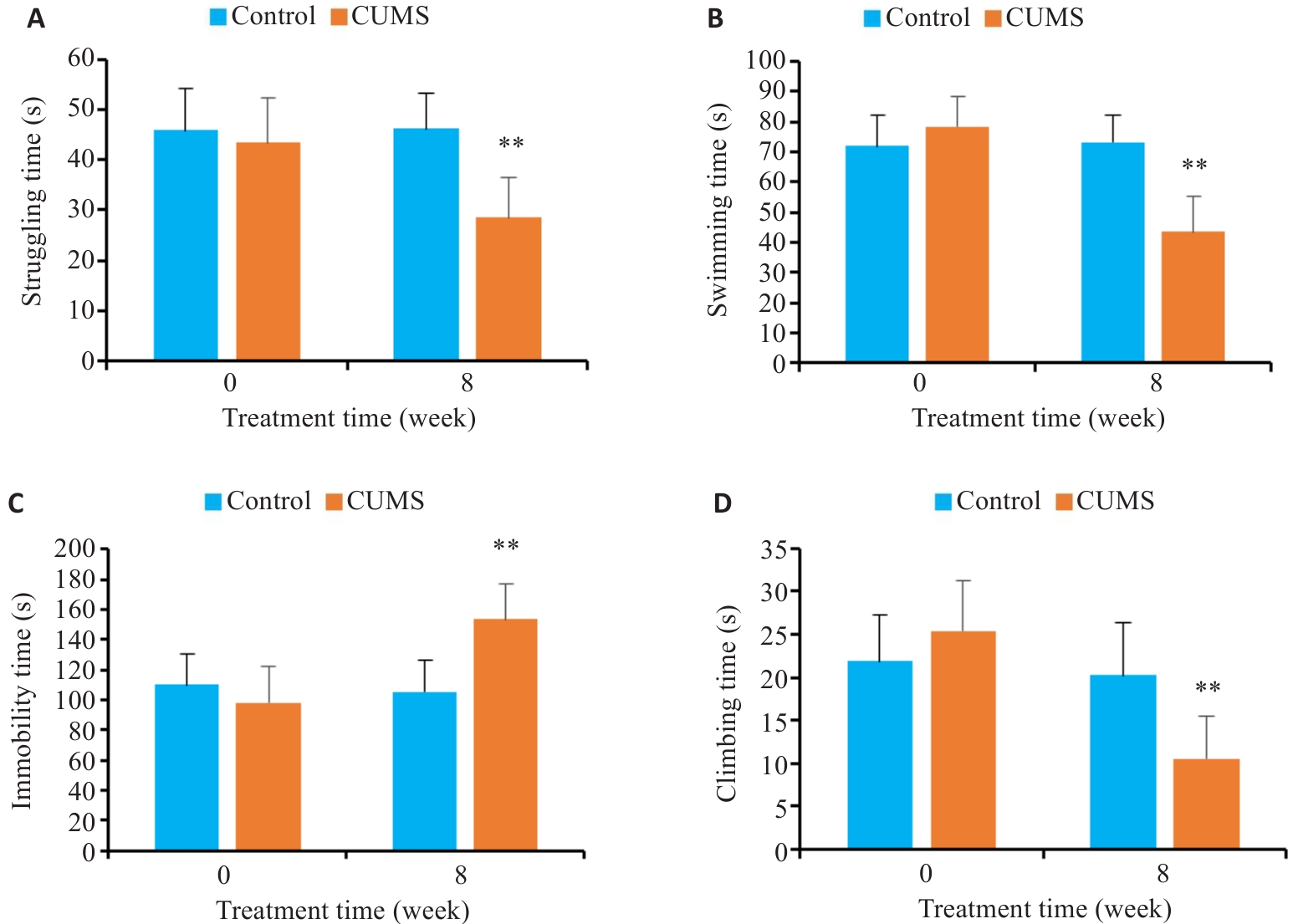
图3 CUMS对小鼠强迫游泳行为的影响
Fig.3 Effects of CUMS on performance of the mice in forced swimming test. A: Struggling time. B: Swimming time. C: Immobility time. D: Climbing time. **P<0.01 vs control group.
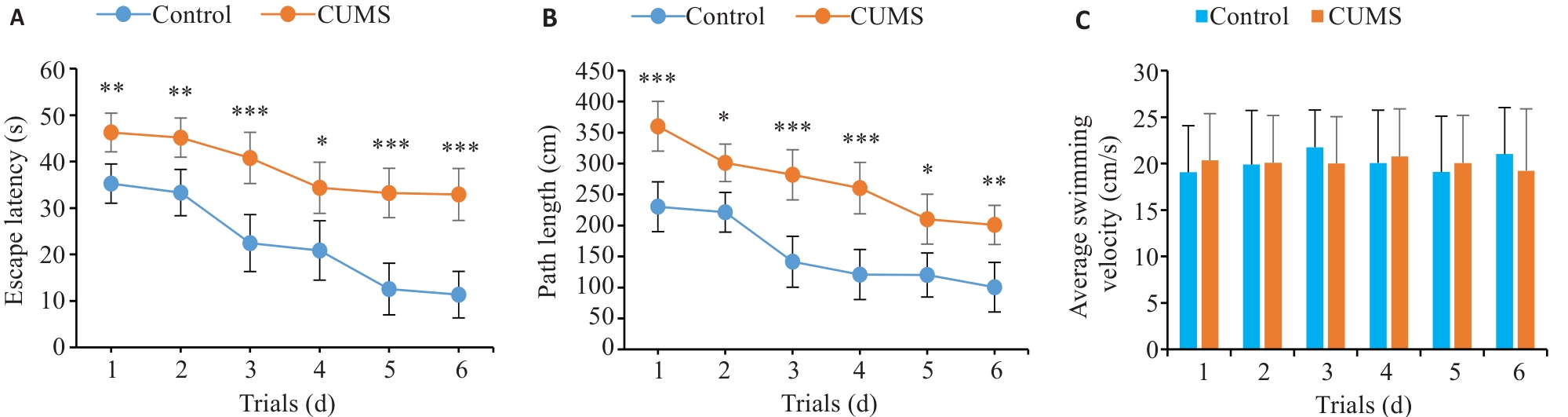
图4 CUMS组和对照组小鼠在Morris水迷宫定位实验中的表现
Fig.4 Performance of CUMS and control mice for localization in Morris water maze test. A: Escape latency. B: Path length. C: Average swimming velocity. *P<0.05, **P<0.01, ***P<0.001 vs control group.
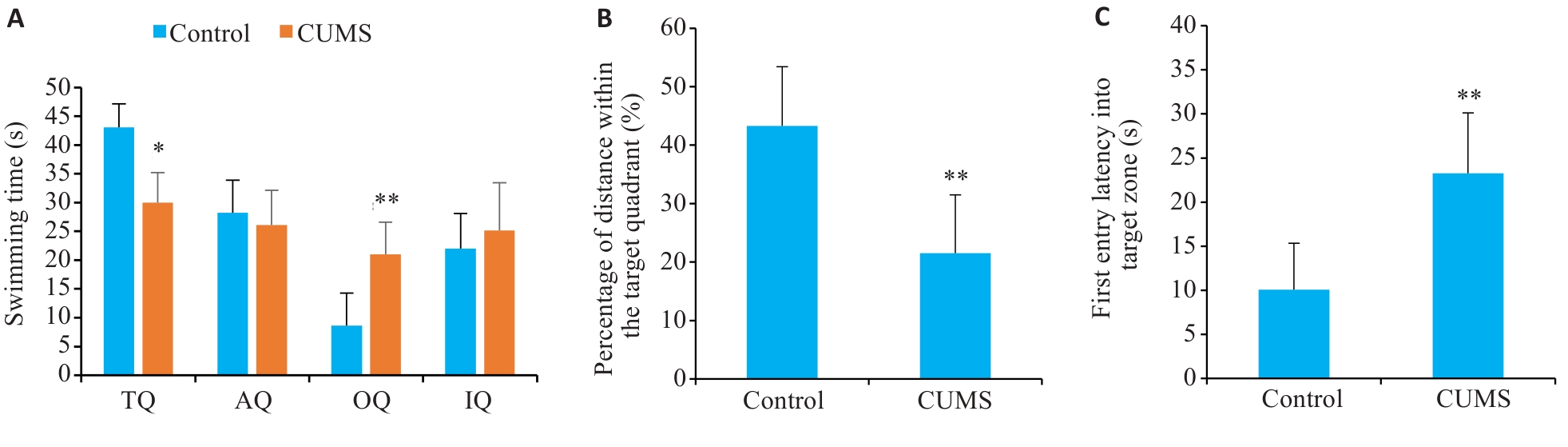
图5 CUMS组和对照组小鼠在Morris水迷宫空间探索实验中的表现
Fig.5 Performance of CUMS and control mice for spatial exploration in Morris water maze test. A: Swimming time. B: Percentage of distance within the target quadrant. C: First entry latency into target zone. TQ: Target quadrant. AQ: Adjacent quadrant. OQ: Opposite quadrant. IQ: Irrelevant quadrant. *P<0.05, **P<0.01 vs control group.
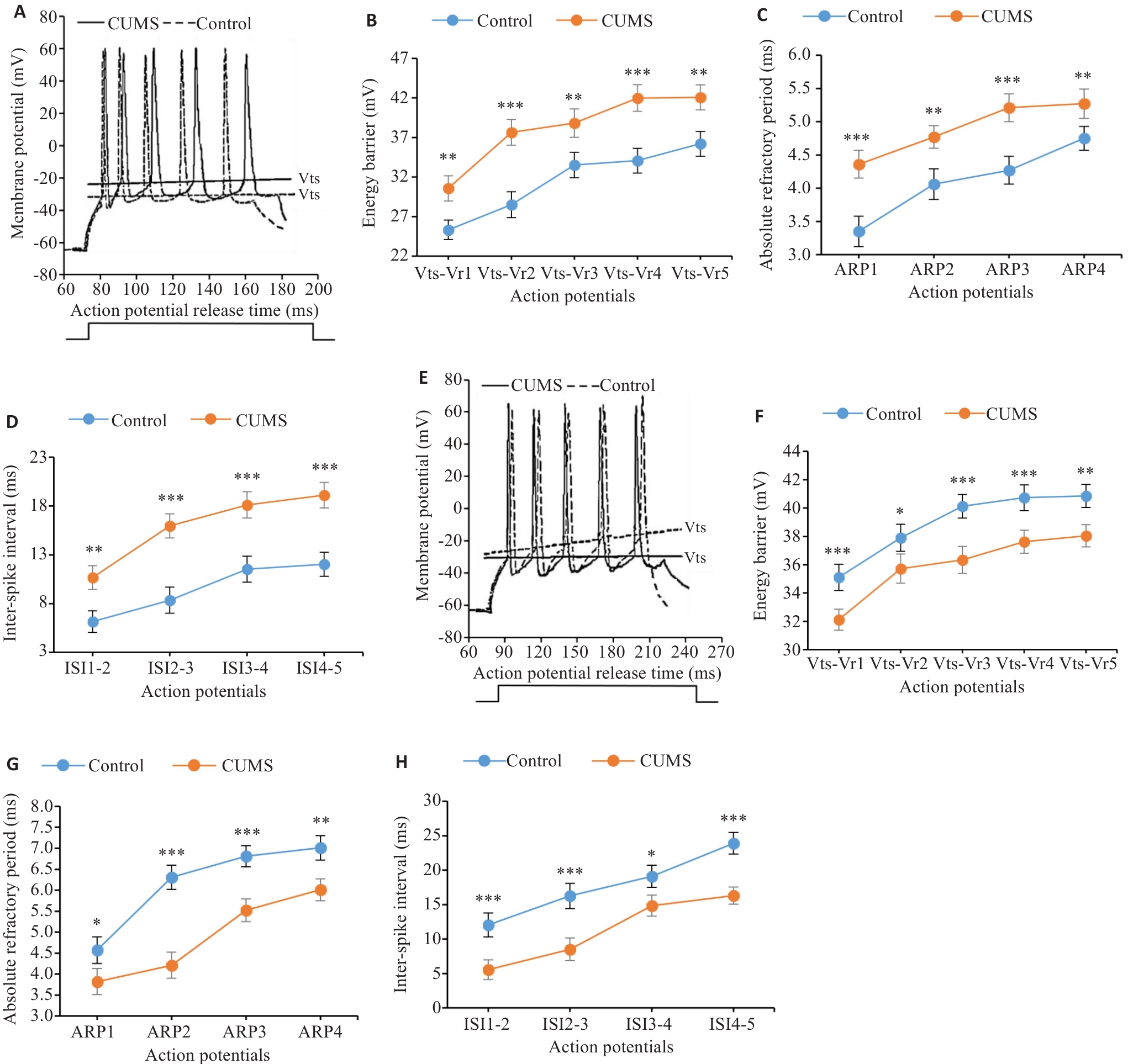
图6 CUMS对小鼠CeA区谷氨酸能和GABA能神经元动作电位发放模式的改变
Fig.6 Changes of action potential firing patterns of glutamatergic and GABAergic neurons in CeA region of CUMS mice. A: Waveforms of sequential action potentials of glutamatergic neurons in CUMS mice (solid line) and control mice (dotted line). B-D: Effects of CUMS on electrophysiological properties of glutamatergic neurons. E: Waveforms of sequential action potentials of GABAergic neurons in CUMS mice (solid line) and control mice (dotted line). F-H: Effects of CUMS on electrophysiological properties of GABAergic neurons. Vts: Threshold potential; Vr: Resting potential; ARP: Absolute refractory period; ISI: Inter-spike interval. *P<0.05, **P<0.01, ***P<0.001 vs control group.

图7 CUMS对小鼠PrL区谷氨酸能和GABA能神经元动作电位发放模式的改变
Fig.7 Changes of action potential firing patterns of glutamatergic and GABAergic neurons in PrL region of CUMS mice. A: Waveforms of sequential action potentials of glutamatergic neurons in CUMS mice (solid line) and control mice (dotted line). B-D: Effects of CUMS on electrophysiological properties of glutamatergic neurons. E: Waveforms of sequential action potentials of GABAergic neurons in CUMS mice (solid line) and control mice (dotted line). F-H: Effects of CUMS on electrophysiological properties of GABAergic neurons. *P<0.05, **P<0.01, ***P<0.001 vs control group.
| 1 | Hu P, Wang Y, Qi XH, et al. SIRT1 in the BNST modulates chronic stress-induced anxiety of male mice via FKBP5 and corticotropin-releasing factor signaling[J]. Mol Psychiatry, 2023, 28(12): 5101-17. |
| 2 | Siopi E, Galerne M, Rivagorda M, et al. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice[J]. Mol Psychiatry, 2023, 28(7): 3002-12. |
| 3 | 崔维维, 龚莉雅, 陈纯辉, 等. 抑郁样小鼠额叶体积的减少与脑源性神经营养因子表达的下降有关[J]. 南方医科大学学报, 2023, 43(6): 1041-6. DOI: 10.12122/j.issn.1673-4254.2023.06.22 |
| 4 | Clement A, Madsen MJ, Kastaniegaard K, et al. Chronic stress induces hippocampal mitochondrial damage in APPPS1 model mice and wildtype littermates[J]. J Alzheimers Dis, 2022, 87(1): 259-72. |
| 5 | Aten S, Du YX, Taylor O, et al. Chronic stress impairs the structure and function of astrocyte networks in an animal model of depression[J]. Neurochem Res, 2023, 48(4): 1191-210. |
| 6 | Zhuang LP, Gao WJ, Chen YB, et al. LHPP in glutamatergic neurons of the ventral hippocampus mediates depression-like behavior by dephosphorylating CaMKIIα and ERK[J]. Biol Psychiatry, 2024, 95(5): 389-402. |
| 7 | Lee J, Yoon KJ, Park P, et al. Neur1 and Neur2 are required for hippocampus-dependent spatial memory and synaptic plasticity[J]. Hippocampus, 2020, 30(11): 1158-66. |
| 8 | Capitano F, Camon J, Ferretti V, et al. MicroRNAs modulate spatial memory in the hippocampus and in the ventral Striatum in a region-specific manner[J]. Mol Neurobiol, 2016, 53(7): 4618-30. |
| 9 | Ireton KE, Xing XM, Kim K, et al. Regulation of the Ca2+ channel CaV1.2 supports spatial memory and its flexibility and LTD[J]. J Neurosci, 2023, 43(30): 5559-73. |
| 10 | Barkus C, McHugh SB, Sprengel R, et al. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion[J]. Eur J Pharmacol, 2010, 626(1): 49-56. |
| 11 | Soltani Zangbar H, Ghadiri T, Seyedi Vafaee M, et al. Theta oscillations through hippocampal/prefrontal pathway: importance in cognitive performances[J]. Brain Connect, 2020, 10(4): 157-69. |
| 12 | Jiang ZJ, Zhu ZM, Zhao MY, et al. H3K9me2 regulation of BDNF expression in the hippocampus and medial prefrontal cortex is involved in the depressive-like phenotype induced by maternal separation in male rats[J]. Psychopharmacology, 2021, 238(10): 2801-13. |
| 13 | Li JJ, Pang YY, Du YH, et al. Lack of interferon regulatory factor 3 leads to anxiety/depression-like behaviors through disrupting the balance of neuronal excitation and inhibition in mice[J]. Genes Dis, 2023, 10(3): 1062-74. |
| 14 | Zhang H, Zhen YF, Yu SJ, et al. Working memory for spatial sequences: developmental and evolutionary factors in encoding ordinal and relational structures[J]. J Neurosci, 2022, 42(5): 850-64. |
| 15 | Ding XB, Lin YW, Chen C, et al. DNMT1 mediates chronic pain-related depression by inhibiting GABAergic neuronal activation in the central amygdala[J]. Biol Psychiatry, 2023, 94(8): 672-84. |
| 16 | Xu LP, Liu Y, Long JY, et al. Loss of spines in the prelimbic cortex is detrimental to working memory in mice with early-life adversity[J]. Mol Psychiatry, 2023, 28(8): 3444-58. |
| 17 | Petrus E, Dembling S, Usdin T, et al. Circuit-specific plasticity of callosal inputs underlies cortical takeover[J]. J Neurosci, 2020, 40(40): 7714-23. |
| 18 | Zhang GJ, Gao ZL, Guan SD, et al. Upregulation of excitatory neurons and downregulation of inhibitory neurons in barrel cortex are associated with loss of whisker inputs[J]. Mol Brain, 2013, 6: 2. |
| 19 | Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso-Nanclares L, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex[J]. Nat Rev Neurosci, 2008, 9(7): 557-68. |
| 20 | Gizewski ER, Gasser T, de Greiff A, et al. Cross-modal plasticity for sensory and motor activation patterns in blind subjects[J]. Neuroimage, 2003, 19(3): 968-75. |
| 21 | Larkum ME, Waters J, Sakmann B, et al. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons[J]. J Neurosci, 2007, 27(34): 8999-9008. |
| 22 | Samad N, Rao T, Rehman MHU, et al. Inhibitory effects of selenium on arsenic-induced anxiety-/ depression-like behavior and memory impairment[J]. Biol Trace Elem Res, 2022, 200(2): 689-98. |
| 23 | 时萌萌, 张 圆, 李 菁, 等. 慢性束缚应激对小鼠海马星形胶质细胞表型转换及抑郁样行为的影响[J]. 解剖学报, 2023, 54(1): 42-9. |
| 24 | Cai YJ, Ji Y, Liu YX, et al. Microglial circ-UBE2K exacerbates depression by regulating parental gene UBE2K via targeting HNRNPU[J]. Theranostics, 2024, 14(10): 4058-75. |
| 25 | Kim J, Kang S, Choi TY, et al. Metabotropic glutamate receptor 5 in amygdala target neurons regulates susceptibility to chronic social stress[J]. Biol Psychiatry, 2022, 92(2): 104-15. |
| 26 | Papp M, Gruca P, Lason M, et al. Insufficiency of ventral hippocampus to medial prefrontal cortex transmission explains antidepressant non-response[J]. J Psychopharmacol, 2021, 35(10): 1253-64. |
| 27 | Zan GY, Sun X, Wang YJ, et al. Amygdala dynorphin/κ opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress[J]. Acta Pharmacol Sin, 2022, 43(3): 577-87. |
| 28 | Folschweiller S, Sauer JF. Behavioral state-dependent modulation of prefrontal cortex activity by respiration[J]. J Neurosci, 2023, 43(26): 4795-807. |
| 29 | Li ZY, Li JX, Wei YX, et al. Anterior and posterior basolateral amygdala projections of cell type-specific D1-expressing neurons from the medial prefrontal cortex differentially control alcohol-seeking behavior[J]. Biol Psychiatry, 2024, 95(10): 963-73. |
| 30 | Wang XL, Miao C, Su YF, et al. MAD2B blunts chronic unpredictable stress and corticosterone stimulation-induced depression-like behaviors in mice[J]. Int J Neuropsychopharmacol, 2023, 26(2): 137-48. |
| 31 | Ge PY, Qu SY, Ni SJ, et al. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting IDO1-associated with tryptophan metabolism[J]. Phytother Res, 2023, 37(1): 342-57. |
| 32 | Hong C, Wang Z, Zheng SL, et al. Metrnl regulates cognitive dysfunction and hippocampal BDNF levels in D-galactose-induced aging mice[J]. Acta Pharmacol Sin, 2023, 44(4): 741-51. |
| 33 | Simonetti M, Paldy E, Njoo C, et al. The impact of Semaphorin 4C/Plexin-B2 signaling on fear memory via remodeling of neuronal and synaptic morphology[J]. Mol Psychiatry, 2021, 26(4): 1376-98. |
| 34 | Hallock HL, 4thQuillian HM, Maynard KR, et al. Molecularly defined hippocampal inputs regulate population dynamics in the prelimbic cortex to suppress context fear memory retrieval[J]. Biol Psychiatry, 2020, 88(7): 554-65. |
| 35 | Mayo LM, Asratian A, Lindé J, et al. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice[J]. Mol Psychiatry, 2020, 25(5): 993-1005. |
| 36 | Elizabeth A, Adegbuyi A, Olusegun A, et al. Morin hydrate attenuates chronic stress-induced memory impairment and degeneration of hippocampal subfields in mice: the role of oxidative, nitrergic and neuroinflammatory pathways[J]. Metab Brain Dis, 2020, 35(7): 1145-56. |
| 37 | Liu L, Zhang R, Chen C, et al. The effect of Banxia-houpo Decoction on CUMS-induced depression by promoting M2 microglia polarization via TrkA/Akt signalling[J]. J Cell Mol Med, 2023, 27(21): 3339-53. |
| 38 | Schmeltzer SN, Vollmer LL, Rush JE, et al. History of chronic stress modifies acute stress-evoked fear memory and acoustic startle in male rats[J]. Stress, 2015, 18(2): 244-53. |
| 39 | Chauveau F, De Job E, Poly-Thomasson B, et al. Procognitive impact of ciproxifan (a histaminergic H3 receptor antagonist) on contextual memory retrieval after acute stress[J]. CNS Neurosci Ther, 2019, 25(8): 832-41. |
| 40 | Leem YH, Park JS, Chang H, et al. Exercise prevents memory consolidation defects via enhancing prolactin responsiveness of CA1 neurons in mice under chronic stress[J]. Mol Neurobiol, 2019, 56(9): 6609-25. |
| 41 | Nakatani Y, Ishikawa K, Aoki Y, et al. Inhibitory effect of atomoxetine on Nav1.2 voltage-gated sodium channel currents[J]. Pharmacol Rep, 2023, 75(3): 746-52. |
| 42 | Liu JJ, Eyring KW, König GM, et al. Oxytocin-modulated ion channel ensemble controls depolarization, integration and burst firing in CA2 pyramidal neurons[J]. J Neurosci, 2022, 42(41): 7707-20. |
| 43 | Routh BN, Brager DH, Johnston D. Ionic and morphological contributions to the variable gain of membrane responses in layer 2/3 pyramidal neurons of mouse primary visual cortex[J]. J Neurophysiol, 2022, 128(4): 1040-50. |
| [1] | 欧阳明子, 崔佳琦, 王慧, 梁正, 皮大锦, 陈利国, 陈前军, 吴迎朝. 开心散通过减轻前额叶皮质铁死亡缓解小鼠的阿霉素化疗性抑郁[J]. 南方医科大学学报, 2024, 44(8): 1441-1449. |
| [2] | 张叶明, 张袁祥, 沈学彬, 王国栋, 朱磊. 在抑郁症大鼠模型中MiRNA-103-3p调控Rab10促进神经细胞自噬[J]. 南方医科大学学报, 2024, 44(7): 1315-1326. |
| [3] | 李明明, 何梁超, 李天雨, 鲍岩, 徐祥, 陈光. 小鼠顶叶皮层反复轻度创伤性脑损伤抑制延髓NLG-1和PSD-95的表达[J]. 南方医科大学学报, 2024, 44(5): 960-966. |
| [4] | 孙一鸣, 张荣, 孟莹, 朱磊, 李明强, 刘哲. 辅酶Q10通过下调焦亡信号通路缓解抑郁小鼠的抑郁样行为[J]. 南方医科大学学报, 2024, 44(5): 810-817. |
| [5] | 熊一凡, 梁小珊, 梁晓涛, 李伟鹏, 钱益啸, 谢 炜. 柴胡皂甙a减轻戊四氮诱发的皮质酮抑郁模型小鼠的急性癫痫发作:基于小胶质细胞介导的炎症反应[J]. 南方医科大学学报, 2024, 44(3): 515-522. |
| [6] | 许光明, 高安迪, 丛 斌. 束缚应激通过激活Rho/ROCK通路诱导大鼠杏仁核血脑屏障的损伤[J]. 南方医科大学学报, 2024, 44(3): 411-419. |
| [7] | 李新翼, 刘玉杰, 邓克崇, 胡义奎. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-410. |
| [8] | 郑轶珺, 侯宇月, 李 帆, 史欣可, 陶雅雯, 赵欣宇, 胡 浩, 杨 林. 近红外光照射可显著改善大鼠抑郁所致的肠功能紊乱[J]. 南方医科大学学报, 2023, 43(9): 1591-1598. |
| [9] | 杨 蕙, 王 华, 李成龙, 何 雄, 雷诗卉, 李 薇, 孟 盼, 王瑾茜, 刘 检, 王宇红. 左归降糖解郁方促进糖尿病并发抑郁症大鼠的海马齿状回神经干细胞的自我更新并激活Shh信号通路[J]. 南方医科大学学报, 2023, 43(5): 694-701. |
| [10] | 杨孟迪, 尹鸿涛, 甄洁玉, 丁雨露, 王玉洁, 孙琳楠, 何风英, 甄东户. 抑郁症状与肾功能快速下降及慢性肾脏病的发生无相关性[J]. 南方医科大学学报, 2023, 43(2): 225-231. |
| [11] | 熊紫玉, 周乐鹏, 陈静芬, 李 萌, 谢日华. 产后抑郁与初乳中转化生长因子-β水平的关系:90例巢氏队列研究[J]. 南方医科大学学报, 2022, 42(9): 1426-1430. |
| [12] | 李佳欣, 肖 艳, 廖 娟, 杨春霞. 我国围绝经期女性抑郁症状变化及影响因素分析:基于CHARLS面板数据[J]. 南方医科大学学报, 2022, 42(7): 1038-1043. |
| [13] | 葛鑫宇, 郭 芳, 范 俊, 陈宝田, 于 林, 任 静, 李纪强, 卢成林, 莫嘉文, 李树基, 袁乐欣, 胡号应, 刘 赟, 周 晓, 崔 娟, 朱志敏, 曹 雄. 柴胡桂枝汤通过sirt1-p53信号通路产生抗抑郁作用[J]. 南方医科大学学报, 2021, 41(3): 399-405. |
| [14] | 王青霞, 曹晓霞, 吴秀颖, 刘江峰, 谢婧雯, 侯德仁. COVID-19流行期间医院急诊患者焦虑与抑郁现状调查[J]. 南方医科大学学报, 2020, 40(09): 1369-1372. |
| [15] | 肖 蓉,于馨淼. 医学生文化信仰及其与抑郁的关系[J]. 南方医科大学学报, 2020, 40(08): 1178-1183. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||