Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (4): 853-861.doi: 10.12122/j.issn.1673-4254.2025.04.21
Previous Articles Next Articles
Yi ZHANG1,2( ), Yu SHEN1,2, Zhiqiang WAN1,2, Song TAO1, Yakui LIU1, Shuanhu WANG1(
), Yu SHEN1,2, Zhiqiang WAN1,2, Song TAO1, Yakui LIU1, Shuanhu WANG1( )
)
Received:2024-09-26
Online:2025-04-20
Published:2025-04-28
Contact:
Shuanhu WANG
E-mail:2826348547@qq.com;knight01030103@126.com
Yi ZHANG, Yu SHEN, Zhiqiang WAN, Song TAO, Yakui LIU, Shuanhu WANG. High expression of CDKN3 promotes migration and invasion of gastric cancer cells by regulating the p53/NF-κB signaling pathway and inhibiting cell apoptosis[J]. Journal of Southern Medical University, 2025, 45(4): 853-861.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.04.21
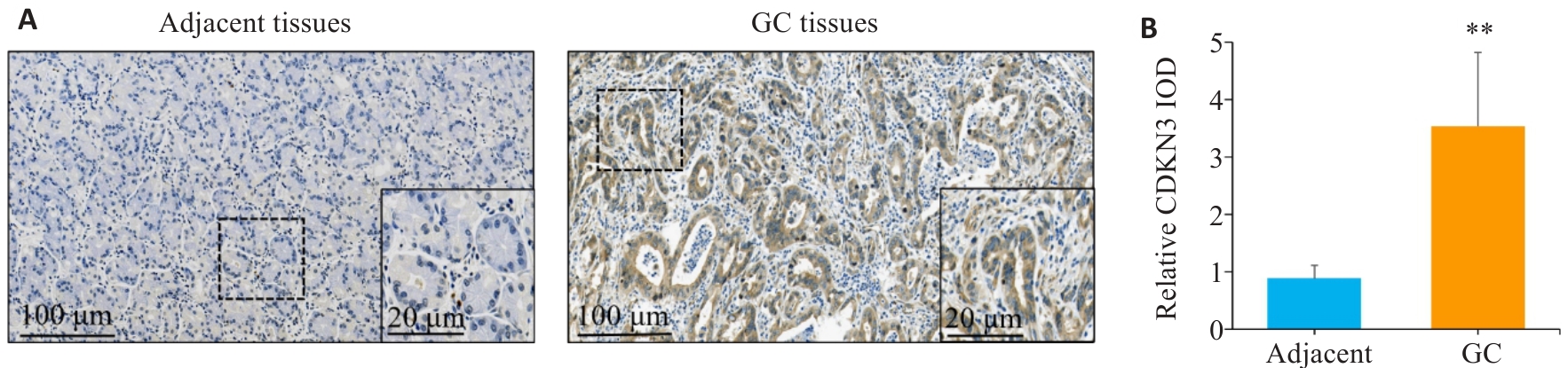
Fig.1 Expression of CDKN3 in gastric cancer (GC) and adjacent tissues. A: Immunohistochemistry of CDKN3 in gastric cancer (GC) and adjacent tissues. B: Relative IOD values of CDKN3 expression. **P<0.01.
| Clinicopatholological parameters | n | CDKN3 | χ2 | P | |
|---|---|---|---|---|---|
| Low expression (n=57) | High expression (n=57) | ||||
| Gender | |||||
| Male | 81 | 43 (53.09%) | 38 (46.91%) | 1.066 | 0.302 |
| Female | 33 | 14 (42.42%) | 19 (57.58%) | ||
| Age (year) | |||||
| <60 | 46 | 22 (47.83%) | 24 (52.17%) | 0.146 | 0.703 |
| ≥60 | 68 | 35 (51.47%) | 33 (48.53%) | ||
| CEA (μg/L) | |||||
| <5 | 69 | 46 (66.67%) | 23 (33.33%) | 19.422 | <0.001 |
| ≥5 | 45 | 11 (24.44%) | 34 (75.56%) | ||
| CA19-9 (kU/L) | |||||
| <37 | 86 | 53 (61.63%) | 33 (38.37%) | 18.937 | <0.001 |
| ≥37 | 28 | 4 (14.29%) | 24 (85.71%) | ||
| Tumor size (cm) | |||||
| <5 | 67 | 38 (56.72%) | 29 (43.28%) | 2.932 | 0.087 |
| ≥5 | 47 | 19 (40.43%) | 28 (59.57%) | ||
| Histological type | |||||
| Adenocarcinoma | 104 | 53 (50.96%) | 51 (49.04%) | 0.438 | 0.508 |
| Other | 10 | 4 (40.00%) | 6 (60.00%) | ||
| Grade of differentiation | |||||
| Well | 10 | 4 (40.00%) | 6 (60.00%) | 4.001 | 0.135 |
| Moderate | 38 | 24 (63.16%) | 14 (36.84%) | ||
| Poor | 66 | 29 (43.94%) | 37 (56.06%) | ||
| T Stage | |||||
| T1-T2 | 37 | 24 (64.86%) | 13 (35.14%) | 4.842 | 0.028 |
| T3-T4 | 77 | 33 (42.86%) | 44 (57.14%) | ||
| N Stage | |||||
| N0-N1 | 71 | 41 (57.75%) | 30 (42.25%) | 4.518 | 0.034 |
| N2-N3 | 43 | 16 (37.21%) | 27 (62.79%) | ||
Tab.1 Relationship between the expression level of CDKN3 in GC tissues and clinicopathological parameters of the patients
| Clinicopatholological parameters | n | CDKN3 | χ2 | P | |
|---|---|---|---|---|---|
| Low expression (n=57) | High expression (n=57) | ||||
| Gender | |||||
| Male | 81 | 43 (53.09%) | 38 (46.91%) | 1.066 | 0.302 |
| Female | 33 | 14 (42.42%) | 19 (57.58%) | ||
| Age (year) | |||||
| <60 | 46 | 22 (47.83%) | 24 (52.17%) | 0.146 | 0.703 |
| ≥60 | 68 | 35 (51.47%) | 33 (48.53%) | ||
| CEA (μg/L) | |||||
| <5 | 69 | 46 (66.67%) | 23 (33.33%) | 19.422 | <0.001 |
| ≥5 | 45 | 11 (24.44%) | 34 (75.56%) | ||
| CA19-9 (kU/L) | |||||
| <37 | 86 | 53 (61.63%) | 33 (38.37%) | 18.937 | <0.001 |
| ≥37 | 28 | 4 (14.29%) | 24 (85.71%) | ||
| Tumor size (cm) | |||||
| <5 | 67 | 38 (56.72%) | 29 (43.28%) | 2.932 | 0.087 |
| ≥5 | 47 | 19 (40.43%) | 28 (59.57%) | ||
| Histological type | |||||
| Adenocarcinoma | 104 | 53 (50.96%) | 51 (49.04%) | 0.438 | 0.508 |
| Other | 10 | 4 (40.00%) | 6 (60.00%) | ||
| Grade of differentiation | |||||
| Well | 10 | 4 (40.00%) | 6 (60.00%) | 4.001 | 0.135 |
| Moderate | 38 | 24 (63.16%) | 14 (36.84%) | ||
| Poor | 66 | 29 (43.94%) | 37 (56.06%) | ||
| T Stage | |||||
| T1-T2 | 37 | 24 (64.86%) | 13 (35.14%) | 4.842 | 0.028 |
| T3-T4 | 77 | 33 (42.86%) | 44 (57.14%) | ||
| N Stage | |||||
| N0-N1 | 71 | 41 (57.75%) | 30 (42.25%) | 4.518 | 0.034 |
| N2-N3 | 43 | 16 (37.21%) | 27 (62.79%) | ||
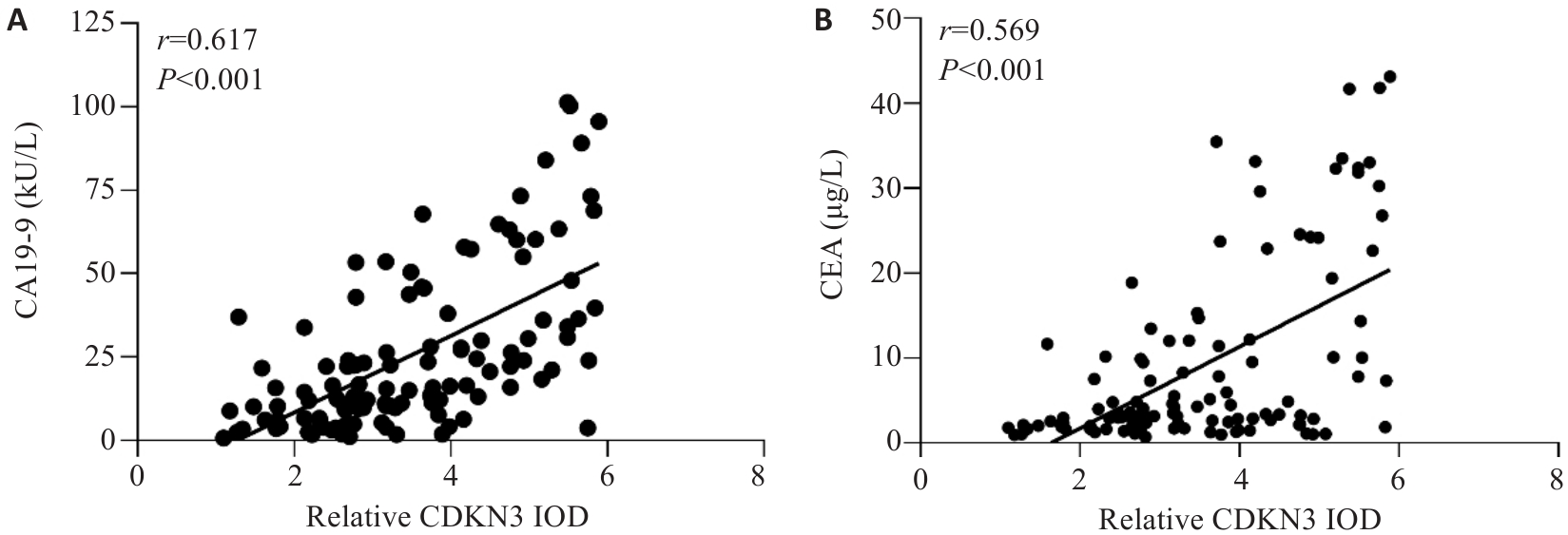
Fig.2 Correlation analysis between the expression level of CDKN3 in GC and the levels of CA19-9 and CEA in peripheral blood. A: Correlation analysis between CDKN3 and CA19-9. B: Correlation analysis between CDKN3 and CEA.
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Log-rank χ2 | P | HR | 95% CI | P | ||
| Gender (female vs male) | 0.108 | 0.742 | - | - | - | |
| Age (≥60 years vs <60 years) | 0.162 | 0.687 | - | - | - | |
| CDKN3 expression (high vs low) | 34.036 | <0.001 | 2.819 | 1.396-5.692 | 0.004 | |
| CEA(≥5 μg/L vs <5 μg/L) | 20.961 | <0.001 | 1.954 | 1.084-3.522 | 0.026 | |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 22.695 | <0.001 | 1.847 | 1.048-3.257 | 0.034 | |
| Tumor size (≥5 cm vs <5 cm) | 6.139 | 0.013 | 0.978 | 0.552-1.730 | 0.938 | |
| Histological type (other vs adenocarcinoma) | 0.309 | 0.578 | - | - | - | |
| Grade of differentiation (well vs moderate vs poor) | 5.623 | 0.060 | - | - | - | |
| T Stage (T3-T4vs T1-T2) | 13.586 | <0.001 | 2.438 | 1.160-5.120 | 0.019 | |
| N Stage (N2-N3vs N0-N1) | 20.178 | <0.001 | 2.099 | 1.177-3.744 | 0.012 | |
Tab.2 Univariate and multivariate analyses of the factors affecting the 5-year survival rate after radical gastrectomy for gastric cancer patients
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Log-rank χ2 | P | HR | 95% CI | P | ||
| Gender (female vs male) | 0.108 | 0.742 | - | - | - | |
| Age (≥60 years vs <60 years) | 0.162 | 0.687 | - | - | - | |
| CDKN3 expression (high vs low) | 34.036 | <0.001 | 2.819 | 1.396-5.692 | 0.004 | |
| CEA(≥5 μg/L vs <5 μg/L) | 20.961 | <0.001 | 1.954 | 1.084-3.522 | 0.026 | |
| CA19-9 (≥37 kU/L vs <37 kU/L) | 22.695 | <0.001 | 1.847 | 1.048-3.257 | 0.034 | |
| Tumor size (≥5 cm vs <5 cm) | 6.139 | 0.013 | 0.978 | 0.552-1.730 | 0.938 | |
| Histological type (other vs adenocarcinoma) | 0.309 | 0.578 | - | - | - | |
| Grade of differentiation (well vs moderate vs poor) | 5.623 | 0.060 | - | - | - | |
| T Stage (T3-T4vs T1-T2) | 13.586 | <0.001 | 2.438 | 1.160-5.120 | 0.019 | |
| N Stage (N2-N3vs N0-N1) | 20.178 | <0.001 | 2.099 | 1.177-3.744 | 0.012 | |
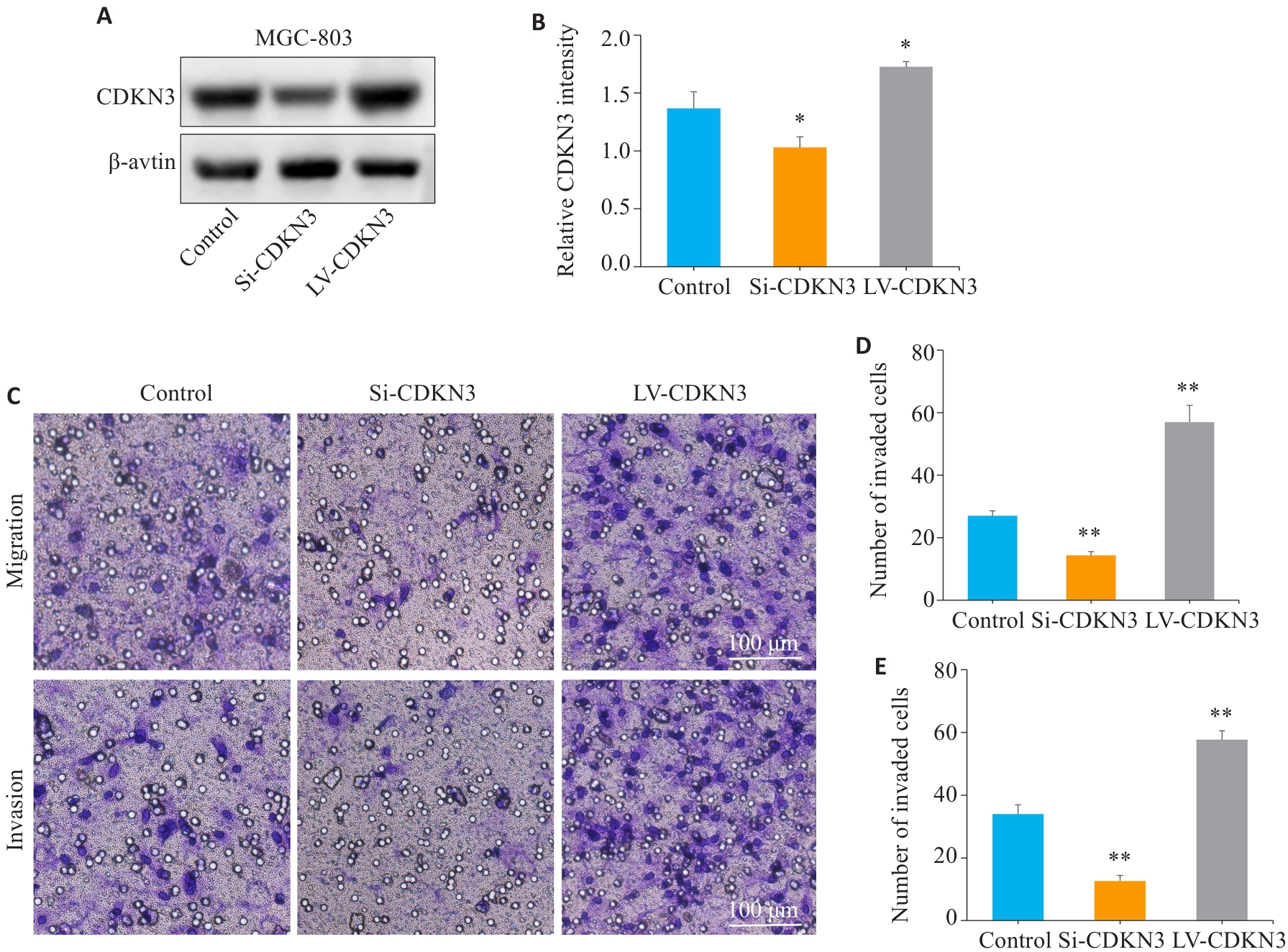
Fig.5 CDKN3 overexpression promotes gastric cancer cell migration and invasion. A,B: The expression of CDKN3 protein after lentivirus transfection was detected by Western blotting. C:Transwell experiment for analyzing the impact of CDKN3 on the migration and invasion abilities of MGC803 cells. D: Quantitative analysis of the number of migrated cells. E: Quantitative analysis of the number of invaded cells. *P<0.05, **P<0.01 vs control.
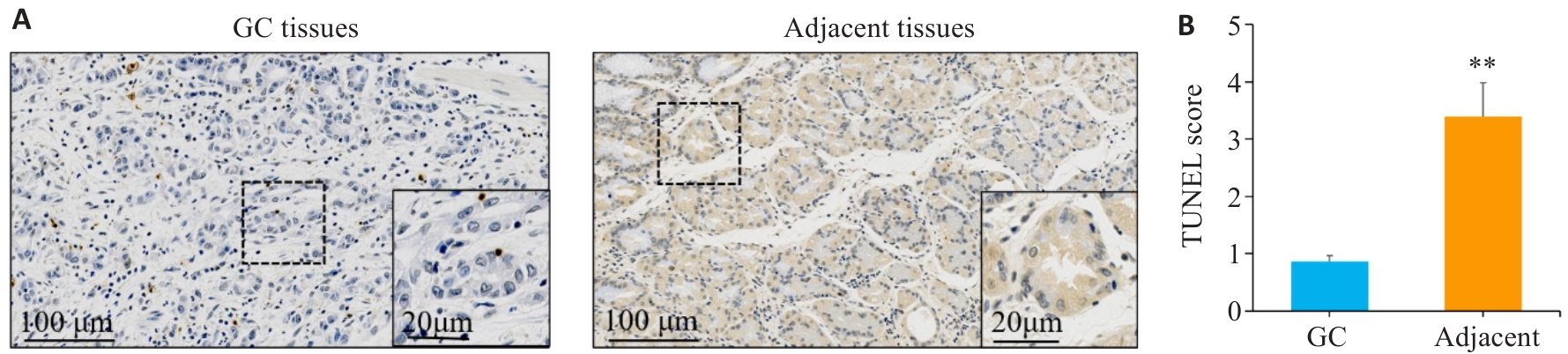
Fig.7 TUNEL staining was used to detect cell apoptosis in gastric cancer and adjacent tissues. A,B: TUNEL staining and scoring of apoptosis in gastric cancer tissues sections and adjacent tissues sections. **P<0.01vs gastric cancer. GC: Gastric cancer.
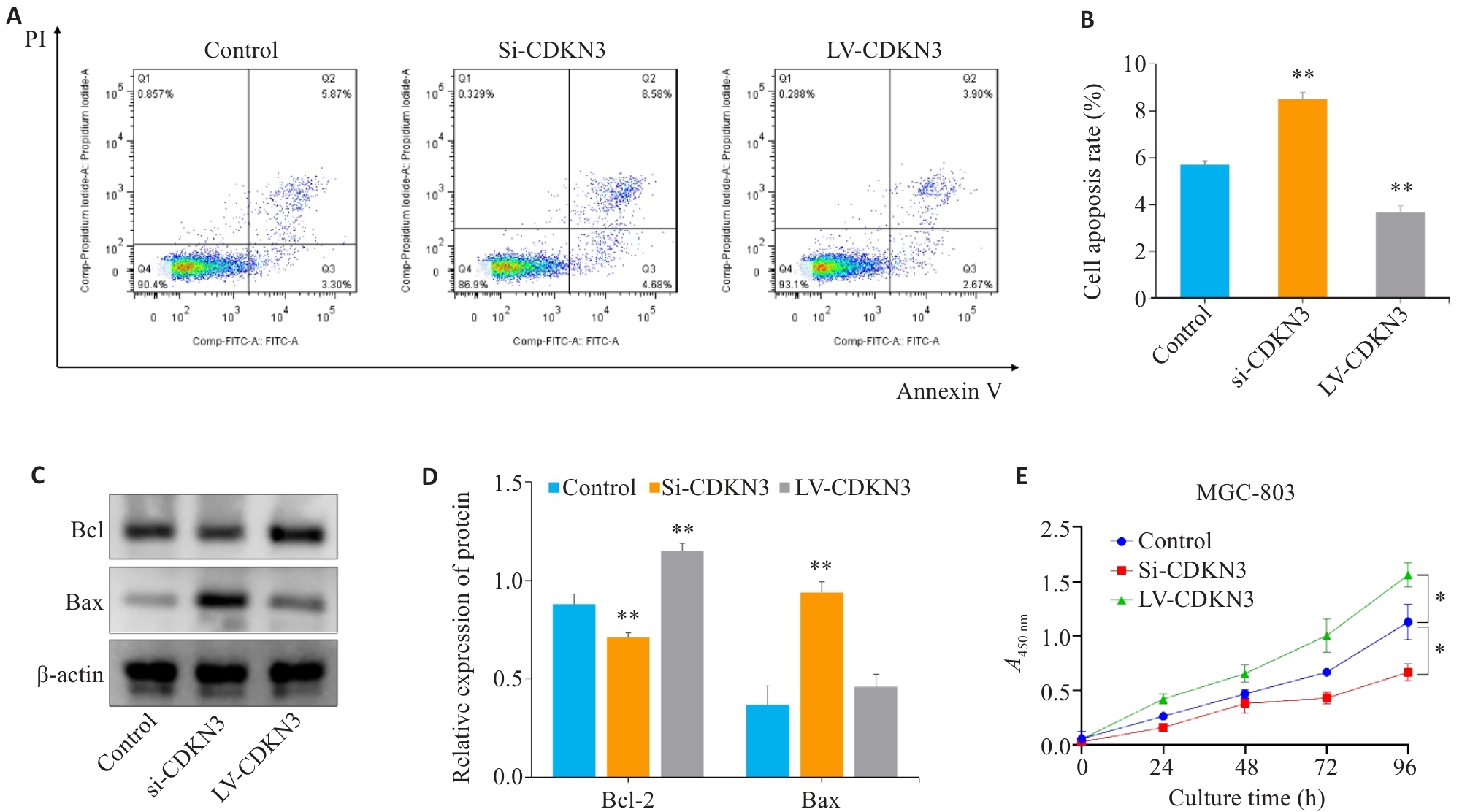
Fig.8 Effect of CDKN3 on apoptosis and proliferation of MGC803 cells. A,B: Cell apoptosis rate detected by flow cytometry after lentivirus transfection. C: Expression of Bcl-2 and Bax in MGC803 cells. D: Quantitative analysis of Bcl-2/Bax ratio. E: Effect of CDKN3 on proliferation of MGC-803 cells. *P<0.05, **P<0.01 vs control.
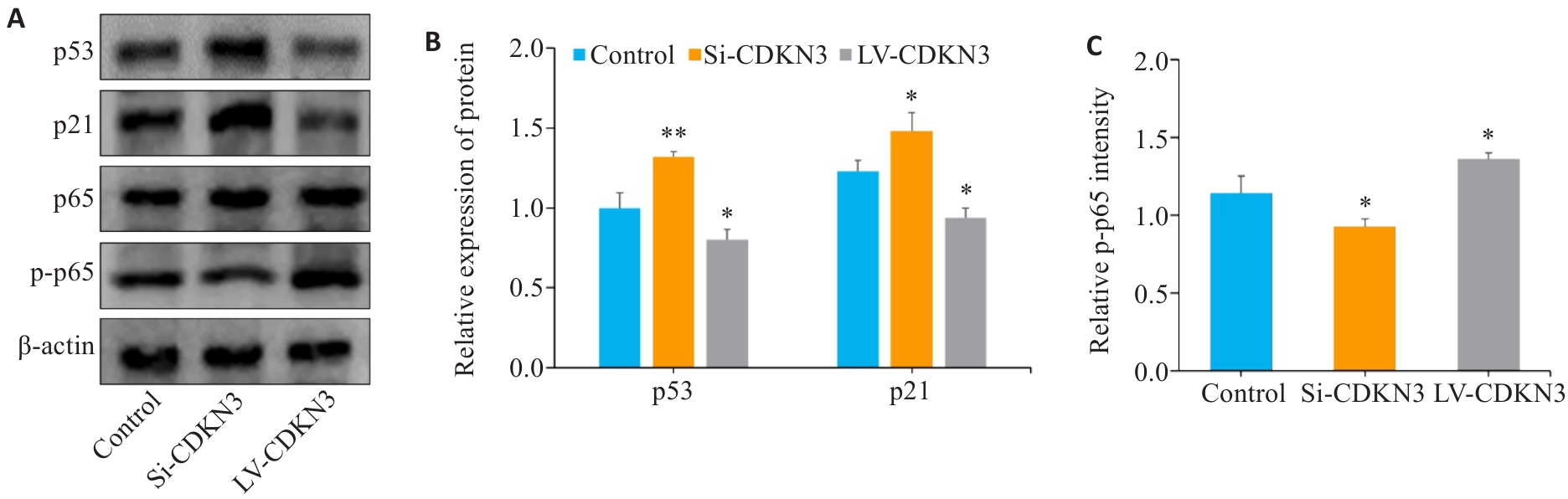
Fig.9 Effect of CDKN3 on p53/NF-κB signaling in MGC803 cells. A: Expression of p53, p21, p65 and p-p65 in MGC803 cells. B, C: Quantitative analysis of p53, p21, p65 and p-p65 ratio. *P<0.05, **P<0.01 vs control.
| 1 | Yang L, Ying X, Liu S, et al. Gastric cancer: Epidemiology, risk factors and prevention strategies[J]. Chin J Cancer Res, 2020, 32(6): 695-704. |
| 2 | Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-63. |
| 3 | Machlowska J, Baj J, Sitarz M, et al. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies[J]. Int J Mol Sci, 2020, 21(11): E4012. |
| 4 | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention[J]. Nat Rev Clin Oncol, 2023, 20(5): 338-49. |
| 5 | Li WH, Zhang L, Wu YH. CDKN3 regulates cisplatin resistance to colorectal cancer through TIPE1[J]. Eur Rev Med Pharmacol Sci, 2020, 24(7): 3614-23. |
| 6 | Dai W, Miao H, Fang S, et al. CDKN3 expression is negatively associated with pathological tumor stage and CDKN3 inhibition promotes cell survival in hepatocellular carcinoma[J]. Mol Med Rep, 2016, 14(2): 1509-14. |
| 7 | Dai W, Fang S, Cai G, et al. CDKN3 expression predicates poor prognosis and regulates adriamycin sensitivity in hepatocellular carcinoma in vitro [J]. J Int Med Res, 2020, 48(7): 300060520936879. |
| 8 | Chang SL, Chen TJ, Lee YE, et al. CDKN3 expression is an independent prognostic factor and associated with advanced tumor stage in nasopharyngeal carcinoma[J]. Int J Med Sci, 2018, 15(10): 992-8. |
| 9 | Wang J, Che W, Wang W, et al. CDKN3 promotes tumor progression and confers cisplatin resistance via RAD51 in esophageal cancer[J]. Cancer Manag Res, 2019, 11: 3253-64. |
| 10 | 李苗苗, 王海啸, 陶国全. CDKN3基因在肝癌中的表达及其对肝癌细胞生长、细胞周期的影响[J]. 山西医科大学学报, 2016, 47(6): 496-501. |
| 11 | 朱 慧, 陆欢平, 李天佑, 等. CDKN3在口腔鳞状细胞癌中的预后价值及免疫细胞浸润分析[J]. 国际检验医学杂志, 2024, 45(11): 1302-1307. |
| 12 | 胡 赞, 孙 锐, 程方雄, 等. 胰腺癌中细胞周期依赖激酶抑制剂3的表达及意义[J]. 实用医学杂志, 2018, 34(20): 3403-5. |
| 13 | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth[J]. Cancer Cell, 2023, 41(3): 374-403. |
| 14 | Ma J, Zhou W, Yuan Y, et al. PSMD12 interacts with CDKN3 and facilitates pancreatic cancer progression[J]. Cancer Gene Ther, 2023, 30(8): 1072-83. |
| 15 | Srinivas V, Kitagawa M, Wong J, et al. The tumor suppressor Cdkn3 is required for maintaining the proper number of centrosomes by regulating the centrosomal stability of Mps1[J]. Cell Rep, 2015, 13(8): 1569-77. |
| 16 | Zhang CL, Shen Q, Gao MQ, et al. The role of Cyclin Dependent Kinase Inhibitor 3 (CDKN3) in promoting human tumors: Literature review and pan-cancer analysis[J]. Heliyon, 2024, 10(4): e26061. |
| 17 | Li Y, Ji S, Fu LY, et al. Knockdown of cyclin-dependent kinase inhibitor 3 inhibits proliferation and invasion in human gastric cancer cells[J]. Oncol Res, 2017, 25(5): 721-31. |
| 18 | Zhou Y, Bian S, Zhou X, et al. Single-cell multiomics sequencing reveals prevalent genomic alterations in tumor stromal cells of human colorectal cancer[J]. Cancer Cell, 2020, 38(6): 818-28. e5. |
| 19 | Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance[J]. Cancer Cell, 2019, 35(3): 347-67. |
| 20 | Yan CS, Zheng L, Jiang ST, et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity[J]. Cancer Cell, 2023, 41(7): 1276-93.e11. |
| 21 | 米贤良, 魏子白, 杨长青. 基于生物信息学分析胃癌与癌旁组织中差异基因的表达[J]. 现代消化及介入诊疗, 2022, 27(11): 1419-23. |
| 22 | Ucaryilmaz Metin C, Ozcan G. Comprehensive bioinformatic analysis reveals a cancer-associated fibroblast gene signature as a poor prognostic factor and potential therapeutic target in gastric cancer[J]. BMC Cancer, 2022, 22(1): 692. |
| 23 | Pistritto G, Trisciuoglio D, Ceci C, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies[J]. Aging: Albany NY, 2016, 8(4): 603-19. |
| 24 | Patriarca C, Pini GM, Conti G. Invasion and metastasis: a historical perspective[J]. Pathologica, 2020, 112(4): 229-33. |
| 25 | Park W, Wei S, Kim BS, et al. Diversity and complexity of cell death: a historical review[J]. Exp Mol Med, 2023, 55(8): 1573-94. |
| 26 | Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications[J]. Nat Rev Mol Cell Biol, 2020, 21(11): 678-95. |
| 27 | Vaghari-Tabari M, Ferns GA, Qujeq D, et al. Signaling, metabolism, and cancer: an important relationship for therapeutic intervention[J]. J Cell Physiol, 2021, 236(8): 5512-32. |
| 28 | Herriage HC, Huang YT, Calvi BR. The antagonistic relationship between apoptosis and polyploidy in development and cancer[J]. Semin Cell Dev Biol, 2024, 156: 35-43. |
| 29 | Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death[J]. Nat Rev Drug Discov, 2023, 22(9): 723-42. |
| 30 | Kumar S, Dorstyn L, Lim Y. The role of caspases as executioners of apoptosis[J]. Biochem Soc Trans, 2022, 50(1): 33-45. |
| 31 | Li MM, Yang JY, Li J, et al. Epiberberine induced p53/p21-dependent G2/M cell cycle arrest and cell apoptosis in gastric cancer cells by activating γ‑aminobutyric acid receptor‑β3[J]. Phytomedicine, 2024, 123: 155198. |
| 32 | 艾亚楠, 赵唯含. NF-κB信号通路在胃癌前病变中的发病机制及中西医治疗的研究进展[J]. 中国实验方剂学杂志, 2022, 28(24): 237-43. |
| 33 | Schäfer C, Göder A, Beyer M, et al. Class I histone deacetylases regulate p53/NF-κB crosstalk in cancer cells[J]. Cell Signal, 2017, 29: 218-25. |
| [1] | Xiaoyu CHANG, Hanwen ZHANG, Hongting CAO, Ling HOU, Xin MENG, Hong TAO, Yan LUO, Guanghua LI. Heat stress affects expression levels of circadian clock gene Bmal1 and cyclins in rat thoracic aortic endothelial cells [J]. Journal of Southern Medical University, 2025, 45(7): 1353-1362. |
| [2] | Xinyuan CHEN, Chengting WU, Ruidi LI, Xueqin PAN, Yaodan ZHANG, Junyu TAO, Caizhi LIN. Shuangshu Decoction inhibits growth of gastric cancer cell xenografts by promoting cell ferroptosis via the P53/SLC7A11/GPX4 axis [J]. Journal of Southern Medical University, 2025, 45(7): 1363-1371. |
| [3] | Ting XIE, Yunyun WANG, Ting GUO, Chunhua YUAN. The peptide toxin components and nucleotide metabolites in Macrothele raveni venom synergistically inhibit cancer cell proliferation by activating the pro-apoptotic pathways [J]. Journal of Southern Medical University, 2025, 45(7): 1460-1470. |
| [4] | Jinlong PANG, Xinli ZHAO, Zhen ZHANG, Haojie WANG, Xingqi ZHOU, Yumei YANG, Shanshan LI, Xiaoqiang CHANG, Feng LI, Xian LI. Overexpression of multimerin-2 promotes cutaneous melanoma cell invasion and migration and is associated with poor prognosis [J]. Journal of Southern Medical University, 2025, 45(7): 1479-1489. |
| [5] | Xuan WU, Jiamin FANG, Weiwei HAN, Lin CHEN, Jing SUN, Qili JIN. High PRELID1 expression promotes epithelial-mesenchymal transition in gastric cancer cells and is associated with poor prognosis [J]. Journal of Southern Medical University, 2025, 45(7): 1535-1542. |
| [6] | Kang WANG, Haibin LI, Jing YU, Yuan MENG, Hongli ZHANG. High expression of ELFN1 is a prognostic biomarker and promotes proliferation and metastasis of colorectal cancer cells [J]. Journal of Southern Medical University, 2025, 45(7): 1543-1553. |
| [7] | Xinrui HOU, Zhendong ZHANG, Mingyuan CAO, Yuxin DU, Xiaoping WANG. Salidroside inhibits proliferation of gastric cancer cells by regulating the miR-1343-3p-OGDHL/PDHB glucose metabolic axis [J]. Journal of Southern Medical University, 2025, 45(6): 1226-1239. |
| [8] | Yujia YANG, Lifang YANG, Yaling WU, Zhaoda DUAN, Chunze YU, Chunyun WU, Jianyun YU, Li YANG. Cannabidiol inhibits neuronal endoplasmic reticulum stress and apoptosis in rats with multiple concussions by regulating the PERK-eIF2α-ATF4-CHOP pathway [J]. Journal of Southern Medical University, 2025, 45(6): 1240-1250. |
| [9] | Yumei ZENG, Jike LI, Zhongxi HUANG, Yibo ZHOU. Villin-like protein VILL suppresses proliferation of nasopharyngeal carcinoma cells by interacting with LMO7 protein [J]. Journal of Southern Medical University, 2025, 45(5): 954-961. |
| [10] | Yue CHEN, Linyu XIAO, Lü REN, Xue SONG, Jing LI, Jianguo HU. Monotropein improves motor function of mice with spinal cord injury by inhibiting the PI3K/AKT signaling pathway to suppress neuronal apoptosis [J]. Journal of Southern Medical University, 2025, 45(4): 774-784. |
| [11] | Fei CHU, Xiaohua CHEN, Bowen SONG, Jingjing YANG, Lugen ZUO. Moslosooflavone ameliorates dextran sulfate sodium-induced colitis in mice by suppressing intestinal epithelium apoptosis via inhibiting the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2025, 45(4): 819-828. |
| [12] | Yaqing YUE, Zhaoxia MU, Xibo WANG, Yan LIU. Aurora-A overexpression promotes cervical cancer cell invasion and metastasis by activating the NF-κBp65/ARPC4 signaling axis [J]. Journal of Southern Medical University, 2025, 45(4): 837-843. |
| [13] | Shunjie QING, Zhiyong SHEN. High expression of hexokinase 2 promotes proliferation, migration and invasion of colorectal cancer cells by activating the JAK/STAT pathway and regulating tumor immune microenvironment [J]. Journal of Southern Medical University, 2025, 45(3): 542-553. |
| [14] | Qingqing HUANG, Wenjing ZHANG, Xiaofeng ZHANG, Lian WANG, Xue SONG, Zhijun GENG, Lugen ZUO, Yueyue WANG, Jing LI, Jianguo HU. High MYO1B expression promotes proliferation, migration and invasion of gastric cancer cells and is associated with poor patient prognosis [J]. Journal of Southern Medical University, 2025, 45(3): 622-631. |
| [15] | Huali LI, Ting SONG, Jiawen LIU, Yongbao LI, Zhaojing JIANG, Wen DOU, Linghong ZHOU. Prognosis-guided optimization of intensity-modulated radiation therapy plans for lung cancer [J]. Journal of Southern Medical University, 2025, 45(3): 643-649. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||