33.5%的胃肠道手术患者至少出现1种非致死性术后并发症,15%的患者发生2种或2种以上并发症[1],老年患者胃肠道手术后并发症发病率和死亡率显著高于非老年人群。此类患者常合并多种疾病,如冠心病、高血压、糖尿病、脑血管病及肾功能不全等,其生理储备功能下降且组织修复能力减弱,可能进一步增加术后并发症风险[2]。因此,系统性分析老年患者胃肠道手术后综合并发症危险因素具有重要的临床意义。 既往研究表明,高龄、美国麻醉医师协会(ASA)分级≥Ⅲ级、低血清白蛋白水平、术中低血压、围手术期输血和饮酒史与老年患者胃肠道手术后谵妄显著相关[3, 4]。术前白细胞计数、出血量以及高血压病史是老年患者胃肠道手术后认知功能障碍的独立危险因素[5]。手术类型是术后胃肠道功能紊乱的主要预测因素[6]。吸烟、合并症、围手术期失血可增加术后切口感染的风险[7]。然而,目前研究多聚焦于胃肠道手术后某一特定类型或某个系统的并发症,针对老年患者胃肠道手术后综合并发症危险因素的分析仍较缺乏。此外,既往危险因素多集中于难以干预的因素(如年龄、肿瘤类型等),较少关注可干预的因素(如疼痛管理和用药)。值得注意的是,Yokozuka等[8]曾对189例行择期胃肠道手术的老年患者进行相关研究,但其单中心设计及小样本量限制了结论的普适性。曹庆等[9]评估了中性粒细胞计数与淋巴细胞计数的比值/血清白蛋白预测胃肠道手术后综合并发症的价值,其纳入的影响因素仅局限于人口学特征及肿瘤类型。基于此,本研究利用全国多中心前瞻性数据库,全面探索整个围手术期(包括术前、术中及术后)与术后综合并发症相关的危险因素,旨在识别高危患者,为临床针对性改善老年患者胃肠道手术后结局提供可能。
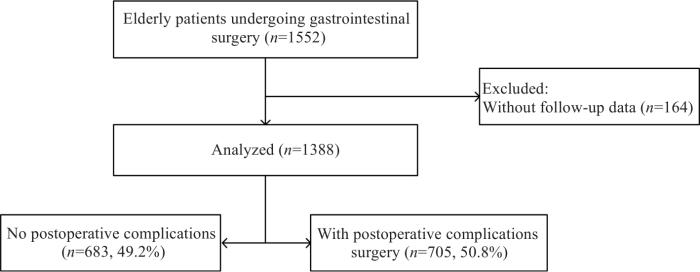
本研究对2020年4月~2022年4月在国内17家医院1388例接受择期胃肠道手术的老年患者资料进行回顾性分析,了解老年患者胃肠道手术后并发症的发生情况,识别术后综合并发症的独立危险因素,并探讨术后急性疼痛与术后各系统并发症的关系。
1 资料和方法
本研究数据来源于“中国老年患者围手术期数据库”,该数据库为一个来自多个地区和中心的围手术期老年患者的前瞻性队列。所有中心研究者均接受标准化操作流程培训,参与中心在线输入数据,并由数据核查人员进行审核和数据质量检查。原始数据库覆盖2020年4月~2022年4月期间接受非心脏手术的10 536例老年患者。本研究提取其中择期胃肠道手术患者数据进行分析,所有个人标识信息均已去标识化处理,以符合《赫尔辛基宣言》隐私保护原则。研究方案通过各参与中心伦理审查委员会批准(伦理批号:S2019-311-03),并在美国临床试验注册中心网站进行注册登记(网址为
1.1 研究对象
纳入标准:年龄≥65岁;接受全身麻醉下择期胃肠肿瘤切除术患者。排除标准:除胃/肠切除外,联合其他脏器的切除术;严重痴呆、语言障碍、听力或视觉严重受损、昏迷、终末期疾病等情况无法完成评估内容;患者或家属拒绝参与研究。剔除标准:术后30 d内并发症结局数据缺失的患者。
1.2 手术方式及围术期处理措施
所有患者均于入院后接受相应常规检查,术前给予预防性抗菌药物,行择期胃肠道手术治疗,术中常规监测心电图、无创血压、脉搏血氧饱和度、呼气末吸入麻醉药浓度、呼气末二氧化碳浓度、尿量等。所有患者均接受全身麻醉,必要时监测有创动脉压和中心静脉压。术中麻醉药物使用由麻醉医师酌情给予。术后实施补液、营养支持、镇痛等常规对症治疗。
1.3 信息收集和随访方法
住院期间收集患者基本信息(性别、年龄、合并症、体质量指数、ASA分级、吸烟饮酒史、术前营养支持、术前运动耐量分级、术前生活质量评价、术前衰弱状态),围手术期信息(手术方式、手术部位、手术时间、出血量、麻醉方式、术中镇痛药物使用情况、术后镇痛情况)和化验指标[血清白蛋白、血红蛋白、血糖、淋巴细胞计数,营养指数(PNI=血清白蛋白+淋巴细胞计数×5)]。麻醉方式分为单纯全身麻醉和全麻联合神经阻滞麻醉,术后是否镇痛分为无术后镇痛、术后采用静脉镇痛方式以及术后采用其他类型镇痛方式。分别在术后1~7 d由专门的访视团队在院内对患者进行随访,术后30 d通过电话随访。随访内容包括术后中枢神经精神系统并发症、心血管系统并发症、呼吸系统并发症、肾脏损伤和疼痛等。其中,谵妄在术后1~7 d采用意识错乱评估法(CAM)进行评估;抑郁在术后7 d和术后30 d采用患者健康问卷-9(PHQ-9)进行评估;生活质量评价在术后7 d和术后30 d采用欧洲五维健康量表(EQ-5D及EQ-VAS)进行评估;术后急性疼痛评分在术后1~7 d和术后30 d采用数字评分法(NRS,0=无痛,10=最大疼痛)进行评估,≥7分,即存在重度疼痛。其他结局通过患者病例资料及化验检查获得。在患者签署知情同意书后,定期联系患者并及时解答患者相关疑问。研究中随访时间设置为访视窗口的±3 d。
1.4 量表评价标准
1.4.1 谵妄
采用CAM进行测量,其诊断需满足以下核心特征:患者存在急性起病且症状呈波动性变化,同时合并注意力不集中,并伴有思维紊乱或意识水平改变中的至少1项。
1.4.2 生活质量评价
采用EQ-5D及EQ-VAS进行评价。EQ-5D从行动能力、自我护理、日常活动、疼痛/不适、焦虑/抑郁5个维度生成健康效用值(范围为-0.391~1.000)。EQ-VAS则要求患者在0(最差健康状态)至100(最佳健康状态)的标尺上标记当前主观健康感受。
1.4.3 运动耐量评估
代谢当量(MET)是评估运动强度和能量代谢的重要指标,以静坐时的能量消耗为基准。根据心肺功能储备分为四级:>10 METs为优秀,7~10 METs为良好,4~7 METs为中等,<4 METs提示功能储备不足。
1.4.4 衰弱评估量表
FRAIL量表涵盖疲乏、阻力增加或耐力减退、自由活动下降、疾病情况、体质量下降5项指标。总分0~5分,0分为健壮,1~2分为衰弱前期,3~5分为衰弱。≥3分可诊断为衰弱。
1.4.5 抑郁评分
采用PHQ-9 评估抑郁症状。总分范围0~27分,0~4分为正常,5~9分提示轻度抑郁,10~14分为中度,≥15分属重度抑郁。将≥5分作为抑郁症状的识别阈值。
1.5 观察指标及评价方法
主要结局指标为术后30 d综合并发症发生率,定义为至少发生1种并发症,合并多系统并发症时仅计为1次事件。包括手术相关并发症:包括手术部位感染(术后30 d内伴脓性渗出或影像学感染证据)、吻合口瘘(影像学异常或二次手术证实)、乳糜瘘(乳糜性引流液)及肠梗阻(腹胀伴CT显示肠管扩张或粘连);神经精神系统并发症:谵妄、脑卒中(神经功能异常>24 h且影像学证实缺血灶)和抑郁;心血管系统并发症:非致命性心梗(肌钙蛋白异常合并缺血症状/心电图改变)、心衰(症状体征伴NT-proBNP>125 ng/L或超声心动图异常)、心律失常(包括非窦性心律失常和传导阻滞)及不稳定性心绞痛(典型症状伴ST-T改变);呼吸系统并发症:术后肺部感染(抗生素调整+发热/白细胞增多/影像学新发浸润影)、呼吸衰竭(PaO₂/FiO₂<300或SPO₂<90%)、胸腔积液/肺不张/气胸(影像学新发或进展性病变)及肺栓塞(肺动脉血栓证据);消化系统并发症:胃瘫(需干预的胃排空延迟)、消化道出血(呕血/黑便/血红蛋白下降)及腹泻(≥3次稀便/日需治疗);急性肾损伤:根据KDIGO标准,术后48 h内血清肌酐升高>26.5 μmol/L或术后7 d内升高至基线值1.5倍以上(基线值为术前30 d内最低值)。
1.6 统计学分析
样本量是基于“中国老年患者围手术期数据库”中所有符合条件的患者数量。采用SPSS 26.0 进行统计分析。通过直方图和Shapiro-Wilk检验评估数据分布的正态性,非正态分布的连续变量(年龄、身高、体质量、体质量指数、EQ-5D、EQ-VAS、PNI、血清白蛋白、血红蛋白、血糖、阿片类药物的消耗、出血量和手术时间)以中位数(四分位距,IQR)表示,组间比较采用Mann-Whitney U检验;分类变量以频率(百分比)表示,组间比较采用χ 2 检验。
采用Logistic回归进行单因素分析,并按照逐步进入法将变量纳入多因素Logistic回归模型,从而明确老年患者胃肠道手术后30 d综合并发症的独立危险因素。Logistic回归分析结果采用比值比(OR)和95%置信区间(CI)报告。采用多因素Logistic回归分析探讨疼痛与术后各系统并发症的相关性,结果以森林图可视化。调整的协变量包括性别、年龄、体质量指数、吸烟、饮酒、术前慢性疼痛病史、术前营养支持、术前运动耐量分级、术前生活质量评价、术前衰弱、术中是否使用非甾体抗炎药物、术中是否使用右美托咪定、术中阿片类药物消耗、手术方式、手术部位、手术时间、出血量、麻醉方式以及术后镇痛情况。采用双侧检验,P<0.05为差异具有统计学意义。缺失数据采用均值插补法进行插补。
2 结果
2.1 入组情况
图1
表1 患者的一般资料及单因素分析
Tab.1
| Parameter | Total (n=1388) | No postoperative complications (n=683) | Have postoperative complications (n=705) | OR (95% CI) | P |
|---|---|---|---|---|---|
| Age (year) | 71.0 (68.0, 76.0) | 71.0 (68.0, 75.0) | 72.0 (68.0, 76.0) | 1.028 (1.008-1.048) | 0.005 |
| Gender [n (%)] | 1.002 (0.804-1.250) | 0.984 | |||
| Female | 906 (65.3%) | 446 (65.3%) | 460 (65.2%) | ||
| Male | 482 (34.7%) | 237 (34.7%) | 245 (34.8%) | ||
| Height (cm) | 164.6 (160.0, 170.0) | 165.0 (160.0, 170.0) | 164.6 (160.0, 170.0) | 1.001 (0.998-1.014) | 0.884 |
| Weight (kg) | 63.7 (57.0, 70.0) | 63.7 (57.0, 70.0) | 63.7 (57.0, 70.0) | 0.997 (0.987-1.007) | 0.541 |
| BMI (kg/m2) | 23.5 (21.3, 25.4) | 23.5 (21.3, 25.6) | 23.5 (21.4, 25.1) | 0.985 (0.953-1.018) | 0.374 |
| ASA | 1.262 (1.001-1.590) | 0.049 | |||
| Ⅰ-Ⅱ | 978 (70.5%) | 498 (72.9%) | 480 (68.1%) | ||
| Ⅲ-Ⅴ | 410 (29.5%) | 185 (27.1%) | 225 (31.9%) | ||
| Medical History [n (%)] | |||||
| Hypertension | 605 (43.6%) | 289 (42.3%) | 316 (44.8%) | 1.107 (0.896-1.369) | 0.346 |
| Diabetes | 290 (20.9%) | 131 (19.2%) | 159 (22.6%) | 1.227 (0.946-1.591) | 0.123 |
| Coronary heart disease | 188 (13.5%) | 88 (12.9%) | 100 (14.2%) | 1.118 (0.821-1.521) | 0.479 |
| Cerebrovascular disease | 79 (5.7%) | 31 (4.5%) | 48 (6.8%) | 1.537 (0.966-2.445) | 0.070 |
| Respiratory disease | 55 (4.0%) | 21 (3.1%) | 34 (4.8%) | 1.597 (0.917-2.781) | 0.098 |
| Kidney disease | 66 (4.8%) | 29 (4.2%) | 37 (5.2%) | 1.249 (0.759-2.055) | 0.381 |
| Smoking | 432 (31.1%) | 218 (31.9%) | 214 (30.4%) | 0.930 (0.741-1.167) | 0.529 |
| Alcohol consumption | 410 (29.5%) | 207 (30.3%) | 203 (28.8%) | 0.930 (0.738-1.171) | 0.537 |
| Preoperative chronic pain | 255 (18.4%) | 106 (15.5%) | 149 (21.1%) | 1.459 (1.108-1.920) | 0.007 |
| Preoperative nutritional support | 163 (11.7%) | 71 (10.4%) | 92 (13.0%) | 1.294 (0.931-1.798) | 0.125 |
| Preoperative psychological and functional status | |||||
| Frailty [n (%)] | 35 (2.5%) | 14 (2.0%) | 21 (3.0%) | 1.467 (0.740-2.909) | 0.272 |
| EQ-5D | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) | 0.9 (0.9, 1.0) | 0.073 (0.014-0.380) | 0.002 |
| EQ-VAS | 76.0 (70.0, 80.0) | 76.0 (75.0, 80.0) | 76.0 (70.0, 80.0) | 0.992 (0.984-1.000) | 0.060 |
| MET | 0.719 (0.483-1.070) | 0.104 | |||
| <4 | 108 (7.8%) | 45 (6.6%) | 63 (8.9%) | ||
| ≥4 | 1280 (92.2%) | 638 (93.4%) | 642 (91.1%) | ||
| Preoperative laboratory test | |||||
| PNI | 42.3 (37.4, 126.3) | 42.9 (38.1, 139.0) | 41.7 (36.8, 89.5) | 0.998 (0.997-1.000) | 0.043 |
| Albumin (g/L) | 39.0 (36.2, 41.2) | 39.0 (36.5, 41.0) | 39.0 (36.0, 41.3) | 0.995 (0.984-1.006) | 0.401 |
| Hemoglobin (g/L) | 120.6 (108.0, 134.0) | 122.0 (109.0, 135.0) | 120.3 (106.0, 134.0) | 0.996 (0.991-1.001) | 0.120 |
| Glucose (µmol/L) | 5.5 (4.8, 5.8) | 5.5 (4.8, 5.8) | 5.5 (4.8, 5.8) | 1.021 (0.956-1.091) | 0.531 |
| Intraoperative medication | |||||
| Consumption of opioids (mg) | 143.5 (50.0, 232.9) | 140.0 (50.0, 230.0) | 148.0 (45.0, 240.0) | 1.000 (0.999-1.001) | 0.796 |
| NSAIDs [n (%)] | 529 (38.1%) | 264 (38.7%) | 265 (37.6%) | 0.956 (0.770-1.187) | 0.683 |
| Dexmedetomidine [n (%)] | 333 (24.0%) | 174 (25.5%) | 159 (22.6%) | 0.852 (0.666-1.090) | 0.203 |
| Surgical/Anesthetic parameters | |||||
| Blood loss (mL) | 100.0 (50.0, 128.7) | 60.0 (50.0, 128.7) | 100.0 (50.0, 128.7) | 1.002 (1.001-1.003) | <0.001 |
| Duration of surgery (min) | 191.5 (143.3, 240.0) | 185.0 (136.0, 230.0) | 198.4 (150.0, 250.0) | 1.002 (1.001-1.003) | 0.002 |
| Location of surgery [n (%)] | 0.930 (0.746-1.160) | 0.521 | |||
| Gastrectomy | 481 (34.7%) | 231 (33.8%) | 250 (35.5%) | ||
| Enterectomy | 907 (65.3%) | 452 (66.2%) | 455 (64.5%) | ||
| Type of surgery [n (%)] | 1.194 (0.934-1.527) | 0.157 | |||
| Open | 1050 (75.6%) | 528 (77.3%) | 522 (74.0%) | ||
| Minimally invasive | 338 (24.4%) | 155 (22.7%) | 183 (26.0%) | ||
| Anesthesia methods [n (%)] | 0.843 (0.621-1.145) | 0.275 | |||
| GA | 1197 (86.2%) | 582 (85.2%) | 615 (87.2%) | ||
| GA+RA | 191 (13.8%) | 101 (14.8%) | 90 (12.8%) | ||
| Postoperative analgesia methods [n (%)] | 0.191 | ||||
| No | 480 (34.6%) | 286 (36.5%) | 194 (34.6%) | ||
| Intravenous analgesia | 784 (56.5%) | 432 (55.2%) | 352 (56.5%) | 1.201 (0.954, 1.512) | |
| Others | 124 (8.9%) | 65 (8.3%) | 59 (9.8%) | 1.338 (0.900, 1.990) | |
| Postoperative acute pain | 1.359 (1.079-1.712) | 0.009 | |||
| Mild to moderate pain | 973 (70.1%) | 501 (73.4%) | 472 (67.0%) | ||
| Severe pain | 415 (29.9%) | 182 (26.6%) | 233 (33.0%) |
ASA: American standards association; BMI: Body mass index; CI: Confidence interval; EQ-5D: Euro quality of life-5 dimension; EQ-VAS: Euro quality of life-visual analogue scale; GA: General anesthesia; IQR: Interquartile range; MET: Metabolic equivalent of task; NSAIDs: Non-steroidal antiinflammatory drugs; RA: Regional anesthesia; OR: Odds ratio; PNI: Prognostic nutritional index. PNI=Albumin+Lymphocyte count ×5. Morphine equivalent doses were calculated to compare opioid consumption.
2.2 术后30 d综合并发症发生情况
1388例老年患者中,术后综合并发症705例(50.8%),其中呼吸系统并发症451例(32.5%),神经精神系统并发症306例(22.0%),手术相关并发症171例(12.3%),急性肾损伤53例(3.8%),需干预的消化系统并发症32例(2.3%),心血管系统并发症32例(2.3%)。发生率最高的并发症是肺部感染341例(24.6%),其次是谵妄231例(16.6%)和手术部位感染143例(10.3%)(表2)。
表2 术后并发症发生情况
Tab.2
| Postoperative complications | n (%) |
|---|---|
| Overall complications | 705 (50.8%) |
| Respiratory complications | 451 (32.5%) |
| Pulmonary infection | 341 (24.6%) |
| Respiratory failure | 7 (0.5%) |
| Pleural effusion | 60 (4.3%) |
| Atelectasis | 19 (1.4%) |
| Pneumothorax | 20 (1.4%) |
| Bronchiostenosis | 3 (0.2%) |
| Aspiration pneumonia | 2 (0.1%) |
| Pulmonary embolism | 3 (0.2%) |
| Pulmonary edema | 2 (0.1%) |
| Neuropsychiatric complications | 306 (22.0%) |
| Stroke | 12 (0.9%) |
| Delirium | 231 (16.6%) |
| Depression | 91 (6.6%) |
| Procedure-related complications | 171 (12.3%) |
| Surgical site infection | 143 (10.3%) |
| Anastomotic leakage | 47 (3.4%) |
| Ileus | 21 (1.5%) |
| Chylous fistula | 2 (0.1%) |
| Acute Kidney Injury | 53 (3.8%) |
| Gastrointestinal complications | 32 (2.3%) |
| Gastroparesis | 9 (0.6%) |
| Diarrhea | 16 (1.2%) |
| Gastrointestinal bleeding | 10 (0.7%) |
| Cardiovascular complications | 32 (2.3%) |
| Myocardial infarction | 4 (0.3%) |
| Heart failure | 3 (0.2%) |
| Angina | 1 (0.1%) |
| Arrhythmia | 25 (1.8%) |
| Cardiac arrest or death | 2 (0.1%) |
2.3 胃肠道手术老年患者的一般资料及术后并发症的单因素分析
单因素分析显示,年龄、ASA分级、术前慢性疼痛史、术前EQ-5D评分、术前PNI、失血量、手术时间以及术后急性疼痛与术后综合并发症发生率显著相关,差异有统计学意义(P<0.05,表1)。
2.4 老年患者胃肠手术后综合并发症的多因素分析
多因素Logistic回归分析显示,高龄、较低的PNI、较低的EQ-5D评分、更多的失血量以及术后急性疼痛是术后综合并发症的独立危险因素(P<0.05,表3)。
表3 术后综合并发症的多因素分析
Tab.3
| Factor | b | SE | Wald | OR (95% CI) | P |
|---|---|---|---|---|---|
| Age (year) | 0.026 | 0.010 | 6.710 | 1.026(1.006-1.046) | 0.010 |
| Preoperative PNI | -0.002 | 0.001 | 4.352 | 0.998(0.997-1.000) | 0.037 |
| Preoperative EQ-5D | -2.369 | 0.855 | 7.681 | 0.094(0.018-0.500) | 0.006 |
| Blood loss (mL) | 0.002 | 0.000 | 21.013 | 1.002(1.001-1.003) | <0.001 |
| Postoperative acute pain (Mild to moderate pain/severe pain) | 0.269 | 0.121 | 4.959 | 1.308(1.033-1.657) | 0.026 |
2.5 急性疼痛与各系统并发症的多因素分析
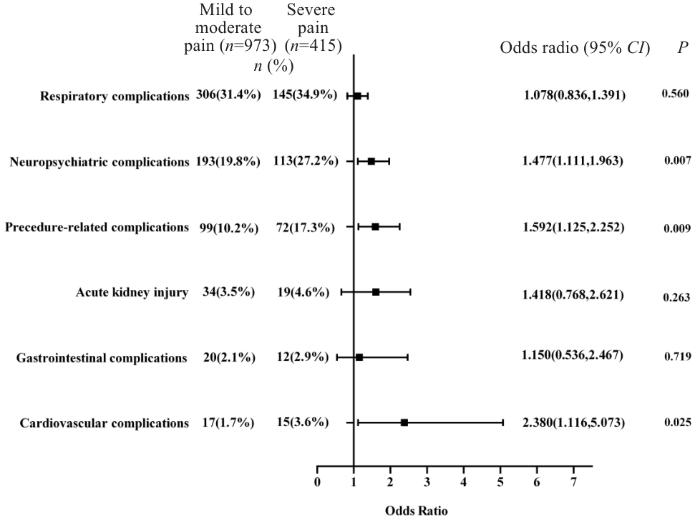
重度疼痛患者术后30 d神经精神系统并发症(27.2% vs 19.8%)、手术相关并发症(17.3% vs 10.2%)以及心血管系统并发症(3.6% vs 1.7%)发生率高于轻中度疼痛患者,差异有统计学意义(P<0.01)。多因素Logistic回归分析显示,术后急性疼痛显著增加术后神经精神系统并发症 [OR(95% CI): 1.477(1.111~1.963)]、手术相关并发症[OR(95% CI):1.592,(1.125~2.252)]以及心血管系统并发症[OR(95% CI):2.380(1.116~5.073)]发生风险(图2)。
图2
图2
术后急性疼痛对各系统并发症影响的森林图
Fig.2
Forest plot of the effect of acute postoperative pain on postoperative complications in elderly patients undergoing gastrointestinal surgeries.
3 讨论
随着人口老龄化进程的加快,老年患者的围手术期管理和术前风险评估已成为临床实践的核心挑战。作为围手术期手术安全的重要参与者,麻醉医师通过识别高危患者,积极干预,有利于患者预后。本研究基于全国多中心前瞻性数据库,首次将麻醉可干预的围手术期指标(如术后急性疼痛)与传统风险因素(高龄、营养状态等)纳入同一分析框架。结果显示,高龄、预后营养指数、生活质量评分、失血量以及术后急性疼痛与术后30 d并发症存在显著相关性,这与既往文献一致[10-12]。此外,术后急性疼痛与多系统并发症的强关联性(OR=1.078~ 2.380),提示疼痛管理在老年患者围术期管理的重要地位。这一发现为麻醉医师主导的多学科干预提供了重要循证依据,也为个体化围手术期策略的制定指明了方向。
EQ-5D是一种评估患者生活质量的量表,涵盖行动能力、疼痛感知及心理状态等多维度,是反映患者功能状态的综合指标。既往研究表明[19],EQ-5D评分与衰弱呈负相关。患者行动能力减弱,消耗量降低,生活自理能力下降,而日常活动受影响则进一步导致耐力和行动力的减退,这可能是EQ-5D与衰弱密切相关的重要原因。本研究也评估了患者术前衰弱状态,多因素分析未观察到其与结局的相关性,这可能与患者的衰弱评估多为正常或仅为衰弱前期有关。此外,术前较低的EQ-5D评分不仅仅提示患者身体处于衰弱状态,可能还受情绪或疼痛的困扰,多系统储备能力减弱,极易受到应激源的影响,增加不良预后的风险。这也提示,传统以器官功能为核心的评估体系需向“躯体-心理-社会”多维模式转型。改善患者术前功能状态,提高患者生活质量评分对患者术后转归具有重要意义。
在胃肠道手术中,约30%的患者会经历严重的术后疼痛,约12%患者因疼痛相关症状就诊急诊[20]。术后疼痛控制不佳与术后并发症的增加相关,对生活质量和功能恢复产生负向影响[21],是持续性疼痛和长期使用阿片类药物的危险因素[22]。本研究发现,术后重度疼痛患者神经精神并发症风险增加37.4%,手术相关并发症风险升高67.6%,这一结果提示在围手术期积极控制患者术后疼痛对患者预后至关重要。其机制可能是急性疼痛通过激活下丘脑-垂体-肾上腺轴,促进皮质醇及儿茶酚胺释放,抑制自然杀伤细胞活性,同时上调Toll样受体4信号通路,导致促炎因子(IL-6、TNF-α)过度表达[23]。动物实验表明,术后疼痛模型中小胶质细胞活化可诱发海马区神经元凋亡,这可能是谵妄高发的神经生物学基础[24]。本研究结果强调了在麻醉期间进行疼痛管理的重要性。这与近期其他几项研究结果一致,这些研究证实了急性疼痛与术后并发症,如谵妄、抑郁、心脏事件等之间存在关联[25-27]。基于加速康复外科的理念,单一的治疗模式无法充分管理疼痛和术后恢复情况。最近的研究表明,炎症-免疫反应不仅在术后疼痛恢复中发挥作用,而且在整个功能恢复中都发挥作用[28]。既往研究表明,微创手术可以减轻术后疼痛,还可减少炎症-免疫反应,从而改善患者预后[29]。此外,其他局部镇痛技术也被证明是有效的。在药物应用方面,术中使用大剂量糖皮质激素具有显著的镇痛作用,并可减轻术后疲劳和手术引起的其他不良病理生理反应[30-34],从而促进患者康复。术中使用右美托咪定也可能改善患者术后疼痛,提高患者预后[35, 36]。因此,了解机体的哪些改变可以减轻患者术后疼痛,加速患者手术康复值得关注。
本研究存在一定的局限性。首先,本研究没有纳入肿瘤分期和新辅助放化疗情况,但纳入了与术后并发症可能相关的多种影响因素,包括营养、药物、功能状态、麻醉手术相关因素等;其次,本研究为观察性研究,虽然规定了严格的纳排标准并进行了多因素逻辑回归分析,但是仍然存在不可预知的混杂因素影响,未来有必要开展前瞻性大规模研究来提高结论的可靠性;第三,缺乏术后炎症标志物(如IL-6、C反应蛋白)的动态监测数据,限制了机制的深入探讨;最后,本研究发现术后急性疼痛与多系统并发症发生相关,但未对术后急性疼痛相关影响因素做进一步分析,未来有必要深入研究以通过控制术后急性疼痛来改善患者预后。
综上所述,老年患者胃肠道手术后并发症是多种风险因素交互作用的结果,其中术后急性疼痛作为麻醉可调控的关键节点,与多系统并发症存在强效关联。通过术前优化患者的营养状况、提高生理机能以及积极控制术后急性疼痛的整合策略,有望改善患者预后。未来研究需要进一步阐明疼痛免疫调控分子靶点,为临床制定针对性干预措施、减少并发症的发生提供新思路。
参考文献
Postoperative complications and mortality after major gastrointestinal surgery
[J].
Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways
[J].
Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery
[J].
Risk factors of delirium after gastrointestinal surgery: a meta-analysis
[J].
Risk factors and predictive value of perioperative neurocognitive disorders in elderly patients with gastrointestinal tumors
[J].
Incidence and risk factors for postoperative gastrointestinal dysfunction occurrence after gastrointestinal procedures in US patients
[J].
Prophylactic negative pressure wound therapy following colorectal perforation: defining the risk factors for delayed wound healing
[J].
New risk factors of postoperative complications in elective gastrointestinal surgery of elderly patients: a prospective cohort study
[J].
Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients
[J].
The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset
[J].
Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the postoperative outcomes within enhanced recovery after surgery protocol (POWER) study
[J].
The hallmarks of aging
[J].
Frailty in elderly people
[J].
Association of nutritional status with clinical outcomes of stroke patients with acute anterior circulation large vessel occlusion after emergency endovascular treatment
[J].
The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis
[J].
High preoperative prognostic nutritional index is associated with less postoperative complication-related impairment of long-term survival after laparoscopic gastrectomy for gastric cancer
[J].
The dual presence of frailty and locomotive syndrome is associated with a greater decrease in the EQ-5D-5L index
[J].
Chronic pain, healthcare utilization, and quality of life following gastrointestinal surgery
[J].
Risk of pain and gastrointestinal complaints at 6Months after elective abdominal surgery
[J].
Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature
[J].
Nociplastic pain mechanisms and toll-like receptors as promising targets for its management
[J].
Chronic pain and neuroinflammation
[J].
Postoperative delirium: perioperative assessment, risk reduction, and management
[J].
The influence of anesthesia and pain management on cognitive dysfunction after joint arthroplasty: a systematic review
[J].
Association between pre-operative depression and postoperative pain outcomes
[J].
Clinical recovery from surgery correlates with single-cell immune signatures
[J].
Enhanced recovery after surgery: which components, if any, impact on the systemic inflammatory response following colorectal surgery? : a systematic review
[J].
Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double-blind, placebo-controlled clinical trial
[J].
The effect of pre-operative methylprednisolone on early endothelial damage after total knee arthroplasty: a randomised, double-blind, placebo-controlled trial
[J].
Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial
[J].
Surgical stress response and the potential role of preoperative glucocorticoids on post-anesthesia care unit recovery
[J].
Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis
[J].
Intraoperative dexmedetomidine on postoperative pain in gastrointestinal surgery: an observational study
[J].
Effect of intraoperative dexmedetomidine on recovery of gastrointestinal function after abdominal surgery in older adults: a randomized clinical trial
[J].
Effect of esketamine on postoperative analgesia and postoperative delirium in elderly patients undergoing gastrointestinal surgery
[J].
Correlation and influencing factors of preoperative anxiety, postoperative pain, and delirium in elderly patients undergoing gastrointestinal cancer surgery
[J].
Depression and neurological disorders
[J].
Depression and cognitive decline in elderly: causes and consequences
[J].