Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (8): 1553-1560.doi: 10.12122/j.issn.1673-4254.2024.08.14
Previous Articles Next Articles
Xiaofan CONG( ), Teng CHEN, Shuo LI, Yuanyuan WANG, Longyun ZHOU, Xiaolong LI, Pei ZHANG, Xiaojin SUN(
), Teng CHEN, Shuo LI, Yuanyuan WANG, Longyun ZHOU, Xiaolong LI, Pei ZHANG, Xiaojin SUN( ), Surong ZHAO(
), Surong ZHAO( )
)
Received:2024-03-15
Online:2024-08-20
Published:2024-09-06
Contact:
Xiaojin SUN, Surong ZHAO
E-mail:736422754@qq.com;aijosxj@163.com;inwindangel@ qq.com
Supported by:Xiaofan CONG, Teng CHEN, Shuo LI, Yuanyuan WANG, Longyun ZHOU, Xiaolong LI, Pei ZHANG, Xiaojin SUN, Surong ZHAO. Dihydroartemisinin enhances sensitivity of nasopharyngeal carcinoma HNE1/DDP cells to cisplatin-induced apoptosis by promoting ROS production[J]. Journal of Southern Medical University, 2024, 44(8): 1553-1560.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.08.14
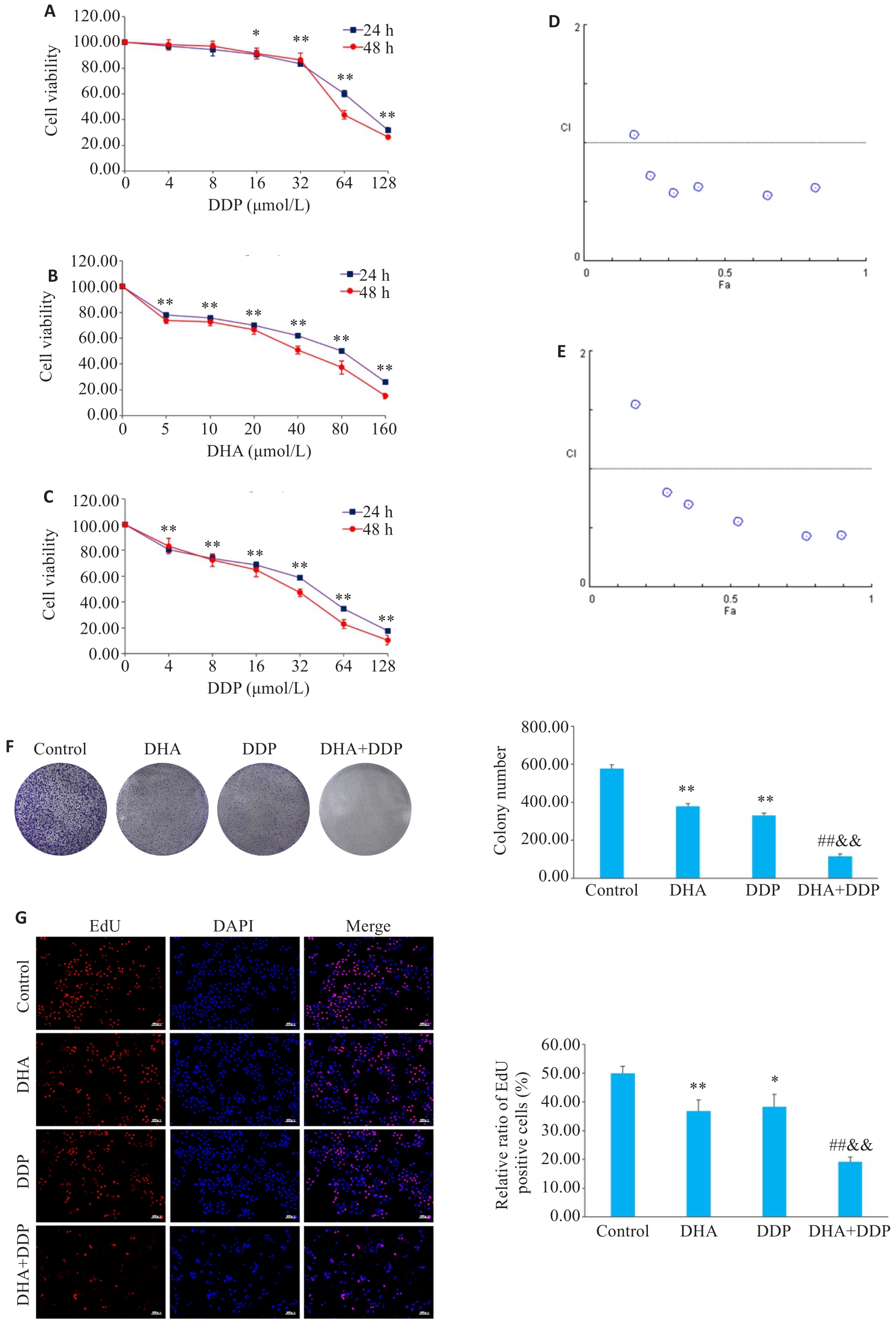
Fig.2 Inhibitory effects of DHA, DDP, and their combination on viability and proliferation of HNE1/DDP cells. A, B: CCK-8 assay for detecting viability of HNE1/DDP cells treated with DHA and DDP for 24 and 48 h. C: CCK-8 assay for detecting viability of HNE1/DDP cells treated with DHA (5 μmol/L) combined with DDP (0, 4, 8, 16, 32, 64, 128 μmol/L) for 24 and 48 h. *P<0.05, **P<0.01 vs 0 μmol/L. D, E: Combination index (CI) of combination treatment with DHA and DDP for 24, 48 h. F: Colony formation ability of cells treated with DHA or/and DDP for 24 h. G: EdU test for detecting proliferation of HNE1/DDP cells treated with DHA or/and DDP for 24 h (Original magnification: ×10). *P<0.05, **P<0.01 vs Control group; ##P<0.01 vs DHA group; &&P<0.01 vs DDP group.
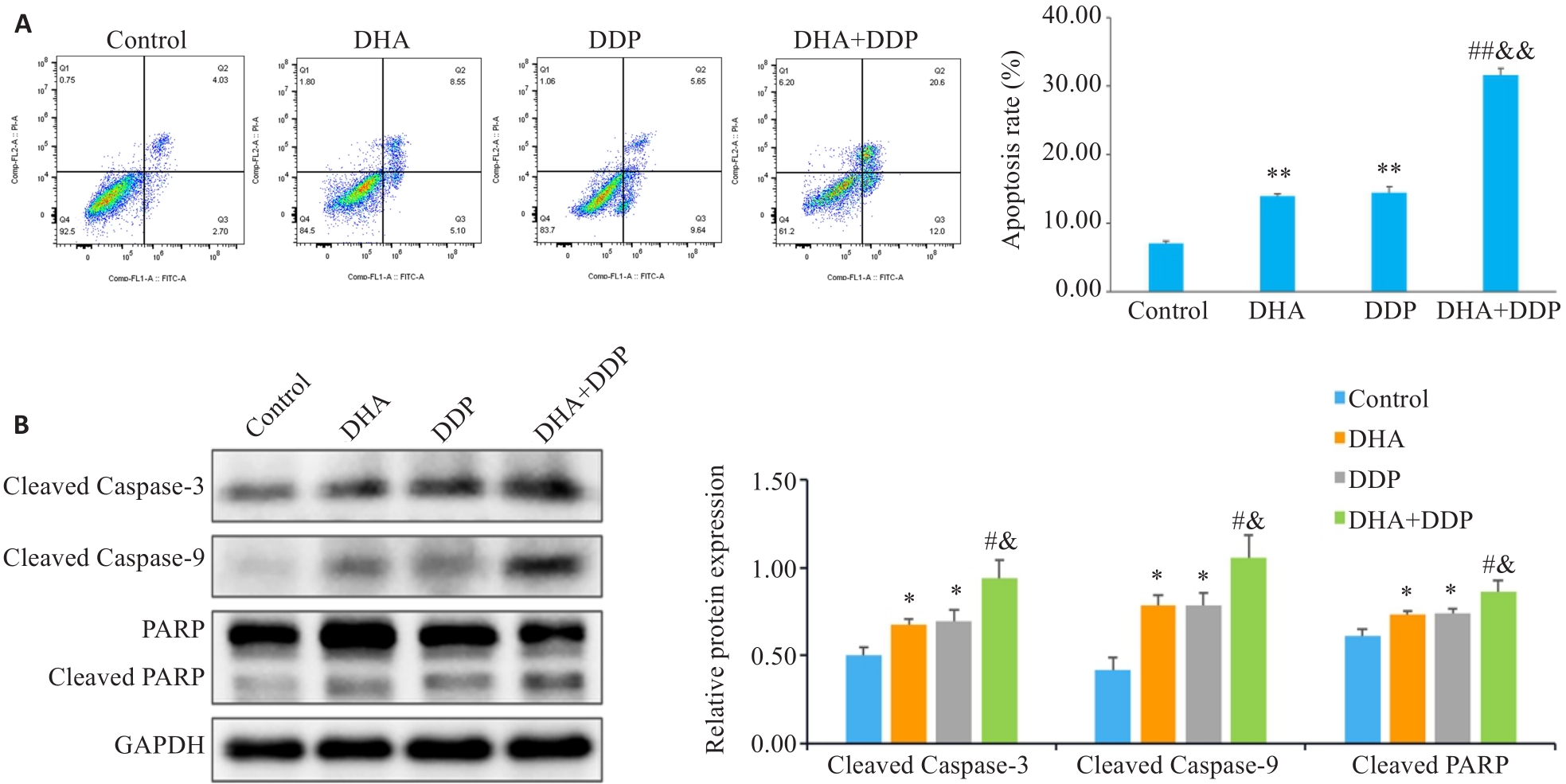
Fig.3 DHA combined with DDP more potently induces apoptosis in HNE1/DDP cells. A: Apoptosis of HNE1/DDP cells treated with DHA or/and DDP for 24 h detected by flow cytometry. B: Protein levels of cleaved PARP, cleaved caspase-9, and cleaved caspase-3 in HNE1/DDP cells detected by Western blotting. *P<0.05, **P<0.01 vs Control group; #P<0.05, ##P<0.01 vs DHA group; &P<0.05, &&P<0.01 vs DDP group.
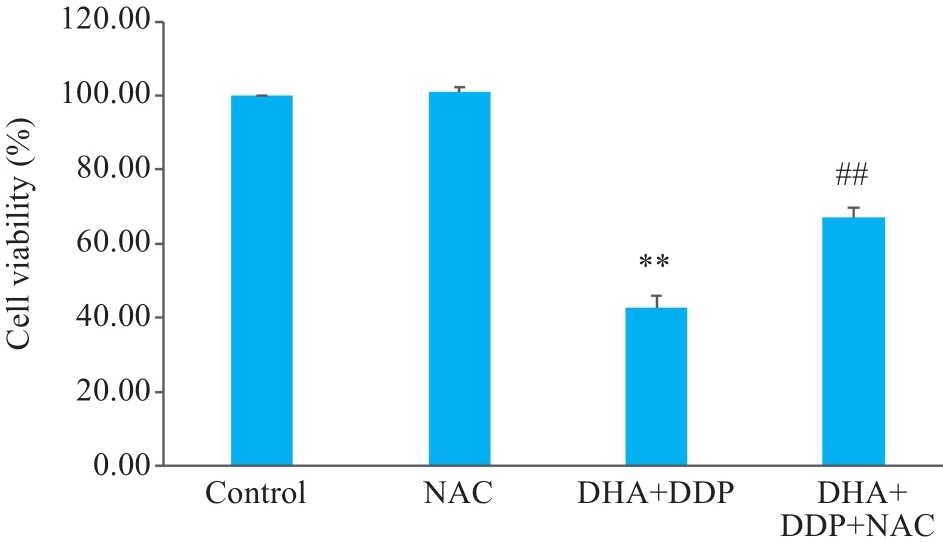
Fig.5 DHA combined with DDP more strongly inhibits proliferation of HNE1/DDP cells by promoting ROS accumulation. **P<0.01 vs Control group; ##P<0.01 vs DHA+DDP group.
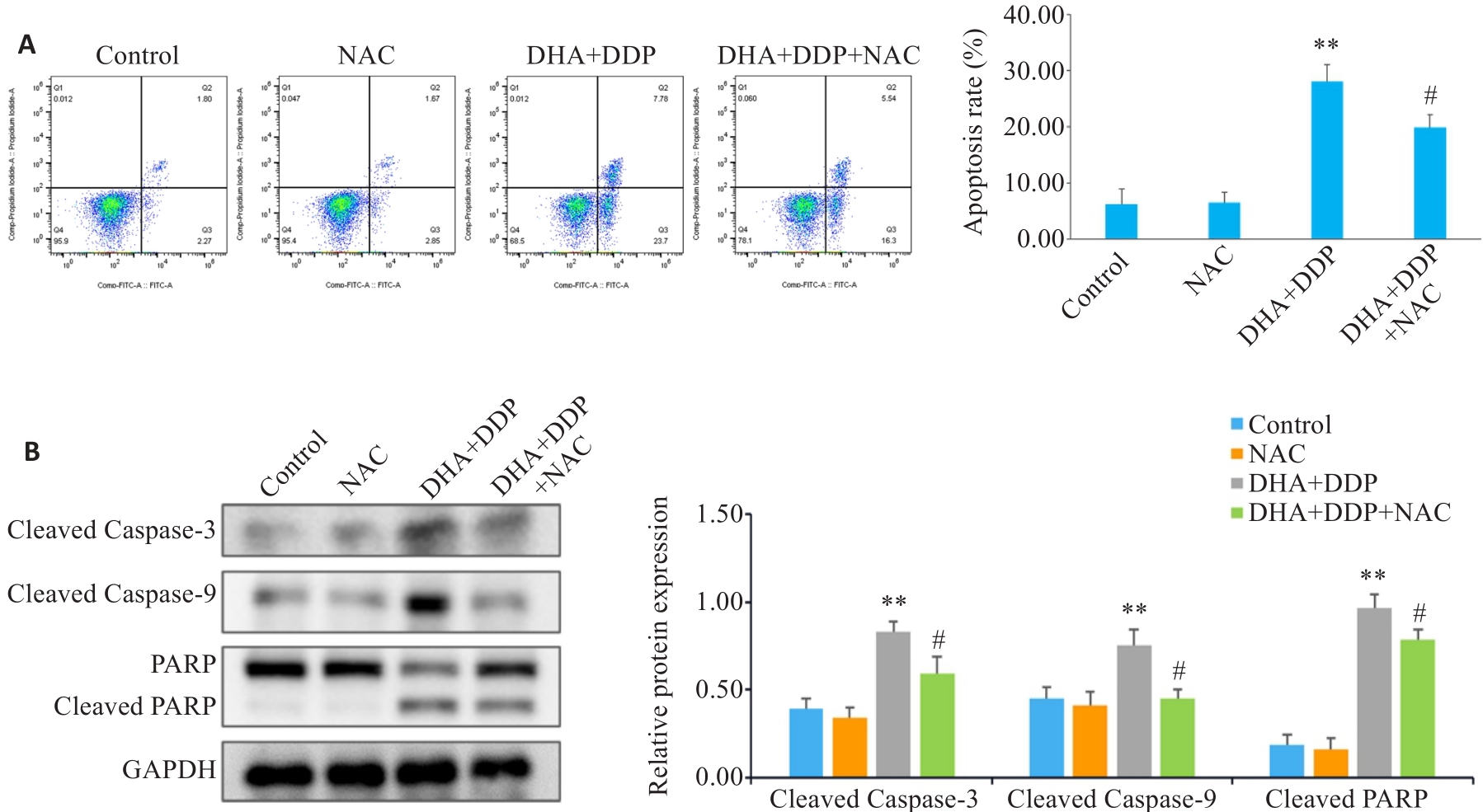
Fig.6 Effects of inhibiting ROS production on apoptosis and expression of apoptosis-related proteins in HNE1/DDP cells following combined treatment with DHA and DDP for 24 h. A: Apoptosis of HNE1/DDP cells treated with NAC (5 mmol/L) followed by combined treatment with DHA and DDP for 24 h was detected by flow cytometry. B: Protein levels of cleaved PARP, cleaved caspase-9, and cleaved caspase-3 in HNE1/DDP cells detected by Western blotting. **P<0.01 vs Control group; #P<0.05 vs DHA+DDP group.
| 1 | Wang SM, Claret FX, Wu WY. MicroRNAs as therapeutic targets in nasopharyngeal carcinoma[J]. Front Oncol, 2019, 9: 756. |
| 2 | Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. |
| 3 | Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma[J]. Lancet, 2019, 394(10192): 64-80. |
| 4 | Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2020, 18(7): 873-98. |
| 5 | Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis[J]. Lancet Oncol, 2015, 16(6): 645-55. |
| 6 | Perri F, Bosso D, Buonerba C, et al. Locally advanced nasopharyngeal carcinoma: current and emerging treatment strategies[J]. World J Clin Oncol, 2011, 2(12): 377-83. |
| 7 | Perri F, Della Vittoria Scarpati G, Caponigro F, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives[J]. Onco Targets Ther, 2019, 12: 1583-91. |
| 8 | Cui ZQ, Pu T, Zhang YJ, et al. Long non-coding RNA LINC00346 contributes to cisplatin resistance in nasopharyngeal carcinoma by repressing miR-342-5p[J]. Open Biol, 2020, 10(5): 190286. |
| 9 | Dutta S, Mahalanobish S, Saha S, et al. Natural products: an upcoming therapeutic approach to cancer[J]. Food Chem Toxicol, 2019, 128: 240-55. |
| 10 | Zhang J, Li Y, Wang JG, et al. Dihydroartemisinin affects STAT3/DDA1 signaling pathway and reverses breast cancer resistance to cisplatin[J]. Am J Chin Med, 2023, 51(2): 445-59. |
| 11 | Malami I, Bunza AM, Alhassan AM, et al. Dihydroartemisinin as a potential drug candidate for cancer therapy: a structural-based virtual screening for multitarget profiling[J]. J Biomol Struct Dyn, 2022, 40(3): 1347-62. |
| 12 | Liu YM, Gao SJ, Zhu J, et al. Dihydroartemisinin induces apoptosis and inhibits proliferation, migration, and invasion in epithelial ovarian cancer via inhibition of the hedgehog signaling pathway[J]. Cancer Med, 2018, 7(11): 5704-15. |
| 13 | Hu H, Wang ZD, Tan CL, et al. Dihydroartemisinin/miR-29b combination therapy increases the pro-apoptotic effect of dihydroartemisinin on cholangiocarcinoma cell lines by regulating Mcl-1 expression[J]. Adv Clin Exp Med, 2020, 29(8): 911-9. |
| 14 | Lu YY, Chen TS, Wang XP, et al. Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques[J]. J Biomed Opt, 2010, 15(4): 046028. |
| 15 | Li PC, Yang S, Dou MM, et al. Synergic effects of artemisinin and resveratrol in cancer cells[J]. J Cancer Res Clin Oncol, 2014, 140(12): 2065-75. |
| 16 | Ni LL, Zhu XP, Zhao Q, et al. Dihydroartemisinin, a potential PTGS1 inhibitor, potentiated cisplatin-induced cell death in non-small cell lung cancer through activating ROS-mediated multiple signaling pathways[J]. Neoplasia, 2024, 51: 100991. |
| 17 | 刘艳艳, 安 倩, 海 波, 等. 双氢青蒿素联合顺铂对人神经母细胞瘤细胞增殖及凋亡的影响[J]. 现代肿瘤医学, 2023, 31(22): 4129-35. |
| 18 | Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China[J]. Chin J Cancer, 2011, 30(2): 114-9. |
| 19 | Hong Y, Che SM, Hui BN, et al. Combination therapy of lung cancer using layer-by-layer cisplatin prodrug and curcumin co-encapsulated nanomedicine[J]. Drug Des Devel Ther, 2020, 14: 2263-74. |
| 20 | Yang ML, Li HR, Li YR, et al. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis[J]. Mol Med Rep, 2018, 17(5): 6211-26. |
| 21 | Kadioglu O, Elbadawi M, Fleischer E, et al. Identification of novel anthracycline resistance genes and their inhibitors[J]. Pharmaceuticals, 2021, 14(10): 1051. |
| 22 | Dama S, Niangaly H, Djimde M, et al. A randomized trial of dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali[J]. Malar J, 2018, 17(1): 347. |
| 23 | Slezakova S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives[J]. Anticancer Res, 2017, 37(11): 5995-6003. |
| 24 | Zhang CZ, Zhang HT, Yun JP, et al. Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo [J]. Biochem Pharmacol, 2012, 83(9): 1278-89. |
| 25 | Qin GQ, Zhao CB, Zhang LL, et al. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells[J]. Apoptosis, 2015, 20(8): 1072-86. |
| 26 | Im E, Yeo C, Lee HJ, et al. Dihydroartemisinin induced caspase-dependent apoptosis through inhibiting the specificity protein 1 pathway in hepatocellular carcinoma SK-Hep-1 cells[J]. Life Sci, 2018, 192: 286-92. |
| 27 | Dong J, Yang WH, Han JQ, et al. Effect of dihydroartemisinin on epithelial-to-mesenchymal transition in canine mammary tumour cells[J]. Res Vet Sci, 2019, 124: 240-7. |
| 28 | Li N, Zhang SY, Luo Q, et al. The effect of dihydroartemisinin on the malignancy and epithelial-mesenchymal transition of gastric cancer cells[J]. Curr Pharm Biotechnol, 2019, 20(9): 719-26. |
| 29 | Yao YY, Guo QL, Cao Y, et al. Artemisinin derivatives inactivate cancer-associated fibroblasts through suppressing TGF‑β signaling in breast cancer[J]. J Exp Clin Cancer Res, 2018, 37(1): 282. |
| 30 | Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro [J]. Pharmacol Res, 2003, 48(3): 231-6. |
| 31 | Lu JJ, Chen SM, Zhang XW, et al. The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells[J]. Invest New Drugs, 2011, 29(6): 1276-83. |
| 32 | Feng X, Li L, Jiang H, et al. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: involvement of apoptosis and autophagy[J]. Biochem Biophys Res Commun, 2014, 444(3): 376-81. |
| 33 | Dai XS, Chen W, Qiao Y, et al. Dihydroartemisinin inhibits the development of colorectal cancer by GSK-3β/TCF7/MMP9 pathway and synergies with capecitabine[J]. Cancer Lett, 2024, 582: 216596. |
| 34 | Li XM, Hou YN, Zhao JT, et al. Combination of chemotherapy and oxidative stress to enhance cancer cell apoptosis[J]. Chem Sci, 2020, 11(12): 3215-22. |
| 35 | Tian YZ, Liu YP, Tian SC, et al. Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of Caspase-3 and Caspase-9[J]. Pharmazie, 2020, 75(4): 147-50. |
| 36 | NavaneethaKrishnan S, Rosales JL, Lee KY. Loss of Cdk5 in breast cancer cells promotes ROS-mediated cell death through dysregulation of the mitochondrial permeability transition pore[J]. Oncogene, 2018, 37(13): 1788-804. |
| 37 | Olliaro PL, Haynes RK, Meunier B, et al. Possible modes of action of the artemisinin-type compounds[J]. Trends Parasitol, 2001, 17(3): 122-6. |
| 38 | Li X, Liu CQ, Zhang X, et al. Bruceine A: Suppressing metastasis via MEK/ERK pathway and invoking mitochondrial apoptosis in triple-negative breast cancer[J]. Biomedecine Pharmacother, 2023, 168: 115784. |
| 39 | Zhu JW, Sun Y, Lu Y, et al. Glaucocalyxin A exerts anticancer effect on osteosarcoma by inhibiting GLI1 nuclear translocation via regulating PI3K/Akt pathway[J]. Cell Death Dis, 2018, 9(6): 708. |
| 40 | Perillo B, di Donato M, Pezone A, et al. ROS in cancer therapy: the bright side of the moon[J]. Exp Mol Med, 2020, 52(2): 192-203. |
| 41 | Zhou Q, Ye FF, Qiu JX, et al. Dihydroartemisinin induces ER stress-mediated apoptosis in human tongue squamous carcinoma by regulating ROS production[J]. Anticancer Agents Med Chem, 2022, 22(16): 2902-8. |
| 42 | Liu ZY, Huang ML, Hong Y, et al. Isovalerylspiramycin I suppresses non-small cell lung carcinoma growth through ROS-mediated inhibition of PI3K/AKT signaling pathway[J]. Int J Biol Sci, 2022, 18(9): 3714-30. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||