南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (9): 1696-1703.doi: 10.12122/j.issn.1673-4254.2024.09.09
董梦璐1( ), 朱恬1, 马俊文2, 杜晓红3, 冯媛1,4,5(
), 朱恬1, 马俊文2, 杜晓红3, 冯媛1,4,5( )
)
收稿日期:2024-04-08
出版日期:2024-09-20
发布日期:2024-09-30
通讯作者:
冯媛
E-mail:dongmlu@163.com;yuanstar@smu.edu.cn
作者简介:董梦璐,硕士,E-mail: dongmlu@163.com
基金资助:
Menglu DONG1( ), Tian ZHU1, Junwen MA2, Xiaohong DU3, Yuan FENG1,4,5(
), Tian ZHU1, Junwen MA2, Xiaohong DU3, Yuan FENG1,4,5( )
)
Received:2024-04-08
Online:2024-09-20
Published:2024-09-30
Contact:
Yuan FENG
E-mail:dongmlu@163.com;yuanstar@smu.edu.cn
Supported by:摘要:
目的 使用饮食诱导肥胖(DIO)大鼠模型,探索间歇性缺氧-复氧(IHR)对肥胖大鼠体质量、食水摄入量、循环代谢因子和中枢瘦素注射反应的影响。 方法 通过12周高脂饮食(HFD)喂养建立DIO大鼠模型,将其随机分为3组并继续HFD喂养:常氧组(NM,n=15)、间歇性缺氧组(IH:6%O2,30周期/h,8 h/d,4周,n=15),IHR组(缺氧2周后复氧2周,n=15)。记录大鼠体质量、饮食饮水情况,检测循环瘦素、IL-6、Ang-II含量。IHR干预结束后,大鼠接受4 μg瘦素侧脑室注射,1 h后处死取材下丘脑及肝脏。通过免疫组化观察下丘脑POMC、FRA-1、FRA-2表达,Western blotting检测下丘脑POMC、pSTAT3、LepR表达,RT-PCR检测下丘脑和肝脏中LepR mRNA含量,对比各组大鼠下丘脑瘦素受体(LepR)及下游通路蛋白的变化。 结果 IH暴露导致DIO大鼠体质量 (P=0.001)和摄食量(P=0.001)增加,全身炎症因子升高(瘦素P=0.004;IL-6 P=0.008;Ang-II P<0.001)。IH抑制下丘脑食欲抑制肽POMC表达(P<0.001 vs NM组),降低反映瘦素反应性神经元活性的FRA-1表达(P<0.001 vs NM组),抑制对瘦素响应的pSTAT3表达(瘦素+vs瘦素-,P=0.241),降低对外源性瘦素给药的反应性(P<0.001 vs NM组),并下调下丘脑和肝脏LepR mRNA含量(P<0.001 vs NM组)。经过2周的复氧治疗后,IH加剧的体质量增加和代谢紊乱能够得到改善,下丘脑瘦素反应性也有所提高。 结论 IH可能通过下调LepR表达损害下丘脑瘦素信号传导,从而促进肥胖大鼠增重,这可以通过复氧治疗得到改善。
董梦璐, 朱恬, 马俊文, 杜晓红, 冯媛. 复氧改善间歇性缺氧所致的肥胖大鼠下丘脑瘦素反应性降低[J]. 南方医科大学学报, 2024, 44(9): 1696-1703.
Menglu DONG, Tian ZHU, Junwen MA, Xiaohong DU, Yuan FENG. Reoxygenation improves reduced hypothalamic leptin responsiveness induced by intermittent hypoxia in obese rats[J]. Journal of Southern Medical University, 2024, 44(9): 1696-1703.
| Primer | Sequence (5'-3') | Product size (bp) |
|---|---|---|
| β-actin F | GCCATGTACGTAGCCATCCA | 375 |
| β-actin R | GAACCGCTCATTGCCGATAG | |
| LepR F | TGGCCCATGAGTAAAGTGAATGCT | 274 |
| LepR R | TCTTTGGTTTTCCAACTCCTTCCA |
表1 PCR引物序列
Tab.1 Primer sequences for RT-qPCR
| Primer | Sequence (5'-3') | Product size (bp) |
|---|---|---|
| β-actin F | GCCATGTACGTAGCCATCCA | 375 |
| β-actin R | GAACCGCTCATTGCCGATAG | |
| LepR F | TGGCCCATGAGTAAAGTGAATGCT | 274 |
| LepR R | TCTTTGGTTTTCCAACTCCTTCCA |
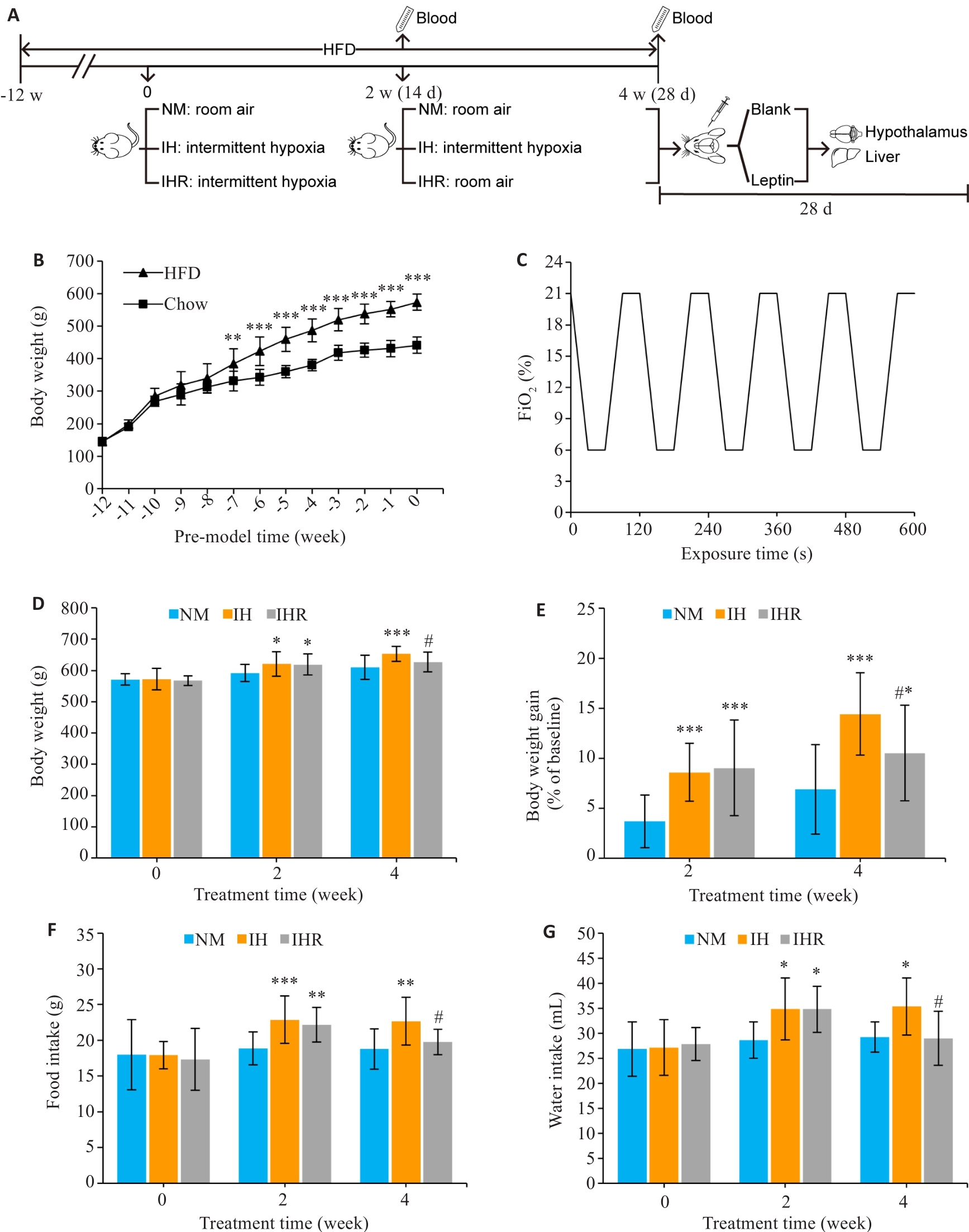
图1 IH导致DIO大鼠体质量、摄食和饮水量增加,而复氧则起相反的作用
Fig.1 Intermittent hypoxia (IH) increases weight gain and diet and water intake of DIO rats, which can be reduced by reoxygenation treatment. A: Protocol for intermittent hypoxia-reoxygenation (IHR) treatment in DIO rat models. Four-week-old male SD rats were fed HFD for 12 weeks and then separately treated with normoxia (NM), IH, and IHR for 4 weeks (n=15 per group). Following hypoxia treatment, 9 rats in each group were randomly selected to sacrifice, while the remaining rats were given intracerebroventricular injection of 4 μg leptin and sacrificed 1 h later. B: Body weight changes in HFD group (n=30) and normal chow group (n=10). C: IH cycle setting. D-G: Changes in body weight, weight gain and daily food and water intake in DIO rats with different treatments. Data are presented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001 vs NM group; #P<0.05 vs IH group.
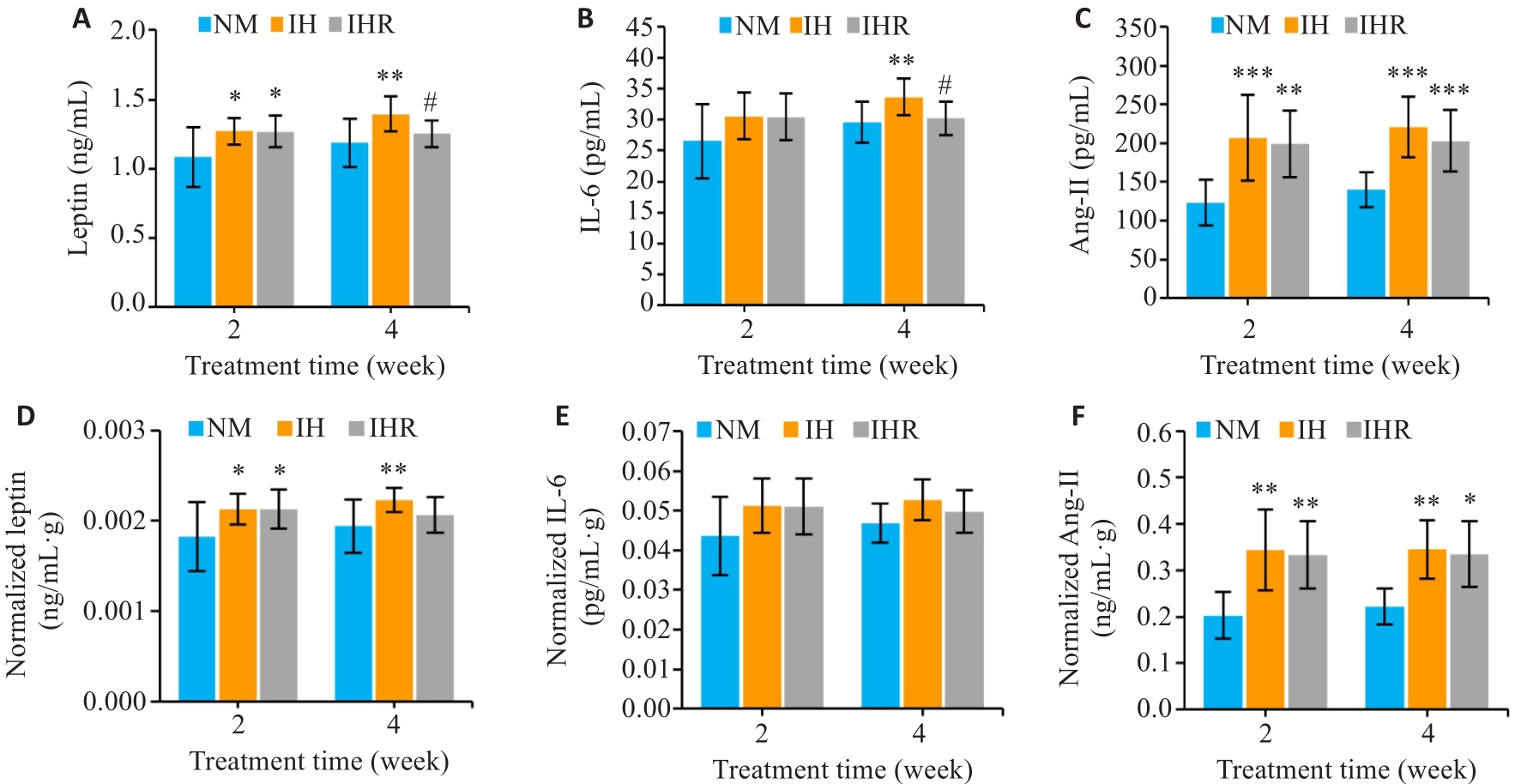
图2 IH显著升高DIO大鼠循环瘦素、IL-6和Ang-II水平,复氧使其降低
Fig.2 IH results in obvious elevation of leptin, IL-6, and Ang-II levels in DIO rats, which are lowered by reoxygenation. A-C: Changes in circulating leptin, IL-6, and Ang-II in each group after hypoxia treatment. D-F: Serum leptin, IL-6, and Ang-II levels normalized by body weight. Data are presented as Mean±SD (n=9). *P<0.05, **P<0.01, ***P<0.001 vs NM group; #P<0.05 vs IH group.
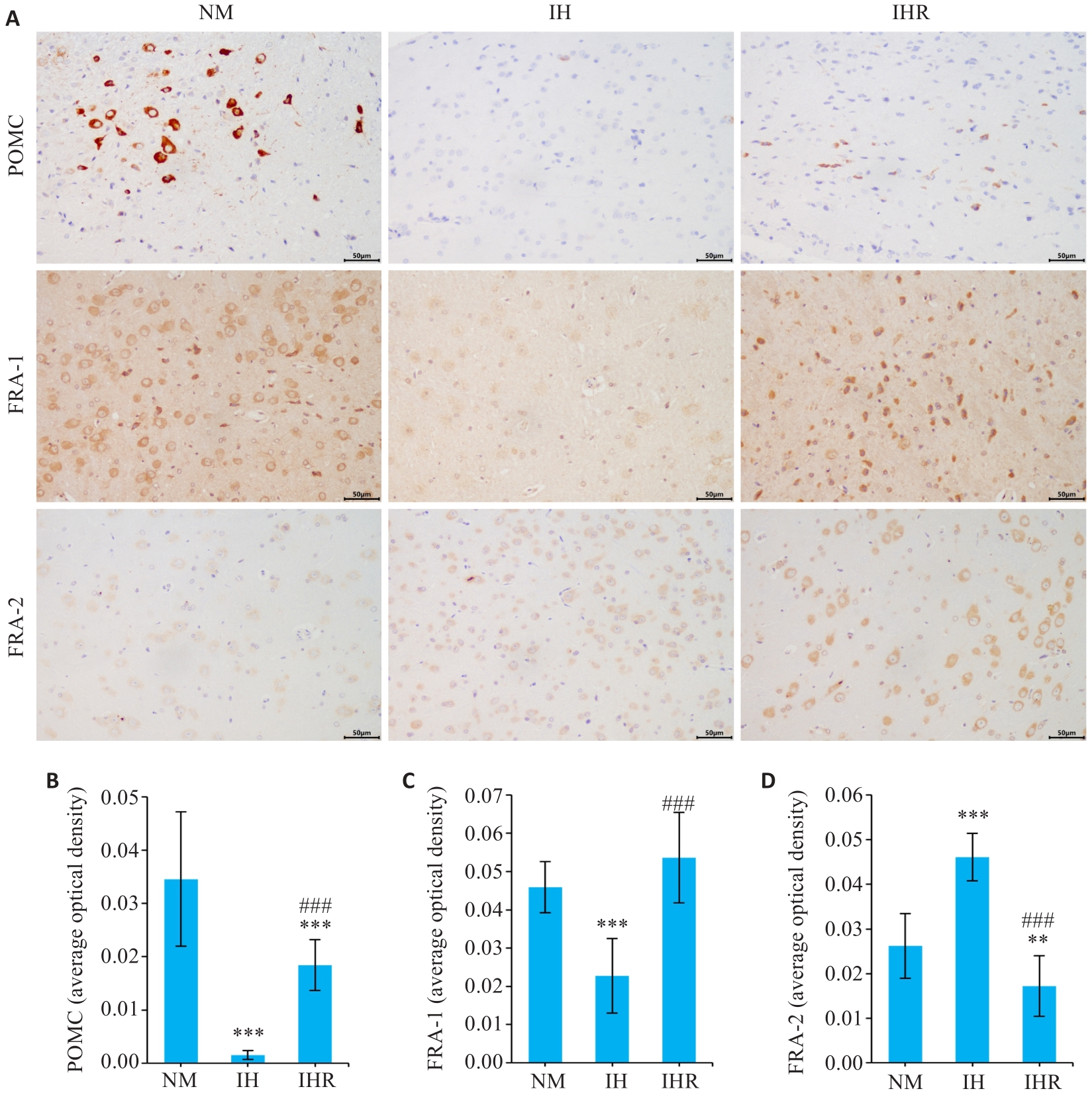
图3 IH抑制DIO大鼠下丘脑食欲抑制肽POMC产生和瘦素反应性神经元活性,复氧有助于缓解
Fig.3 IH inhibits the production of the hypothalamic appetite-suppressing peptide POMC and the activity of leptin-responsive neurons in DIO rats, while reoxygenation promotes remission of these changes. A: Immunohistochemical analysis of expressions of POMC and FRA-1/2 in the hypothalamus (scale bar=50 μm). B-D: Semi-quantitative evaluation of POMC, FRA-1, and FRA-2 expressions (Mean±SD, n=9). **P<0.01, ***P<0.001 vs NM group; ###P<0.001 vs IH group.
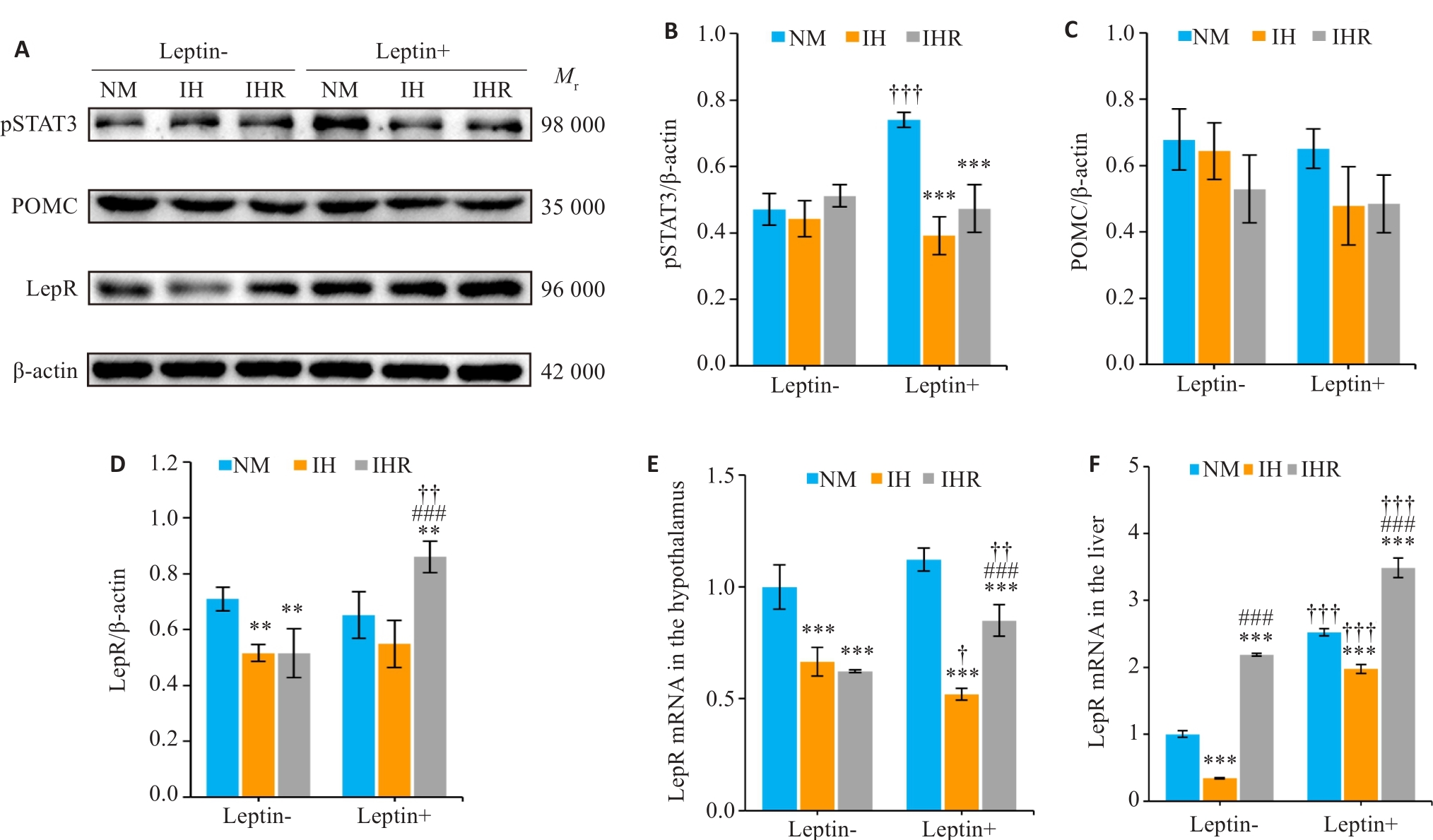
图4 IH损害DIO大鼠下丘脑瘦素反应性,而复氧不能完全恢复
Fig.4 IH impairs hypothalamic leptin responsiveness in the DIO rats, and reoxygenation treatment fails to achieve full restoration. A: Western blotting of pSTAT3, POMC, and LepR in the hypothalamus 1 h after leptin ICV injection. B-D: Relative expressions of pSTAT3, POMC, and LepR normalized by β-actin. E, F: Relative mRNA expression of hypothalamic and hepatic LepR. Data are presented as Mean±SD (n=6). **P<0.01, ***P<0.001 vs NM group; ###P<0.01 vs IH group; †P<0.05, ††P<0.01, †††P<0.001 vs leptin ICV injection.
| 1 | PATEL S R. Obstructive Sleep Apnea [J]. Ann Intern Med, 2019, 171(11): ITC81-96. |
| 2 | Lv RJ, Liu XY, Zhang Y, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome[J]. Signal Transduct Target Ther, 2023, 8(1): 218. |
| 3 | Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review[J]. JAMA, 2020, 323(14): 1389-400. |
| 4 | Giampá SQC, Lorenzi-Filho G, Drager LF. Obstructive sleep apnea and metabolic syndrome[J]. Obesity, 2023, 31(4): 900-11. |
| 5 | Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults[J]. Am J Epidemiol, 2013, 177(9): 1006-14. |
| 6 | Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials[J]. Thorax, 2015, 70(3): 258-64. |
| 7 | Feng Y, Maislin D, Keenan BT, et al. Physical activity following positive airway pressure treatment in adults with and without obesity and with moderate-severe obstructive sleep apnea[J]. J Clin Sleep Med, 2018, 14(10): 1705-15. |
| 8 | Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea[J]. J Clin Sleep Med, 2013, 9(10): 989-93. |
| 9 | Zhang YY, Chua S Jr. Leptin function and regulation[J]. Compr Physiol, 2017, 8(1): 351-69. |
| 10 | Zhu CJ, Jiang ZY, Xu YZ, et al. Profound and redundant functions of arcuate neurons in obesity development[J]. Nat Metab, 2020, 2(8): 763-74. |
| 11 | Ulukavak Ciftci T, Kokturk O, Bukan N, et al. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome[J]. Respiration, 2005, 72(4): 395-401. |
| 12 | Imayama I, Prasad B. Role of leptin in obstructive sleep apnea[J]. Ann Am Thorac Soc, 2017, 14(11): 1607-21. |
| 13 | Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment[J]. Eur Respir J, 2003, 22(2): 251-7. |
| 14 | Chen X, Niu X, Xiao Y, et al. Effect of continuous positive airway pressure on leptin levels in patients with obstructive sleep apnea: a meta-analysis[J]. Otolaryngol Head Neck Surg, 2015, 152(4): 610-8. |
| 15 | de Santis S, Cambi J, Tatti P, et al. Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in Obstructive Sleep Apnea Syndrome (OSAS) patients[J]. Pol Otolaryngol, 2015, 69(2): 1-8. |
| 16 | Tachikawa R, Ikeda K, Minami T, et al. Changes in energy metabolism after continuous positive airway pressure for obstructive sleep apnea[J]. Am J Respir Crit Care Med, 2016, 194(6): 729-38. |
| 17 | Ciriello J, Moreau JM, McCoy A, et al. Effect of intermittent hypoxia on arcuate nucleus in the leptin-deficient rat[J]. Neurosci Lett, 2016, 626: 112-8. |
| 18 | Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis[J]. Lancet Respir Med, 2019, 7(8): 687-98. |
| 19 | Moreau JM, Ciriello J. Effects of acute intermittent hypoxia on energy balance and hypothalamic feeding pathways[J]. Neuroscience, 2013, 253: 350-60. |
| 20 | Gozal D, Gileles-Hillel A, Cortese R, et al. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice[J]. Am J Respir Cell Mol Biol, 2017, 56(4): 477-87. |
| 21 | Wang HP, Wang Y, Xia TL, et al. Pathogenesis of abnormal hepatic lipid metabolism induced by chronic intermittent hypoxia in rats and the therapeutic effect of N-acetylcysteine[J]. Med Sci Monit, 2018, 24: 4583-91. |
| 22 | Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia[J]. Am J Physiol Lung Cell Mol Physiol, 2014, 307(2): L129-40. |
| 23 | Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose[J]. Am J Physiol Regul Integr Comp Physiol, 2014, 307(10): R1181-97. |
| 24 | Yue XP, Zhou YZ, Qiao M, et al. Intermittent hypoxia treatment alleviates memory impairment in the 6-month-old APPswe/PS1dE9 mice and reduces amyloid beta accumulation and inflammation in the brain[J]. Alzheimers Res Ther, 2021, 13(1): 194. |
| 25 | Rogers RS, Wang H, Durham TJ, et al. Hypoxia extends lifespan and neurological function in a mouse model of aging[J]. PLoS Biol, 2023, 21(5): e3002117. |
| 26 | Panza GS, Puri S, Lin HS, et al. Daily exposure to mild intermittent hypoxia reduces blood pressure in male patients with obstructive sleep apnea and hypertension[J]. Am J Respir Crit Care Med, 2022, 205(8): 949-58. |
| 27 | Saeed O, Bhatia V, Formica P, et al. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure[J]. J Card Fail, 2012, 18(5): 387-91. |
| 28 | Drager LF, Li JG, Reinke C, et al. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity[J]. Obesity, 2011, 19(11): 2167-74. |
| 29 | Valverde-Pérez E, Olea E, Obeso A, et al. Intermittent hypoxia and diet-induced obesity on the intestinal wall morphology in a murine model of sleep apnea[J]. Adv Exp Med Biol, 2023, 1427: 89-97. |
| 30 | Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea[J]. Am J Physiol Heart Circ Physiol, 2000, 279(1): H234-7. |
| 31 | Zhao SG, Zhu Y, Schultz RD, et al. Partial leptin reduction as an insulin sensitization and weight loss strategy[J]. Cell Metab, 2019, 30(4): 706-19.e6. |
| 32 | Zhao SG, Kusminski CM, Elmquist JK, et al. Leptin: less is more[J]. Diabetes, 2020, 69(5): 823-9. |
| 33 | Pretz D, Le Foll C, Rizwan MZ, et al. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity[J]. FASEB J, 2021, 35(2): e21216. |
| 34 | Friedman JM. Leptin and the endocrine control of energy balance[J]. Nat Metab, 2019, 1(8): 754-64. |
| 35 | Pérez-Cadahía B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system[J]. Biochem Cell Biol, 2011, 89(1): 61-73. |
| 36 | de Git KCG, Adan RAH. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation[J]. Obes Rev, 2015, 16(3): 207-24. |
| 37 | Balland E, Cowley MA. New insights in leptin resistance mechanisms in mice[J]. Front Neuroendocrinol, 2015, 39: 59-65. |
| 38 | Gileles-Hillel A, Almendros I, Khalyfa A, et al. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: effect of normoxic recovery[J]. Sleep, 2017, 40(3): 1093. |
| 39 | Bailly S, Daabek N, Jullian-Desayes I, et al. Partial failure of CPAP treatment for sleep apnoea: analysis of the French national sleep database[J]. Respirology, 2020, 25(1): 104-11. |
| 40 | Siopi D, Steiropoulos P. The influence of CPAP therapy on basal metabolic rate and physical activity in obese patients with obstructive sleep apnea[J]. Nutrients, 2023, 15(20): 4446. |
| 41 | Batool-Anwar S, Goodwin JL, Drescher AA, et al. Impact of CPAP on activity patterns and diet in patients with obstructive sleep apnea (OSA)[J]. J Clin Sleep Med, 2014, 10(5): 465-72. |
| 42 | Perrini S, Cignarelli A, Quaranta VN, et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea[J]. JCI Insight, 2017, 2(17): e94379. |
| [1] | 周雪利, 李华, 陈青宇, 靳美娜, 李海波, 白炜, 贾楚璇, 魏翠英. 慢性间歇低氧和复氧对大鼠胰岛素抵抗及骨骼肌miR-27a-3p/PPARγ/IRS1/PI3K/AKT表达的影响[J]. 南方医科大学学报, 2024, 44(9): 1729-1737. |
| [2] | 肖静, 李盈, 方敏, 巩红, 李文, 张春艳, 陈方尧, 张岩, 韩拓. 甘油三酯-葡萄糖指数与非肥胖型非酒精性脂肪性肝病的相关性[J]. 南方医科大学学报, 2024, 44(7): 1266-1271. |
| [3] | 陈洁, 刘晨旭, 王春, 李丽, 陶伟婷, 徐婧茹, 唐红辉, 黄丽. 外源性瘦素通过上调星形胶质细胞GLT-1和GLAST的表达减轻小鼠脑缺血再灌注引起的谷氨酸兴奋毒性损伤[J]. 南方医科大学学报, 2024, 44(6): 1079-1087. |
| [4] | 梁国新, 唐红悦, 郭畅, 张明明. miR-224-5p调控PI3K/Akt/FoxO1轴抑制氧化应激减轻缺氧/复氧诱导的心肌细胞损伤[J]. 南方医科大学学报, 2024, 44(6): 1173-1181. |
| [5] | 包汉生, 王苏童, 吕穆杰, 王永成, 姜 萍, 李 晓. 激活α7nAchR促进肥胖小鼠的脂肪稳态和米色脂肪生成及产热作用[J]. 南方医科大学学报, 2024, 44(3): 499-506. |
| [6] | 朱继伟, 卢曼路, 焦倩倩, 孙运良, 刘 璐, 丁红红, 于 燕, 潘 磊. 基于16S rRNA测序分析阻塞性睡眠呼吸暂停患者肠道靶标菌群的变化[J]. 南方医科大学学报, 2024, 44(1): 146-155. |
| [7] | 周 蓓, 李 静, 方晨圆, 黄亚楠, 桑贵蕊, 陶少平, 何春玲. 二甲双胍/维格列汀和利拉鲁肽对肥胖合并2型糖尿病患者的临床疗效比较[J]. 南方医科大学学报, 2023, 43(3): 436-442. |
| [8] | 叶 潇, 宋迎香, 赵 瑜, 朱大龙. 脂肪组织黏膜相关恒定T细胞通过分泌白介素4调节小鼠脂肪棕色化[J]. 南方医科大学学报, 2023, 43(11): 1881-1885. |
| [9] | 虞亘明, 王鑫玮, 骆金光, 苏 萧, 陶怀祥, 闻志远, 关 翰. SPP1在肾缺血再灌注诱导的急性肾损伤中的作用及机制[J]. 南方医科大学学报, 2023, 43(11): 1947-1954. |
| [10] | 吴江豪, 吴永鑫, 杨韵菲, 余 靖, 傅 饶, 孙 悦, 肖 谦. Mibefradil可改善肥胖小鼠骨骼肌的质量、功能和结构[J]. 南方医科大学学报, 2022, 42(7): 1032-1037. |
| [11] | 苏小凤, 韩继明, 高莹卉, 范 利, 何子君, 赵 哲, 林俊岭, 郭静静, 陈开兵, 高 燕, 刘 霖. 老年阻塞性睡眠呼吸暂停患者远期缺血性脑卒中的风险评分模型: 多中心前瞻性队列研究[J]. 南方医科大学学报, 2022, 42(3): 338-346. |
| [12] | 丁孟汝, 王国栋, 袁平川, 何曙光, 邵太丽, 柳春燕, 孔 祥. 多糖调控糖脂代谢的作用及其机制研究进展[J]. 南方医科大学学报, 2021, 41(3): 471-474. |
| [13] | 张 玲, 李贵芳, 苏莉莉, 杜 磊, 周东雷, 程晓芸, 林紫薇, 曲 伸. 黑棘皮病和非黑棘皮病患者的总睾酮水平与胰岛素抵抗的相关性[J]. 南方医科大学学报, 2021, 41(12): 1780-1786. |
| [14] | 苏小凤, 韩继明, 高莹卉, 何子君, 赵 哲, 林俊岭, 郭静静, 陈开兵, 高 燕, 刘 霖. 老年阻塞性睡眠呼吸暂停与代谢综合征各组分的相关性及其对远期不良心血管事件发生风险的影响[J]. 南方医科大学学报, 2021, 41(11): 1592-1599. |
| [15] | 高莹卉, 温永飞, 钱小顺, 赵力博, 徐 虎, 徐伟豪, 孔晓萱, 车贺宾, 王亚斌, 刘 霖. 无心血管合并症的老年阻塞性睡眠呼吸暂停综合征患者心功能变化及其影响因素分析[J]. 南方医科大学学报, 2020, 40(11): 1587-1592. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||