南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (10): 1866-1873.doi: 10.12122/j.issn.1673-4254.2024.10.04
• • 上一篇
收稿日期:2024-07-23
出版日期:2024-10-20
发布日期:2024-10-31
通讯作者:
杜伯雨
E-mail:xixueyan2001@126.com;du.boyu@hotmail.com
作者简介:郗雪艳,博士,E-mail: xixueyan2001@126.com
基金资助:
Xueyan XI( ), Ting DENG, Boyu DU(
), Ting DENG, Boyu DU( )
)
Received:2024-07-23
Online:2024-10-20
Published:2024-10-31
Contact:
Boyu DU
E-mail:xixueyan2001@126.com;du.boyu@hotmail.com
Supported by:摘要:
目的 探究人结直肠成纤维细胞(CCD18-Co)条件培养基对结直肠癌(CRC)细胞恶性进展的影响,为CRC治疗提供思路。 方法 利用RTCA、克隆形成和创伤愈合实验测定CRC细胞增殖、克隆形成和迁移能力;Western blotting检测CCD18-Co-CM激活的CRC细胞ATK、ERK和STAT3信号通路,同时检测相应信号通路阻断后CRC细胞增殖、克隆形成和迁移能力;肿瘤球形成实验检测CCD18-Co-CM对CRC细胞成球能力的影响;RT-PCR方法检测CRC细胞干性标志物的表达情况。 结果 CCD-18Co-CM能够促进CRC细胞的增殖、克隆形成和迁移能力(P<0.05)。CCD-18Co-CM能够增强CRC细胞的成球能力及干性标志物的表达(P<0.05)。CCD-18Co-CM 能够激活CRC细胞ERK信号通路(P<0.05),ERK信号通路抑制剂SCH772984能够降低CRC细胞增殖、克隆形成和迁移能力及成球能力和干性标志物的表达(P<0.05)。 结论 人正常结直肠成纤维细胞可通过激活ERK通路促进CRC细胞的恶性进展。
郗雪艳, 邓婷, 杜伯雨. 结直肠成纤维细胞通过激活ERK信号通路促进结直肠癌细胞的恶性生物学行为[J]. 南方医科大学学报, 2024, 44(10): 1866-1873.
Xueyan XI, Ting DENG, Boyu DU. Colorectal fibroblasts promote malignant phenotype of colorectal cancer cells by activating the ERK signaling pathway[J]. Journal of Southern Medical University, 2024, 44(10): 1866-1873.
| Gene name | Primer sequences |
|---|---|
| CD29 | Up: 5'-TCCAACCTGATCCTGTGTCC-3' |
| Down: 5'-ACAATTCCAGCAACCACACC-3' | |
| CD44 | Up: 5'-GGCACCCGCTATGTCCAGAA-3' |
| Down: 5'-CCTCCTGAAGTGCTGCTCCT-3' | |
| CD166 | Up: 5'-GTCTGCTCTTCTGCCTCTTG-3' |
| Down: 5'-CGTCAAGTCGGCAAGGTATG-3' | |
| MYC | Up: 5'- CGTCTCCACACATCAGCACAA-3' |
| Down: 5'-TCTTGGCAGCAGGATAGTCCTT -3' | |
| EPCAM | Up: 5'-GGCTCTTTAAGGCCAAGCAG-3' |
| Down: 5'-CCAGTAGGTTCTCACTCGCT-3' | |
| GAPDH | Up:5'-AGCTCACTGGCATGGCCTTC-3' |
| Down: 5'-CGCCTGCTTCACCACCTTCT-3' |
表1 RT-PCR的引物序列
Tab.1 Primer sequences for RT-PCR
| Gene name | Primer sequences |
|---|---|
| CD29 | Up: 5'-TCCAACCTGATCCTGTGTCC-3' |
| Down: 5'-ACAATTCCAGCAACCACACC-3' | |
| CD44 | Up: 5'-GGCACCCGCTATGTCCAGAA-3' |
| Down: 5'-CCTCCTGAAGTGCTGCTCCT-3' | |
| CD166 | Up: 5'-GTCTGCTCTTCTGCCTCTTG-3' |
| Down: 5'-CGTCAAGTCGGCAAGGTATG-3' | |
| MYC | Up: 5'- CGTCTCCACACATCAGCACAA-3' |
| Down: 5'-TCTTGGCAGCAGGATAGTCCTT -3' | |
| EPCAM | Up: 5'-GGCTCTTTAAGGCCAAGCAG-3' |
| Down: 5'-CCAGTAGGTTCTCACTCGCT-3' | |
| GAPDH | Up:5'-AGCTCACTGGCATGGCCTTC-3' |
| Down: 5'-CGCCTGCTTCACCACCTTCT-3' |
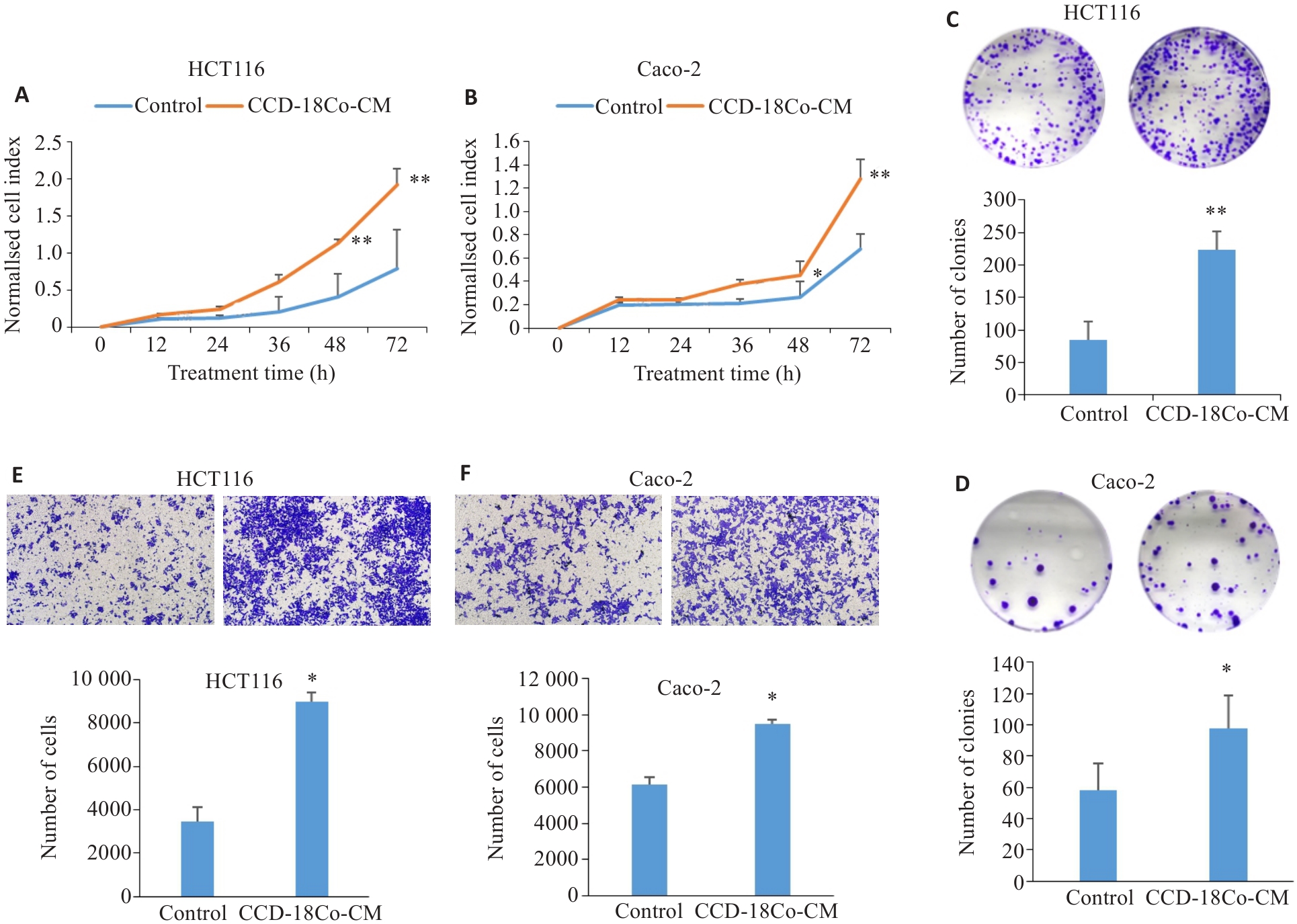
图1 CCD-18Co-CM可增强CRC细胞的增殖、克隆形成及迁能力
Fig.1 CCD-18Co-CM promotes proliferation (A, B), clone formation (C, D) and migration (E, F; original magnification: ×100) of CRC cells analyzed using real-time cellular analysis, colony forming assay and Transwell assay (Mean±SD, n=3). *P<0.05, **P<0. 01 vs control.
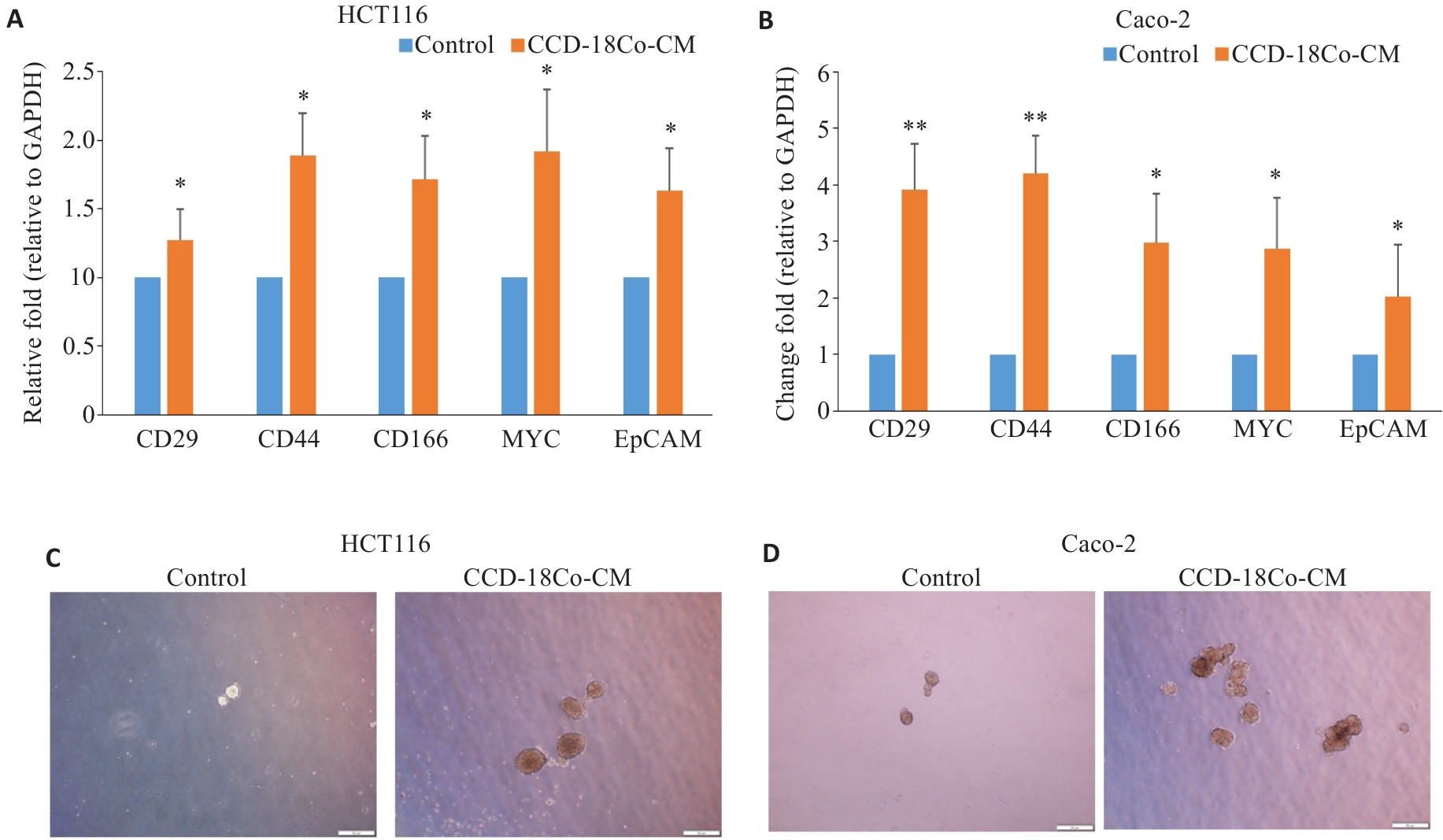
图2 CCD-18Co-CM促进CRC细胞的干性特征
Fig.2 CCD-18Co-CM promotes stemness characteristics of CRC cells. A, B: Relative mRNA expression levels of CD29, CD44, CD166, MYC and EPCAM in HCT116 and Caco-2 cells after CCD-18Co-CM treatment detected by RT-qPCR (Mean±SD, n=3). C, D: Morphology of the tumor spheres formed by HCT116 and Caco-2 cells after CCD-18Co-CM treatment (Scale bar = 50 μm). *P<0.05, **P<0.01 vs control.
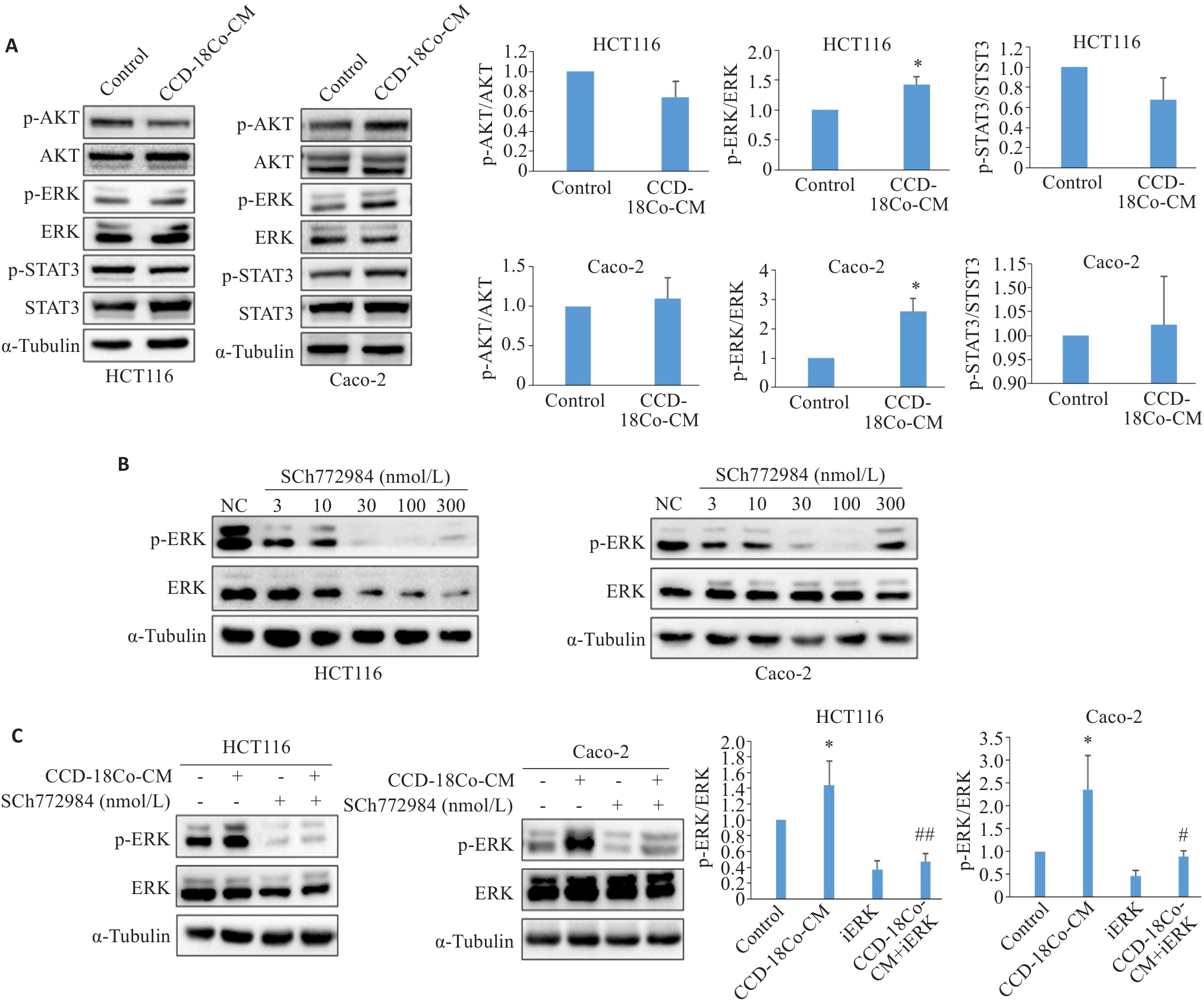
图3 CCD-18Co-CM激活CRC细胞中ERK信号通路
Fig.3 CCD-18Co-CM activates the ERK signaling pathway in CRC cells. A: Western blotting for detecting expression levels of STAT3, p-STAT3, AKT, p-AKT, ERK and p-ERK proteins in HCT116 and Caco-2 cells treated with CCD-18Co-CM. B: Western blotting for detecting expression levels of ERK and p-ERK in HCT116 and Caco-2 cells treated with different concentrations of SCH772984. C: Western blotting for detecting expression levels of ERK and p-ERK in HCT116 and Caco-2 cells after combined treatment with CCD-18Co-CM and 100 nmol/L SCH772984. Data are presented as Mean±SD (n=3). *P<0.05 vs control; #P<0.05, ##P<0.01 vs CCD-18Co-CM.
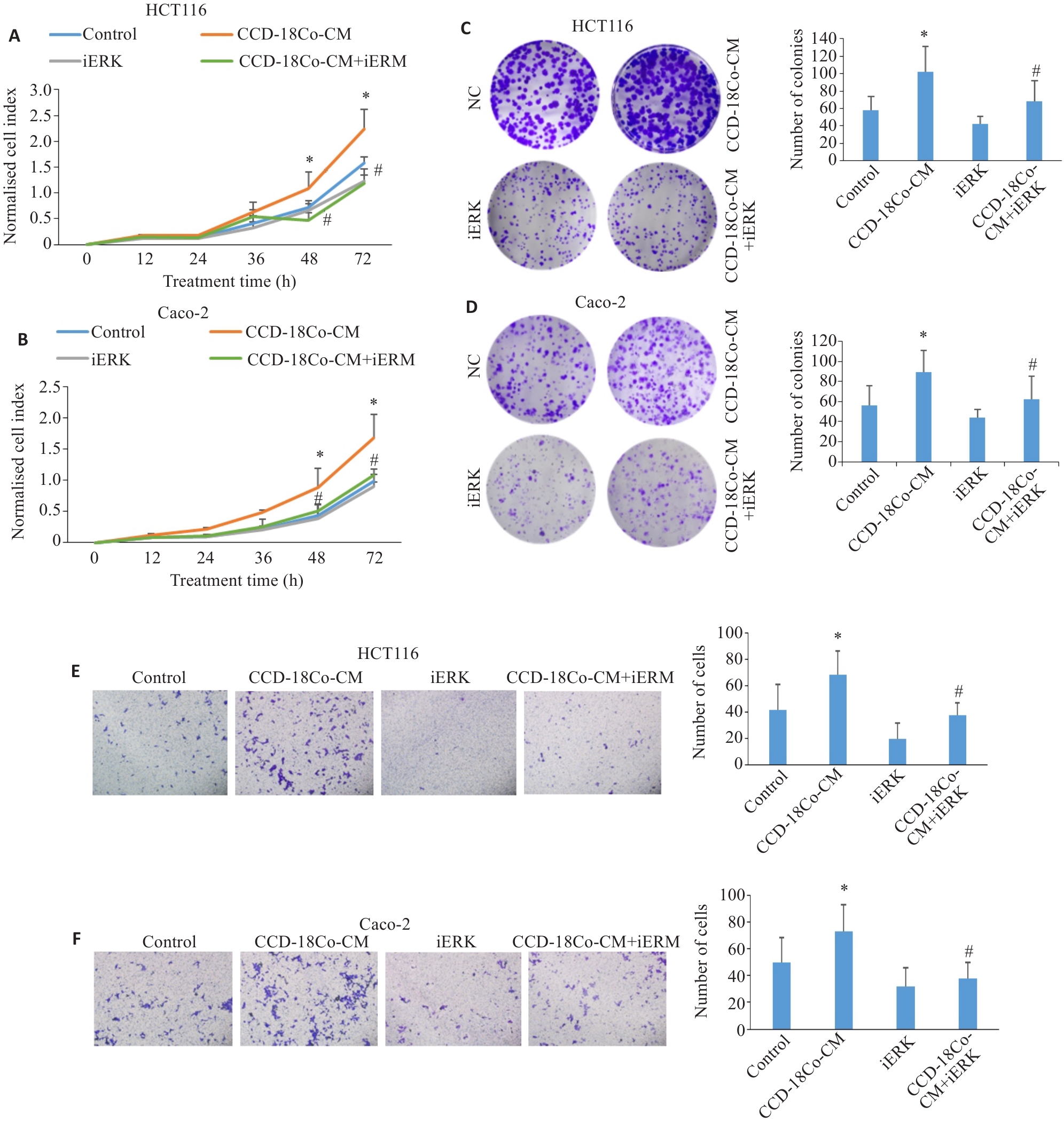
图4 iERK可抑制CCD-18Co-CM诱导的CRC细胞增殖、克隆形成和迁移能力的增强
Fig.4 SCH772984 (iERK) inhibits CCD-18Co-CM-induced proliferation, clone formation, and migration of CRC cells. A, B: RTCA for assessing proliferation of HCT116 and Caco-2 cells treated with CCD-18Co-CM and iERK (100 nmol/L). C, D: Colony-forming assay of HCT116 and Caco-2 cells treated with CCD-18Co-CM and 100 nmol/L iERK (×100). E, F:Transwell assay of HCT116 and Caco-2 cells treated with CCD-18Co-CM and 100 nmol/L iERK (×100). Data are presented as Mean±SD (n=3). *P<0.05 vs control, #P<0.05 vs CCD-18Co-CM.
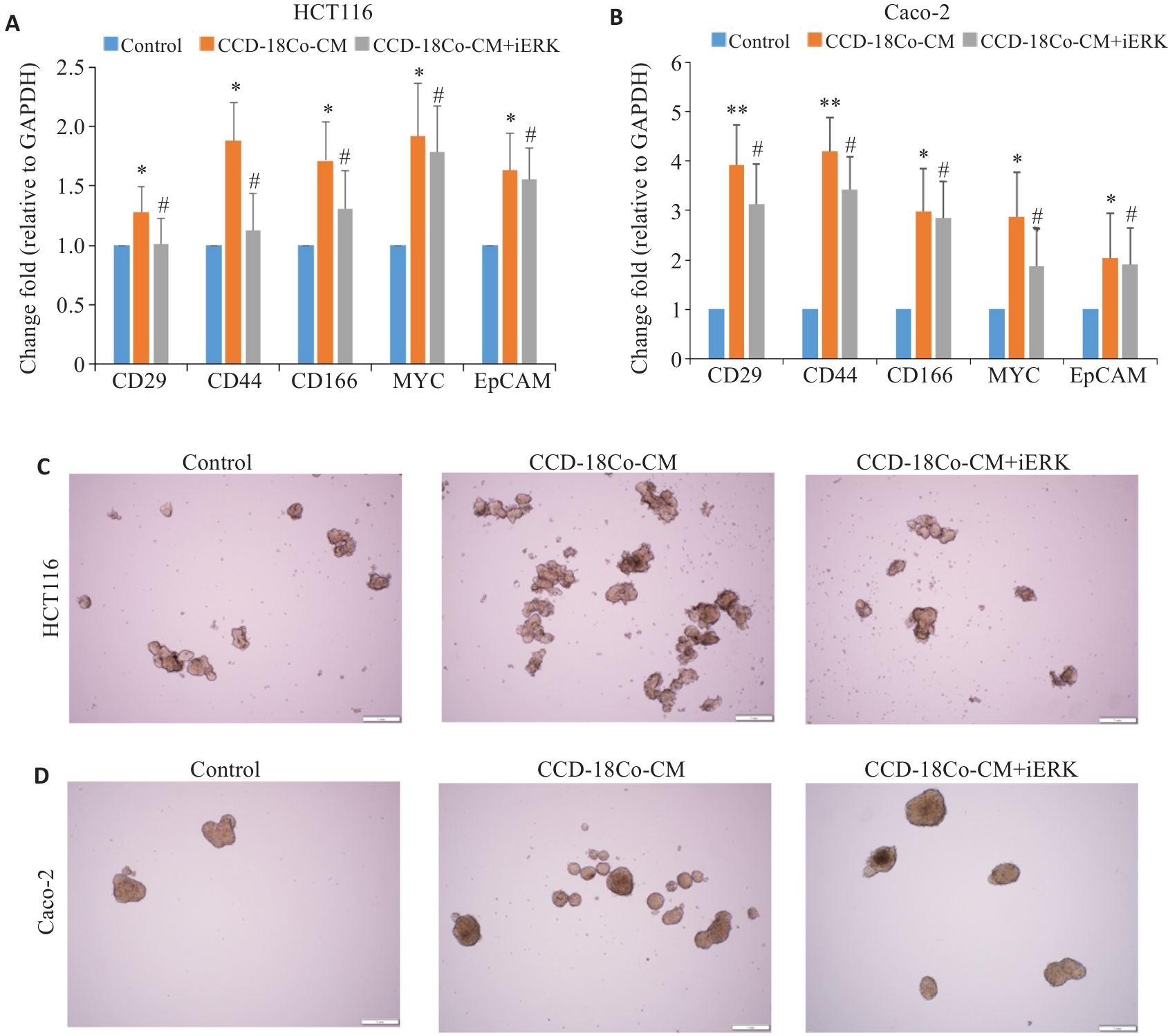
图5 iERK抑制CCD-18Co-CM诱导的CRC细胞干性特征的增强
Fig.5 iERK decreases cancer stemness of CRC cells induced by CCD-18Co-CM. A, B: RT-qPCR for detecting mRNA expression levels of stemness-related genes in HCT116 and Caco-2 cells treated with CCD-18Co-CM and SCH772984 (Mean±SD, n=3). C, D: Morphology of tumor spheres formed by HCT116 and Caco-2 cells after CCD-18Co-CM and SCH772984 treatment (Scale bar=1 mm). *P<0.05, **P<0.01 vs control; #P<0.05 vs CCD-18Co-CM.
| 1 | Dyba T, Randi G, Bray F, et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers[J]. Eur J Cancer, 2021, 157: 308-47. |
| 2 | Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality[J]. Gut, 2017, 66(4): 683-91. |
| 3 | Mao XQ, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives[J]. Mol Cancer, 2021, 20(1): 131. |
| 4 | Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment[J]. Cancer Discov, 2021, 11(4): 933-59. |
| 5 | Tiwari A, Trivedi R, Lin SY. Tumor microenvironment: barrier or opportunity towards effective cancer therapy[J]. J Biomed Sci, 2022, 29(1): 83. |
| 6 | Verginadis II, Avgousti H, Monslow J, et al. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression[J]. Nat Cell Biol, 2022, 24(6): 940-53. |
| 7 | Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts[J]. Nat Rev Cancer, 2020, 20(3): 174-86. |
| 8 | Fang T, Lv HW, Lv GS, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer[J]. Nat Commun, 2018, 9(1): 191. |
| 9 | Chen MH, Gu YY, Zhang AL, et al. Biological effects and mechanisms of matrine and other constituents of Sophora flavescens in colorectal cancer[J]. Pharmacol Res, 2021, 171: 105778. |
| 10 | Rausio H, Cervera A, Heuser VD, et al. PIK3R1 fusion drives chemoresistance in ovarian cancer by activating ERK1/2 and inducing rod and ring-like structures[J]. Neoplasia, 2024, 51: 100987. |
| 11 | 邓 婷, 杜伯雨, 郗雪艳. 结直肠癌细胞通过激活成纤维细胞的ERK通路诱导癌症相关成纤维细胞的形成[J]. 南方医科大学学报, 2023, 43(6): 943-51. |
| 12 | Correa-Gallegos D, Jiang DS, Rinkevich Y. Fibroblasts as confederates of the immune system[J]. Immunol Rev, 2021, 302(1): 147-62. |
| 13 | Gong Z, Li Q, Shi JY, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment[J]. Immunity, 2022, 55(8): 1483-500. e9. |
| 14 | Chen W, Chen YW, Su J, et al. CaMKII mediates TGFβ1-induced fibroblasts activation and its cross talk with colon cancer cells[J]. Dig Dis Sci, 2022, 67(1): 134-45. |
| 15 | Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy[J]. J Cell Physiol, 2019, 234(6): 8381-95. |
| 16 | Angius A, Scanu AM, Arru C, et al. Portrait of cancer stem cells on colorectal cancer: molecular biomarkers, signaling pathways and miRNAome[J]. Int J Mol Sci, 2021, 22(4): 1603. |
| 17 | Ferrer-Mayorga G, Niell N, Cantero R, et al. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts[J]. Sci Rep, 2019, 9(1): 8085. |
| 18 | Dias Carvalho P, Mendonça S, Martins F, et al. Modulation of fibroblast phenotype by colorectal cancer cell-secreted factors is mostly independent of oncogenic KRAS[J]. Cells, 2022, 11(16): 2490. |
| 19 | Hassametto A, Tanomrat R, Muangthong T, et al. Role of oxidative stress-dependent C/EBPβ expression on CAF transformation inducing HCT116 colorectal cancer cell progression; migration and invasion[J]. Asian Pac J Cancer Prev, 2023, 24(11): 3825-35. |
| 20 | Si GF, Li SY, Zheng Q, et al. MiR-1246 shuttling from fibroblasts promotes colorectal cancer cell migration[J]. Neoplasma, 2021, 68(2): 317-24. |
| 21 | Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways[J]. J Exp Clin Cancer Res, 2020, 39(1): 112. |
| 22 | Mhaidly R, Mechta-Grigoriou F. Role of cancer-associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer[J]. Immunol Rev, 2021, 302(1): 259-72. |
| 23 | Widjaja AA, Chothani S, Viswanathan S, et al. IL11 stimulates IL33 expression and proinflammatory fibroblast activation across tissues[J]. Int J Mol Sci, 2022, 23(16): 8900. |
| 24 | Pein M, Insua-Rodríguez J, Hongu T, et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs[J]. Nat Commun, 2020, 11(1): 1494. |
| 25 | Takatsu F, Suzawa K, Tomida S, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer progression and drug resistance in non-small cell lung cancer[J]. J Mol Med, 2023, 101(12): 1603-14. |
| 26 | Tian BQ, Chen XJ, Zhang HH, et al. Urokinase plasminogen activator secreted by cancer-associated fibroblasts induces tumor progression via PI3K/AKT and ERK signaling in esophageal squamous cell carcinoma[J]. Oncotarget, 2017, 8(26): 42300-13. |
| 27 | Liu L, Liu SC, Luo HJ, et al. GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERα‑positive breast cancer cells[J]. Aging, 2021, 13(12): 16178-97. |
| 28 | Tayama H, Karasawa H, Yamamura A, et al. The association between ERK inhibitor sensitivity and molecular characteristics in colorectal cancer[J]. Biochem Biophys Res Commun, 2021, 560: 59-65. |
| 29 | Lin K, Zhao Y, Tang YQ, et al. Collagen I-induced VCAN/ERK signaling and PARP1/ZEB1-mediated metastasis facilitate OSBPL2 defect to promote colorectal cancer progression[J]. Cell Death Dis, 2024, 15(1): 85. |
| 30 | Liu W, Tang JT, Gao W, et al. PPP2R1B abolishes colorectal cancer liver metastasis and sensitizes Oxaliplatin by inhibiting MAPK/ERK signaling pathway[J]. Cancer Cell Int, 2024, 24(1): 90. |
| [1] | 纪凯, 于冠宇, 周乐其, 张天帅, 凌潜龙, 满文江, 朱冰, 张卫. HNRNPA1基因在结直肠癌组织中高表达及其潜在的诊断和治疗价值[J]. 南方医科大学学报, 2024, 44(9): 1685-1695. |
| [2] | 张银亮, 骆泽谭, 赵睿, 赵娜, 徐志东, 奥迪, 丛古一, 刘新宇, 郑海伦. 血根碱通过调控STUB1/GPX4诱导直肠癌细胞发生铁死亡[J]. 南方医科大学学报, 2024, 44(8): 1537-1544. |
| [3] | 戎圣炜, 李宏芳, 魏怡然, 冯子航, 甘 露, 邓仲豪, 赵 亮. 锌指蛋白-36缺陷抑制小鼠的成骨细胞分化:基于激活ERK/MAPK通路[J]. 南方医科大学学报, 2024, 44(4): 697-705. |
| [4] | 颜 畅, 刘 爽, 宋庆志, 胡艺冰. 二甲双胍通过抑制线粒体氧化磷酸化降低结直肠癌干细胞的自我更新能力[J]. 南方医科大学学报, 2023, 43(8): 1279-1286. |
| [5] | 魏 可, 石纪雯, 肖雨寒, 王文锐, 杨清玲, 陈昌杰. miR-30e-5p过表达促进结直肠癌细胞的增殖和迁移:基于下调PTEN激活CXCL12轴[J]. 南方医科大学学报, 2023, 43(7): 1081-1092. |
| [6] | 张雪芳, 陈延华, 李宗恒, 尚 靖, 袁泽婷, 邓皖利, 骆 莺, 韩 娜, 殷佩浩, 殷 军. 六神丸治疗小鼠结肠炎相关性结直肠癌的作用机制:基于网络药理学和体内验证方法[J]. 南方医科大学学报, 2023, 43(7): 1051-1062. |
| [7] | 邓 婷, 杜伯雨, 郗雪艳. 结直肠癌细胞通过激活成纤维细胞的ERK通路诱导癌症相关成纤维细胞的形成[J]. 南方医科大学学报, 2023, 43(6): 943-951. |
| [8] | 马振南, 赵雪峰, 张晓微, 许广大, 刘福全. DTX2通过Notch2/Akt轴促进结直肠癌细胞的迁移和侵袭[J]. 南方医科大学学报, 2023, 43(3): 340-348. |
| [9] | 王旋成, 朱一帆, 周海琳, 黄宗声, 陈鸿炜, 张嘉豪, 杨珊伊, 陈广辉, 张淇淞. 血清非靶向代谢组学联合靶向胆汁酸代谢组学筛查结直肠癌的潜在生物标志物[J]. 南方医科大学学报, 2023, 43(3): 443-453. |
| [10] | 曾心靛, 陈 利, 周 鹏, 唐 婷, 陈 曦, 胡 丹, 王 川, 陈丽丽. 鹦鹉热衣原体III型分泌蛋白SINC通过激活MAPK/ERK信号通路促进宿主细胞自噬[J]. 南方医科大学学报, 2023, 43(2): 294-299. |
| [11] | 吴超英, 程文俊. 载脂蛋白E通过激活ERK/MMP9信号通路促进子宫内膜癌细胞的迁移[J]. 南方医科大学学报, 2023, 43(2): 232-241. |
| [12] | 赵欢灵, 凌羽晓, 宓 帅, 朱家豪, 范佳耀, 杨 叶, 王 晶, 李迎君. Leptin循环水平与结直肠腺瘤及结直肠癌的关联性:一项病例对照和孟德尔随机化研究[J]. 南方医科大学学报, 2023, 43(12): 1989-1997. |
| [13] | 邓金海, 潘 腾, 周广林, 高 悦, 彭伟雄, 魏 玮, 吕纯鑫. 高表达分泌颗粒蛋白II增加结直肠癌细胞对奥沙利铂的耐药性[J]. 南方医科大学学报, 2023, 43(10): 1657-1664. |
| [14] | 姚婉瑜, 汪枭睿, 杨 雨, 游俊雄, 金军国, 曾 平, 韩钦芮, 姚学清, 孙学刚, 周 瑾. 靶向结直肠癌的小白菊内酯脂质体纳米颗粒诱导程序性坏死并改善T细胞耗竭[J]. 南方医科大学学报, 2023, 43(10): 1674-1681. |
| [15] | 丁虹芳, 李肖娟, 周璐炜, 崔 智, 蒙海德, 王 娟. ANP32A沉默体外抑制结直肠癌的生长、侵袭和迁移:基于AKT信号通路活性的下降[J]. 南方医科大学学报, 2023, 43(1): 52-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||