南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (5): 960-966.doi: 10.12122/j.issn.1673-4254.2024.05.18
李明明( ), 何梁超, 李天雨, 鲍岩, 徐祥(
), 何梁超, 李天雨, 鲍岩, 徐祥( ), 陈光(
), 陈光( )
)
收稿日期:2024-01-05
出版日期:2024-05-20
发布日期:2024-06-04
通讯作者:
徐祥,陈光
E-mail:limingmingwnmc@163.com;xuxiang575883@163.com;chenguang210401@163.com
作者简介:李明明,在读硕士研究生,E-mail: limingmingwnmc@163.com
基金资助:
Mingming LI( ), Liangchao HE, Tianyu LI, Yan BAO, Xiang XU(
), Liangchao HE, Tianyu LI, Yan BAO, Xiang XU( ), Guang CHEN(
), Guang CHEN( )
)
Received:2024-01-05
Online:2024-05-20
Published:2024-06-04
Contact:
Xiang XU, Guang CHEN
E-mail:limingmingwnmc@163.com;xuxiang575883@163.com;chenguang210401@163.com
摘要:
目的 研究反复轻度创伤性脑损伤(rmTBI)对延髓神经元形态和突触可塑性的影响。 方法 32只雄性ICR小鼠随机分为假手术组(SHAM组,n=8)和rmTBI组(n=24)。使用自由落体打击装置建立rmTBI模型,打击后存活小鼠为rmTBI存活组(rmTBI-S),打击后死亡小鼠为rmTBI死亡组(rmTBI-D)。使用神经损伤评分、翻正反射和强迫游泳实验检测小鼠神经功能障碍,使用苏木素-伊红及尼氏染色观察神经细胞病理形态改变,使用免疫印迹及免疫荧光染色检测神经连接蛋白1(NLG-1)和突触后致密蛋白95(PSD-95)表达水平。 结果 SHAM组小鼠无死亡,rmTBI组死亡率41.67%,差异有统计学意义(P<0.05);和SHAM组相比,rmTBI-S组小鼠神经损伤评分降低(P<0.001)、翻正反射恢复时间(P<0.001)和强迫游泳不动时间(P<0.05)增加,神经元尼氏小体消失、肿胀坏死;延髓NLG-1(P<0.05)和PSD-95(P<0.05)表达水平降低;和rmTBI-S组相比,rmTBI-D组NLG-1(P<0.01)和PSD-95(P<0.01)表达水平降低。rmTBI-D组小鼠延髓神经纤维扭曲水肿,神经元密度降低。 结论 延髓突触结构异常和功能障碍可能与rmTBI导致的死亡及神经功能损害相关。
李明明, 何梁超, 李天雨, 鲍岩, 徐祥, 陈光. 小鼠顶叶皮层反复轻度创伤性脑损伤抑制延髓NLG-1和PSD-95的表达[J]. 南方医科大学学报, 2024, 44(5): 960-966.
Mingming LI, Liangchao HE, Tianyu LI, Yan BAO, Xiang XU, Guang CHEN. Repeated mild traumatic brain injury in the parietal cortex inhibits expressions of NLG-1 and PSD-95 in the medulla oblongata of mice[J]. Journal of Southern Medical University, 2024, 44(5): 960-966.
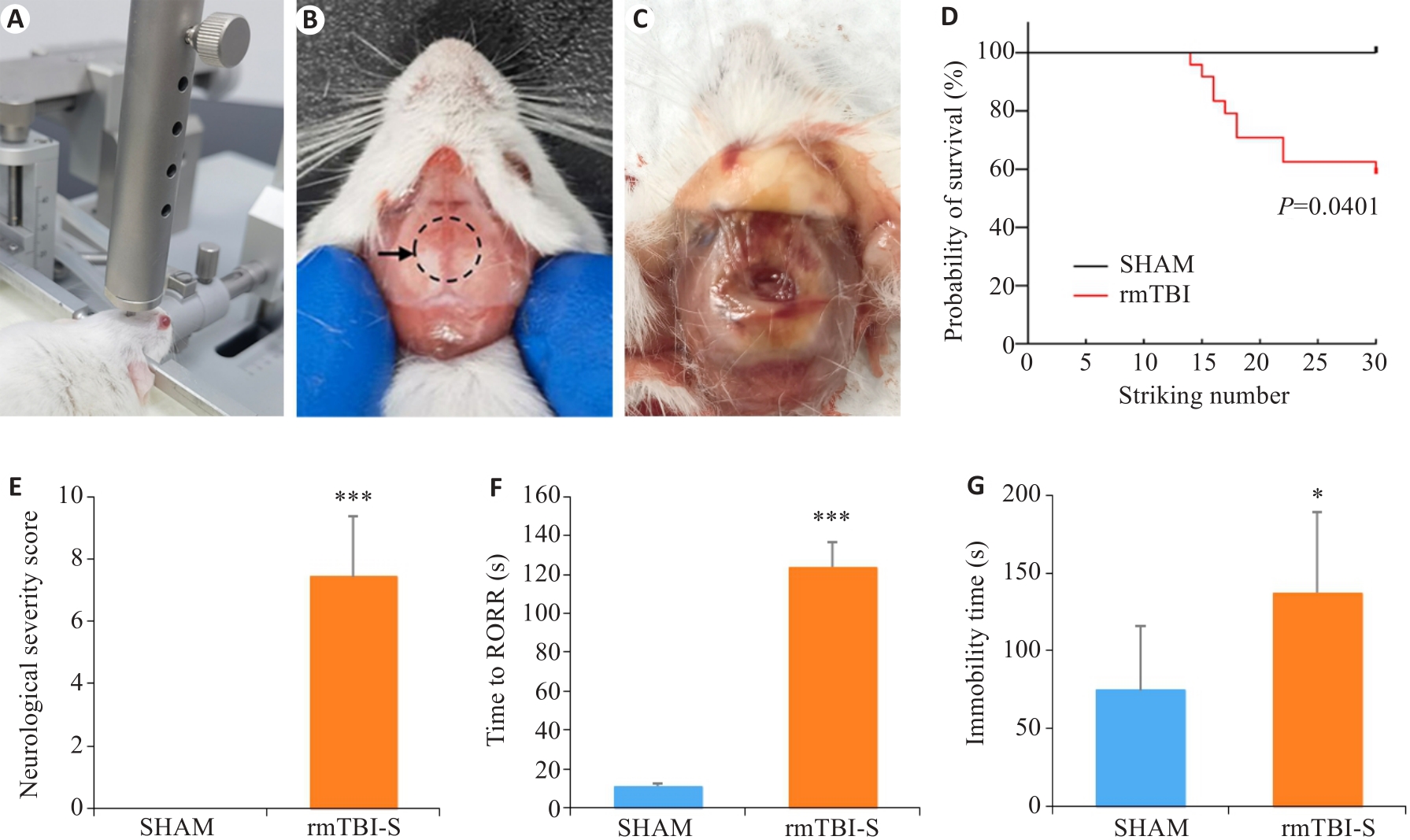
图1 反复轻度脑损伤导致小鼠死亡和行为学改变
Fig.1 Repeated mild traumatic brain injury (rmTBI) results in death and behavioral changes of mice. A: The free-fall device for inducing rmTBI in mice. B: Impact site on the skull of mice shown by the dashed line circle. C: Wound in the skull after rmTBI. D: The mortality rate of mice increases with the strike number. E: Neurological severity scores of the mice surviving rmTBI. F: The mice surviving rmTBI show delayed recovery of righting reflex (RORR). G: The mice surviving rmTBI show increased immobility time in forced swimming test. *P<0.05, ***P<0.001 vs sham group (nsham=8, nrmTBI-S=10-14).
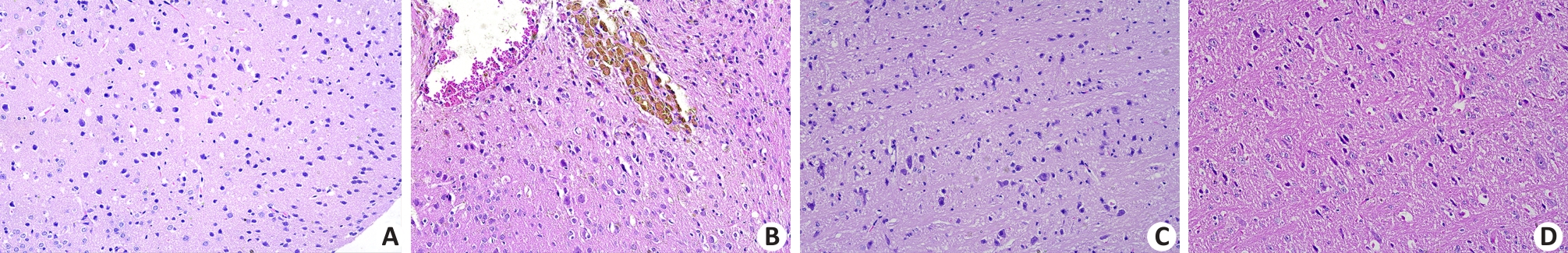
图2 反复轻度脑损伤死亡组小鼠顶叶皮层和延髓组织病理学改变
Fig.2 Histopathological changes in the parietal cortex and medulla oblongata of the mice that died after rmTBI (Original magnification: ×20). A: No abnormalities are observed in the parietal cortical neurons in the sham group. B: Tissue defects, bleeding, neuronal edema and deposition of hemosiderin particles are observed in the parietal cortex of a mouse that did not survive rmTBI. C: No abnormalities are observed in the neurons and nerve fibers in the medulla oblongata of a sham-operated mouse. D: Loose, twisted and broken nerve fibers and swollen neurons are observed in the medulla oblongata of a mouse that did not survive rmTBI.
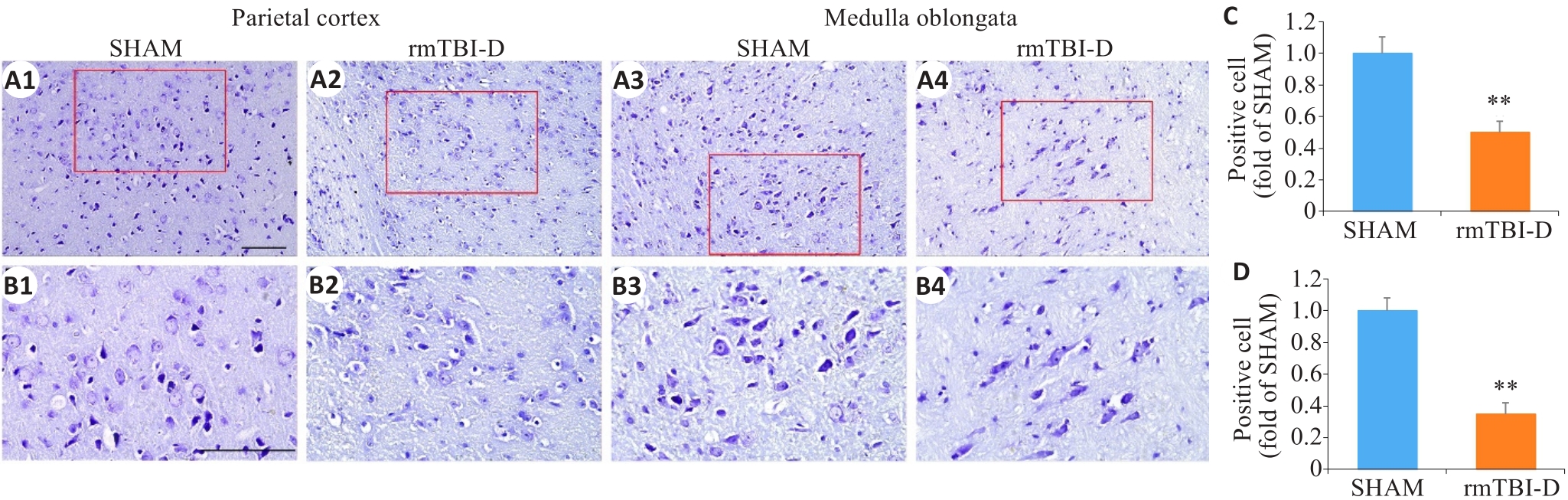
图3 反复轻度脑损伤死亡组小鼠顶叶皮层和延髓神经元尼氏小体消失
Fig.3 Loss of Nissl bodies in the neurons in the parietal cortex and medulla oblongata of the mice that did not survive rmTBI. The figures in the lower panel (B1-B4; ×40, scale bar=100 μm) are enlarged views of the boxed areas in A1-A4 (×20, scale bar=100 μm). C, D: Statistical analysis of positive cells in the parietal cortex (C) and medulla oblongata (D) (n=6). **P<0.01 vs sham group.
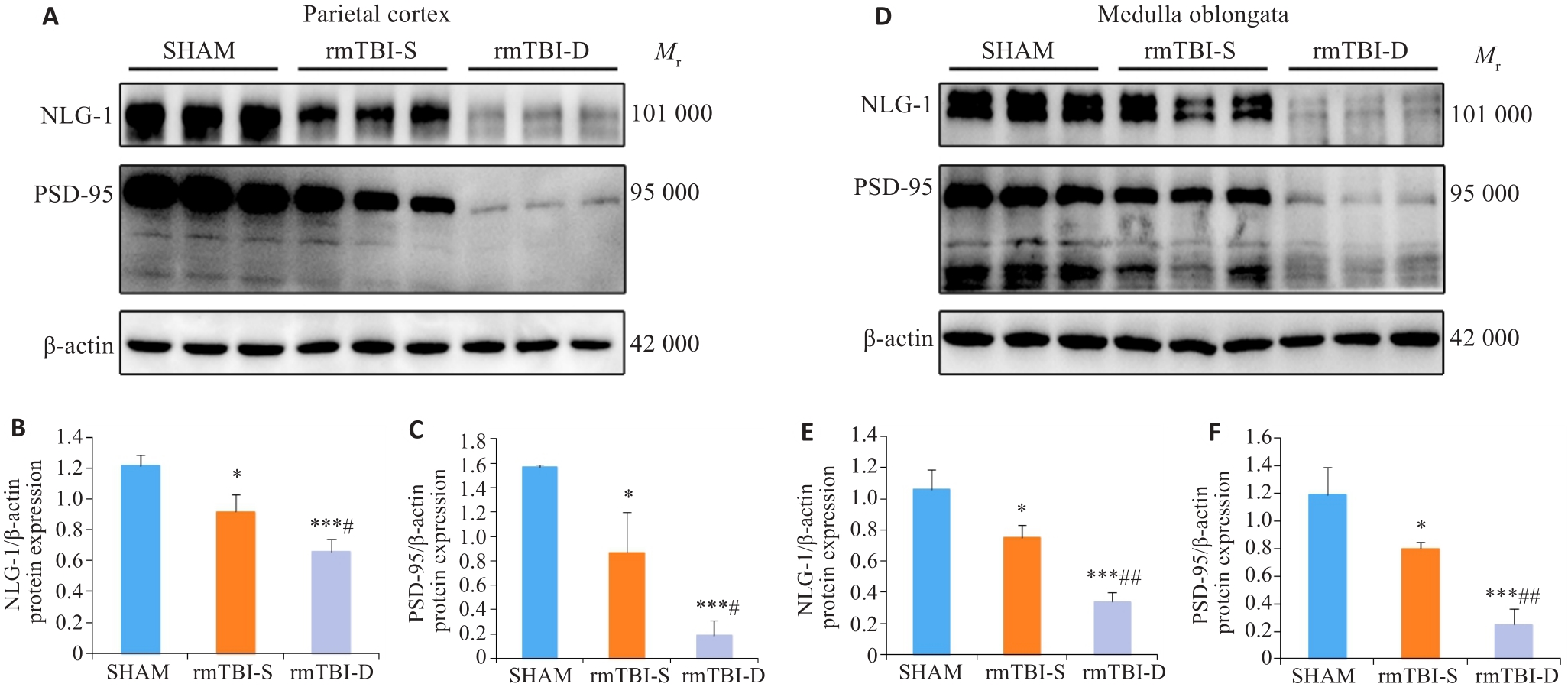
图4 Western blotting分析NLG-1、PSD-95蛋白表达
Fig.4 Western blotting of NLG-1 and PSD-95 in the parietal cortex and medulla oblongata of the mice. A-C: rmTBI decreases the expression levels of NLG-1 and PSD-95 in the parietal cortex, and their expression levels are lower in the mice that died after rmTBI (rmTBI-D group) than in the survivors (rmTBI-S group). D-F: rmTBI decreases the expression levels of NLG-1 and PSD-95 in the medulla oblongata, and their expression levels are lower in rmTBI-D than in rmTBI-S group. *P<0.05, ***P<0.001 vs sham group, #P<0.05, ##P<0.01 vs rmTBI-S group (n=3).
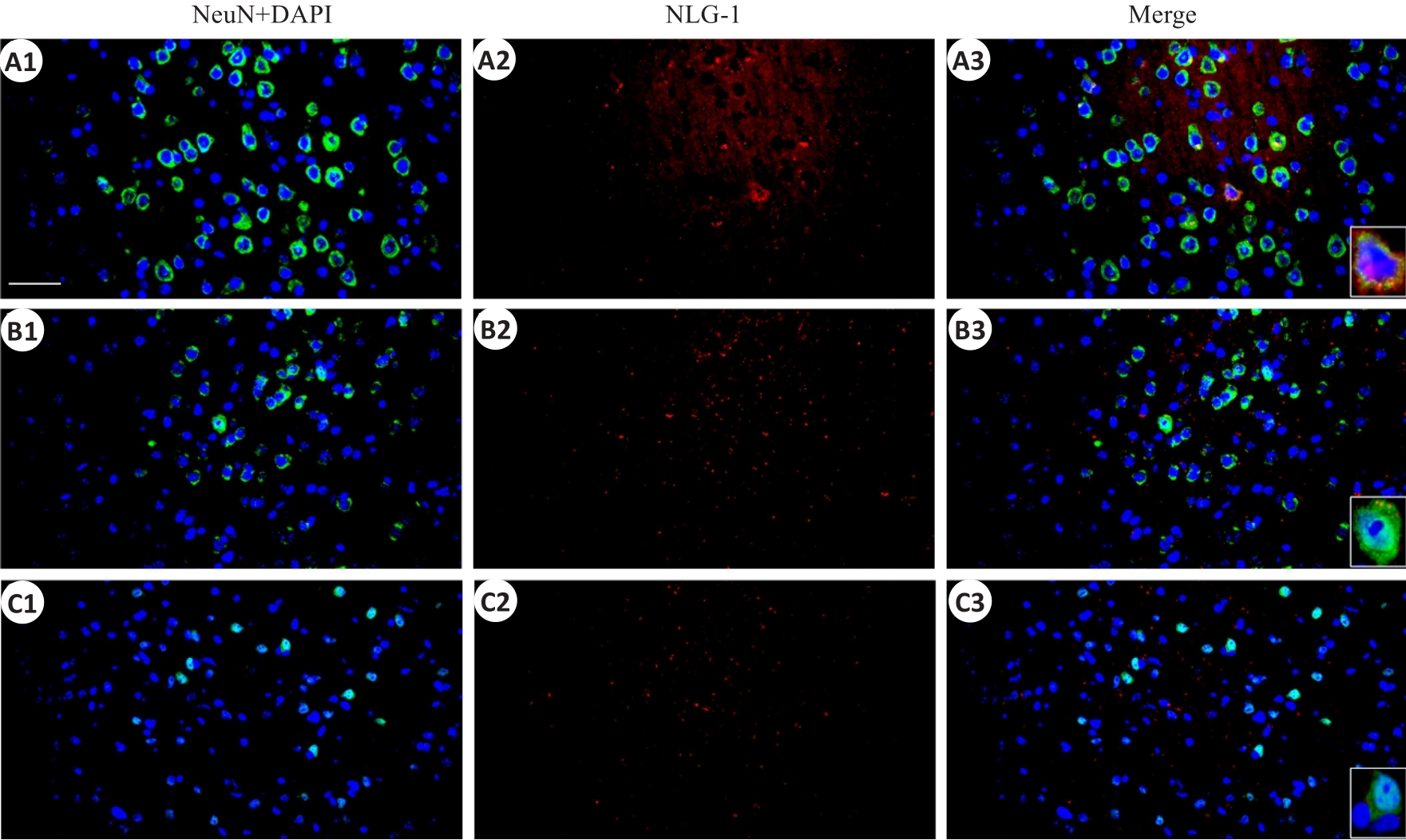
图5 顶叶皮层NLG-1和神经元标记物NeuN免疫荧光染色的共表达
Fig.5 Immunofluorescent co-expression of NLG-1 and neuronal marker NeuN in the parietal cortex (×40, scale bar=100 μm). A1-A3: Sham group; B1-B3: rmTBI-S group; C1-C3: rmTBI-D group; NeuN,DAPI and NLG-1 present green,blue and red fluorescence rspectively.
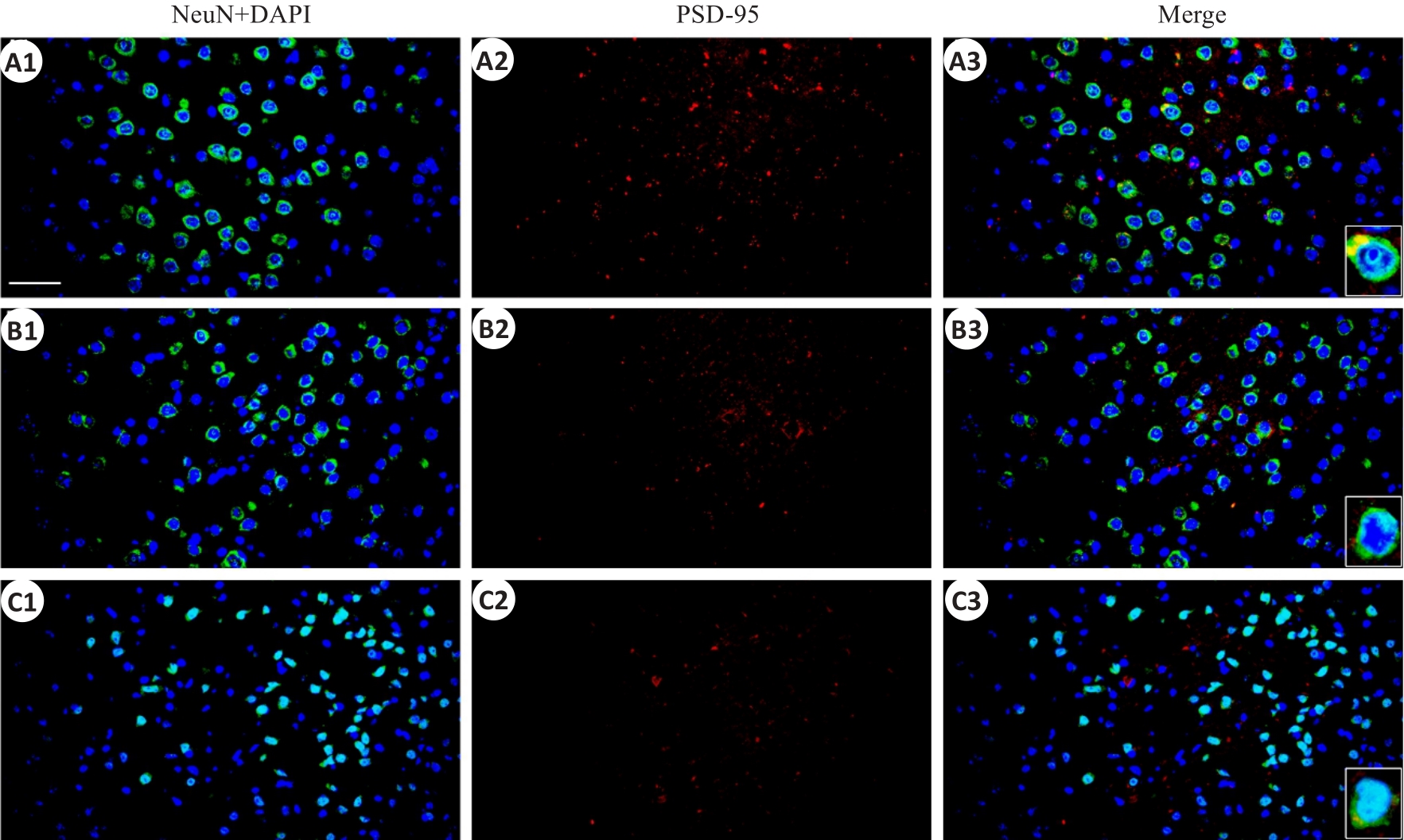
图6 顶叶皮层PSD-95和神经元标记物NeuN免疫荧光染色的共表达
Fig.6 Immunofluorescent co-expression of PSD-95 and NeuN in the parietal cortex (×40, scale bar=100 μm).A1-A3: the sham group; B1-B3: rmTBI-S group; C1-C3: rmTBI-D group; PSD-95 presents red fluorescence.
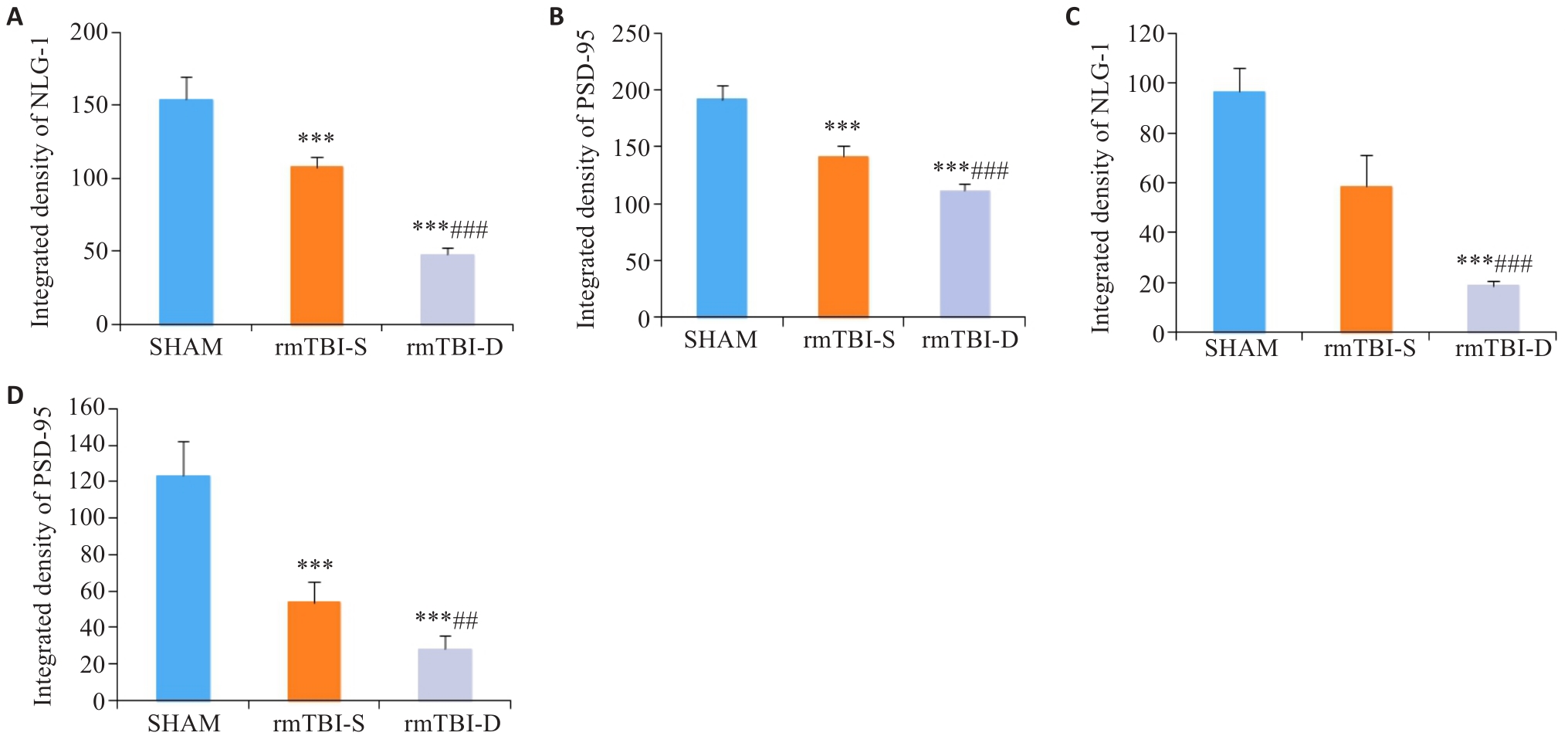
图7 NLG-1、PSD-95荧光强度统计分析
Fig.7 Statistical analysis of immunofluorescence intensity of NLG-1 and PSD-95. A, B: rmTBI decreases the integrated densities of NLG-1 and PSD-95 in the parietal cortex, which are lowered in rmTBI-D group than in rmTBI-S group. C, D: rmTBI decreases the integrated densities of NLG-1 and PSD-95 in the medulla oblongata, which are lower in rmTBI-D group than in rmTBI-S group. ***P<0.001 vs sham group, ##P<0.01, ###P<0.001 vs rmTBI-S group (n=8).
| 1 | 隋立森, 余佳彬, 姜晓丹. 大鼠创伤性颅脑损伤后脑室下区内源性神经干细胞的增殖与分化[J]. 南方医科大学学报, 2016, 36(8): 1094-9. |
| 2 | Skrifvars MB, Luethi N, Bailey M, et al. The effect of recombinant erythropoietin on long-term outcome after moderate-to-severe traumatic brain injury[J]. Intensive Care Med, 2023, 49(7): 831-9. |
| 3 | Ratliff WA, Qubty D, Delic V, et al. Repetitive mild traumatic brain injury and transcription factor modulation[J]. J Neurotrauma, 2020, 37(17): 1910-7. DOI: 10.1089/neu.2020.7005 |
| 4 | Kelly JP, Priemer DS, Perl DP, et al. Sports concussion and chronic traumatic encephalopathy: finding a path forward[J]. Ann Neurol, 2023, 93(2): 222-5. DOI: 10.1002/ana.26566 |
| 5 | Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion[J]. Br J Sports Med, 2017, 51(12): 969-77. DOI: 10.1136/bjsports-2017-097791 |
| 6 | Kawata K, Rubin LH, Wesley L, et al. Acute changes in plasma total tau levels are independent of subconcussive head impacts in college football players[J]. J Neurotrauma, 2018, 35(2): 260-6. DOI: 10.1089/neu.2017.5376 |
| 7 | Gold EM, Vasilevko V, Hasselmann J, et al. Repeated mild closed head injuries induce long-term white matter pathology and neuronal loss that are correlated with behavioral deficits[J]. ASN Neuro, 2018, 10: 1759091418781921. DOI: 10.1177/1759091418781921 |
| 8 | Greco T, Ferguson L, Giza C, et al. Mechanisms underlying vulnerabilities after repeat mild traumatic brain injuries[J]. Exp Neurol, 2019, 317: 206-13. DOI: 10.1016/j.expneurol.2019.01.012 |
| 9 | Omalu BI, Bailes J, Hammers JL, et al. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist[J]. Am J Forensic Med Pathol, 2010, 31(2): 130-2. DOI: 10.1097/paf.0b013e3181ca7f35 |
| 10 | McMillan TM, Weir CJ, Wainman-Lefley J. Mortality and morbidity 15 years after hospital admission with mild head injury: a prospective case-controlled population study[J]. J Neurol Neurosurg Psychiatry, 2014, 85(11): 1214-20. DOI: 10.1136/jnnp-2013-307279 |
| 11 | Wenker IC, Abe C, Viar KE, et al. Blood pressure regulation by the rostral ventrolateral medulla in conscious rats: effects of hypoxia, hypercapnia, baroreceptor denervation, and anesthesia[J]. J Neurosci, 2017, 37(17): 4565-83. DOI: 10.1523/jneurosci.3922-16.2017 |
| 12 | Song JY, Ichtchenko K, Südhof TC, et al. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses[J]. Proc Natl Acad Sci USA, 1999, 96(3): 1100-5. DOI: 10.1073/pnas.96.3.1100 |
| 13 | Levinson JN, Chéry N, Huang K, et al. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity[J]. J Biol Chem, 2005, 280(17): 17312-9. DOI: 10.1074/jbc.m413812200 |
| 14 | Zhao JY, Duan XL, Yang L, et al. Activity-dependent synaptic recruitment of neuroligin 1 in spinal dorsal horn contributed to inflammatory pain[J]. Neuroscience, 2018, 388: 1-10. DOI: 10.1016/j.neuroscience.2018.06.047 |
| 15 | Coley AA, Gao WJ. PSD95: a synaptic protein implicated in schizophrenia or autism[J]? Prog Neuro Psychopharmacol Biol Psychiatry, 2018, 82: 187-94. DOI: 10.1016/j.pnpbp.2017.11.016 |
| 16 | Petkova-Tuffy A, Gödecke N, Viotti J, et al. Neuroligin-1 mediates presynaptic maturation through brain-derived neurotrophic factor signaling[J]. BMC Biol, 2021, 19(1): 215. DOI: 10.1186/s12915-021-01145-7 |
| 17 | Dinda B, Dinda M, Kulsi G, et al. Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: a review[J]. Eur J Med Chem, 2019, 169: 185-99. DOI: 10.1016/j.ejmech.2019.03.009 |
| 18 | Cai WM, Wang GJ, Wu H, et al. Identifying traumatic brain injury (TBI) by ATR-FTIR spectroscopy in a mouse model[J]. Spectrochim Acta A Mol Biomol Spectrosc, 2022, 274: 121099. DOI: 10.1016/j.saa.2022.121489 |
| 19 | Flierl MA, Stahel PF, Beauchamp KM, et al. Mouse closed head injury model induced by a weight-drop device[J]. Nat Protoc, 2009, 4(9): 1328-37. DOI: 10.1038/nprot.2009.148 |
| 20 | Lasry O, Liu EY, Powell GA, et al. Epidemiology of recurrent traumatic brain injury in the general population: a systematic review[J]. Neurology, 2017, 89(21): 2198-209. DOI: 10.1212/wnl.0000000000004671 |
| 21 | Wall SE, Williams WH, Cartwright-Hatton S, et al. Neuropsychological dysfunction following repeat concussions in jockeys[J]. J Neurol Neurosurg Psychiatry, 2006, 77(4): 518-20. DOI: 10.1136/jnnp.2004.061044 |
| 22 | Iverson GL, Echemendia RJ, Lamarre AK, et al. Possible lingering effects of multiple past concussions[J]. Rehabil Res Pract, 2012, 2012: 316575. DOI: 10.1155/2012/316575 |
| 23 | Hunzinger KJ, Law CA, Elser H, et al. Associations between head injury and subsequent risk of falls: results from the atherosclerosis risk in communities (ARIC) study[J]. Neurology, 2023, 101(22): e2234-e2242. DOI: 10.1212/wnl.0000000000207949 |
| 24 | Huynh LM, Burns MP, Taub DD, et al. Chronic neurobehavioral impairments and decreased hippocampal expression of genes important for brain glucose utilization in a mouse model of mild TBI[J]. Front Endocrinol, 2020, 11: 556380. DOI: 10.3389/fendo.2020.556380 |
| 25 | Zohar O, Schreiber S, Getslev V, et al. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice[J]. Neuroscience, 2003, 118(4): 949-55. DOI: 10.1016/s0306-4522(03)00048-4 |
| 26 | Tsenter J, Beni-Adani L, Assaf Y, et al. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions[J]. J Neurotrauma, 2008, 25(4): 324-33. DOI: 10.1089/neu.2007.0452 |
| 27 | Wang YL, Wang L, Xu W, et al. Paraventricular thalamus controls consciousness transitions during propofol anaesthesia in mice[J]. Br J Anaesth, 2023, 130(6): 698-708. DOI: 10.1016/j.bja.2023.01.016 |
| 28 | Dewitt DS, Perez-Polo R, Hulsebosch CE, et al. Challenges in the development of rodent models of mild traumatic brain injury[J]. J Neurotrauma, 2013, 30(9): 688-701. DOI: 10.1089/neu.2012.2349 |
| 29 | Verley DR, Torolira D, Hessell BA, et al. Cortical neuromodulation of remote regions after experimental traumatic brain injury normalizes forelimb function but is temporally dependent[J]. J Neurotrauma, 2019, 36(5): 789-801. DOI: 10.1089/neu.2018.5769 |
| 30 | Ong LK. Beyond the primary infarction: focus on mechanisms related to secondary neurodegeneration after stroke[J]. Int J Mol Sci, 2022, 23(24): 16024. DOI: 10.3390/ijms232416024 |
| 31 | Varoqueaux F, Aramuni G, Rawson RL, et al. Neuroligins determine synapse maturation and function[J]. Neuron, 2006, 51(6): 741-54. DOI: 10.1016/j.neuron.2006.09.003 |
| 32 | Cao C, Wang LW, Zhang J, et al. Neuroligin-1 plays an important role in methamphetamine-induced hippocampal synaptic plasticity[J]. Toxicol Lett, 2022, 361: 1-9. DOI: 10.1016/j.toxlet.2022.03.007 |
| 33 | Camporesi E, Lashley T, Gobom J, et al. Neuroligin-1 in brain and CSF of neurodegenerative disorders: investigation for synaptic biomarkers[J]. Acta Neuropathol Commun, 2021, 9(1): 19. DOI: 10.1186/s40478-021-01119-4 |
| 34 | Lai XP, Yu XJ, Qian H, et al. Chronic alcoholism-mediated impairment in the medulla oblongata: a mechanism of alcohol-related mortality in traumatic brain injury[J]? Cell Biochem Biophys, 2013, 67(3): 1049-57. DOI: 10.1007/s12013-013-9603-y |
| 35 | Levy AM, Gomez-Puertas P, Tümer Z. Neurodevelopmental disorders associated with PSD-95 and its interaction partners[J]. Int J Mol Sci, 2022, 23(8): 4390. (编辑:吴锦雅) |
| [1] | 葛鑫宇, 郭 芳, 范 俊, 陈宝田, 于 林, 任 静, 李纪强, 卢成林, 莫嘉文, 李树基, 袁乐欣, 胡号应, 刘 赟, 周 晓, 崔 娟, 朱志敏, 曹 雄. 柴胡桂枝汤通过sirt1-p53信号通路产生抗抑郁作用[J]. 南方医科大学学报, 2021, 41(3): 399-405. |
| [2] | 魏雯雯,吴琼波,郑 超,汪萌芽,张环环. 神经性厌食症大鼠伏隔核与延髓头端腹外侧区的神经通路联系[J]. 南方医科大学学报, 2020, 40(05): 609-615. |
| [3] | 姬明丽,吴云红,千智斌. 孕期酒精暴露对新生大鼠延髓基本节律性呼吸放电的作用[J]. 南方医科大学学报, 2015, 35(04): 598-. |
| [4] | 孙 娜,孔令恒,牛利刚,朱娟霞,徐 燕,杜剑青. 中脑导水管周围灰质腹外侧区促代谢型谷氨酸受体亚型7和8对大鼠心脏-躯体运动反射的双向调节作用[J]. 南方医科大学学报, 2014, 34(01): 8-. |
| [5] | 孙娜,孔令恒,牛利刚,朱娟霞,徐燕,杜剑青. 延髓头端腹内侧区对大鼠心脏伤害性感受的下行调控作用[J]. 南方医科大学学报, 2013, 33(11): 1611-. |
| [6] | 郑奇辉 李国才 方芳 吴中海 焦永钢. Ⅱ组代谢性谷氨酸受体参与新生鼠离体延髓脑片呼吸节律性放电的调节[J]. 南方医科大学学报, 2010, 30(08): 1813-1816. |
| [7] | 陈娟; 邹志鹏; 吴中海;. 苯巴比妥钠对新生大鼠离体延髓脑片节律性呼吸的影响[J]. 南方医科大学学报, 2006, 26(09): 1273-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||