Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (8): 1545-1552.doi: 10.12122/j.issn.1673-4254.2024.08.13
Previous Articles Next Articles
Shuo LIU1( ), Jing LI2,3, Xingwang WU4(
), Jing LI2,3, Xingwang WU4( )
)
Received:2024-05-06
Online:2024-08-20
Published:2024-09-06
Contact:
Xingwang WU
E-mail:ls1603814410@163.com;duobi2004@126.com
Shuo LIU, Jing LI, Xingwang WU. Swertiamarin ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by inhibiting intestinal epithelial cell apoptosis[J]. Journal of Southern Medical University, 2024, 44(8): 1545-1552.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.08.13
| Primers | Forward (5'-3') | Reverse (5'-3') |
|---|---|---|
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| CCL3 | CTCCCAGCCAGGTGTCATTTT | CTTGGACCCAGGTCTCTTTGG |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
Tab.1 Primers Sequence
| Primers | Forward (5'-3') | Reverse (5'-3') |
|---|---|---|
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| CCL3 | CTCCCAGCCAGGTGTCATTTT | CTTGGACCCAGGTCTCTTTGG |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
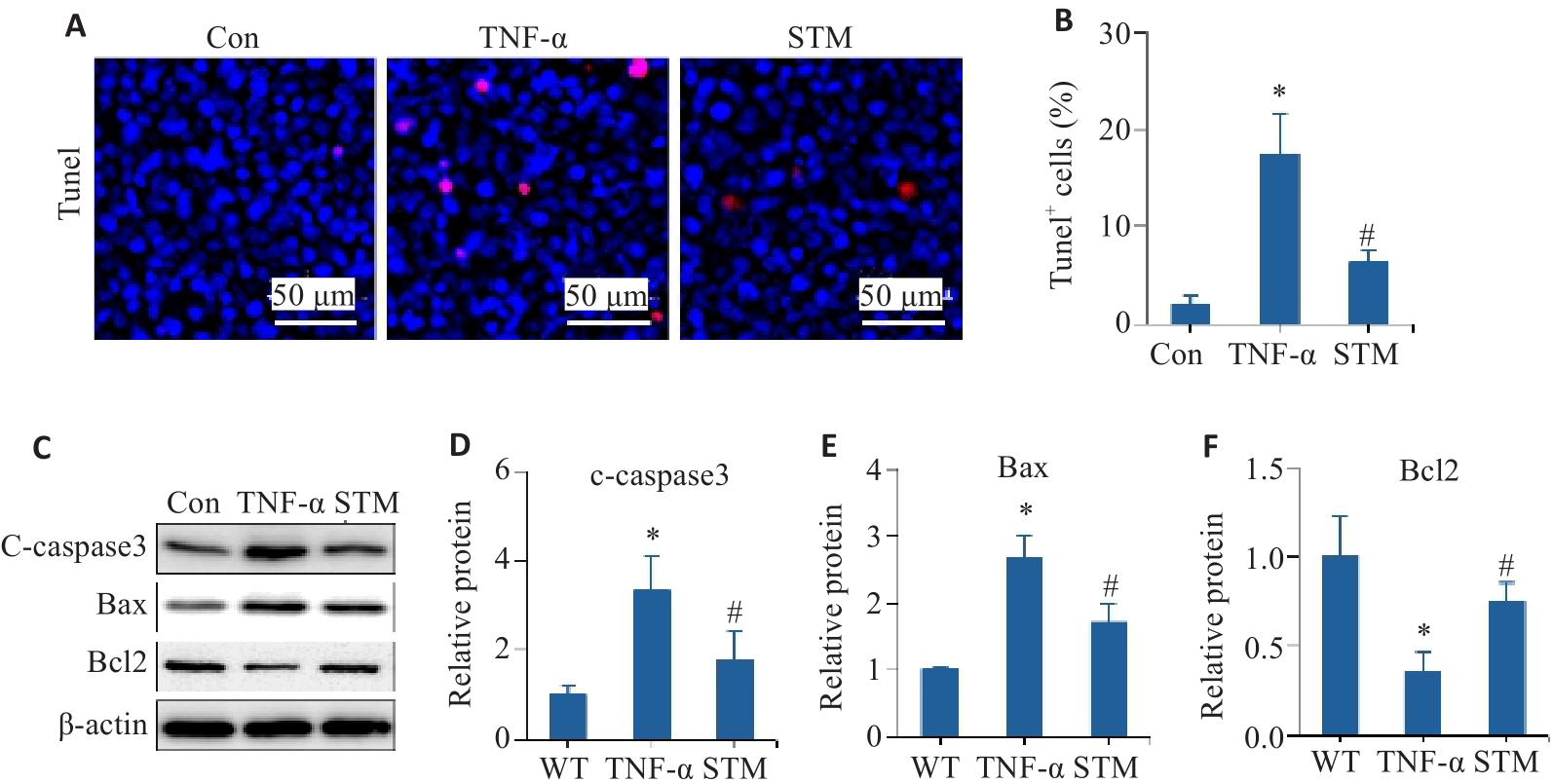
Fig.1 Effect of STM on TNF-α-induced apoptosis in Caco-2 cells. A, B: Representative images of TUNEL staining and statistical analysis of the positively stained cells. C-F: Levels of c-caspase 3, Bax and Bcl2 detected by Western blotting. *P<0.05 vs Control group/WT group. #P<0.05 vs TNF-α group.
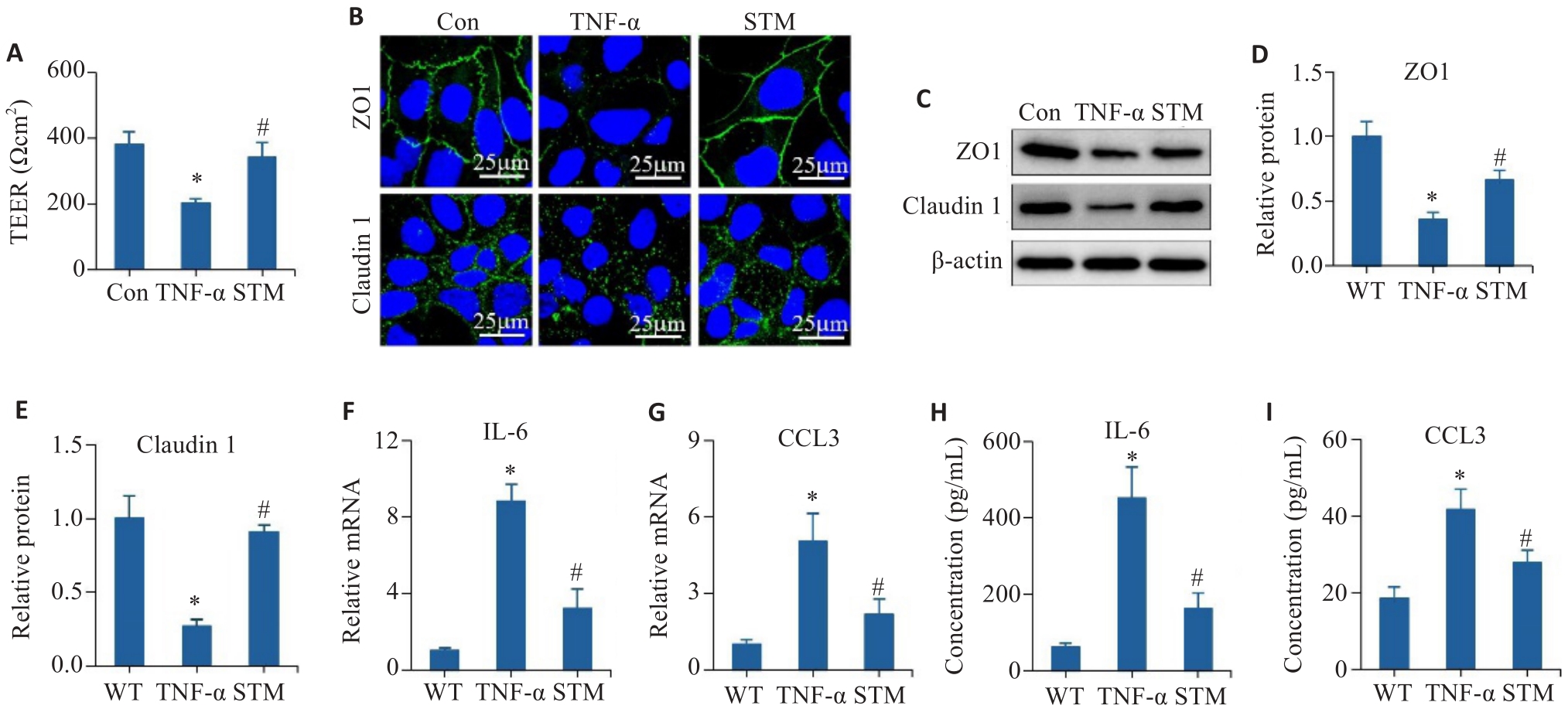
Fig.2 Effect of STM on TNF-α-induced barrier dysfunction and inflammatory responses in Caco-2 cells. A: Detection of TEER values. B: Immunofluorescence staining of ZO1 and claudin 1. C-E: Levels of ZO1 and claudin 1 detected by Western blotting. F, G: qRT-PCR for detecting expressions of IL-6 and CCL3. H, I: Levels of IL-6 and CCL3 detected by ELISA. *P<0.05 vs Control group, WT group. #P<0.05 vs TNF-α group.
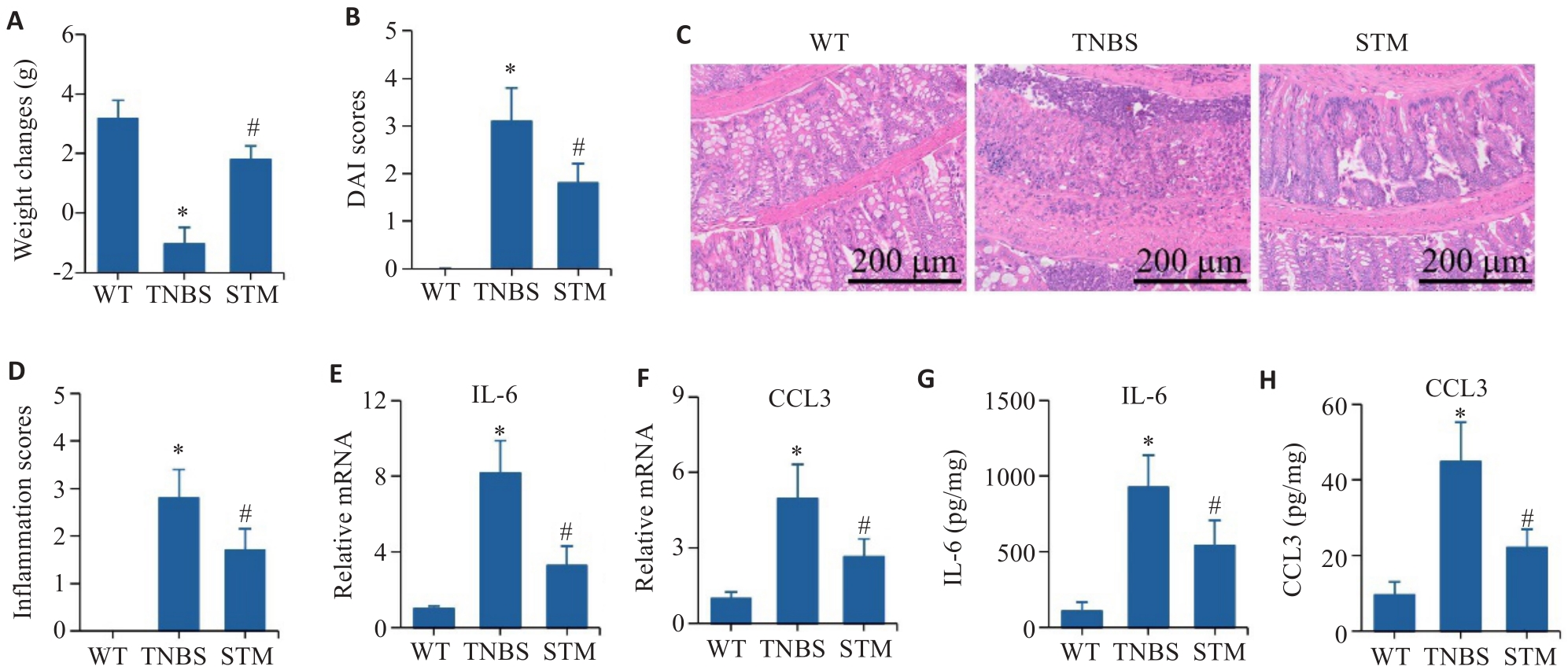
Fig.3 Effect of STM on TNBS-induced CD-like colitis in mice. A: Body weight changes of the mice. B: DAI score. C, D: HE staining and inflammation score of the colonic tissue. E, F: qRT-PCR for detecting mRNA expressions of IL-6 and CCL3. G, H: Levels of IL-6 and CCL3 detected by ELISA. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
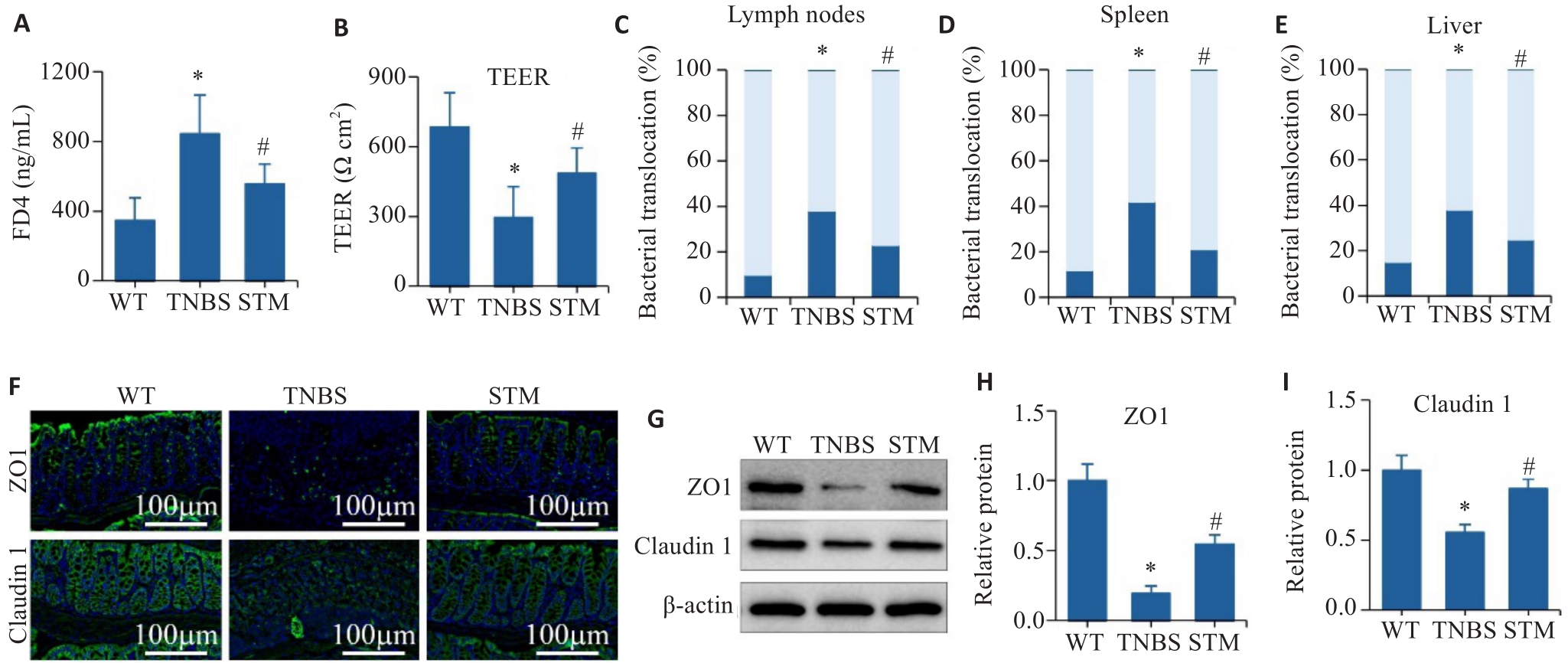
Fig.4 Effect of STM on intestinal barrier in TNBS-induced mice. A: Intestinal barrier permeability assay. B: TEER analysis of the colonic tissues. C-E: Assessment of the percentage of bacterial translocation in the intestinal lymph nodes, spleen and liver. F: Immunofluorescence staining of ZO1 and claudin 1. G-I: Levels of ZO1 and claudin 1 detected by Western blotting. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
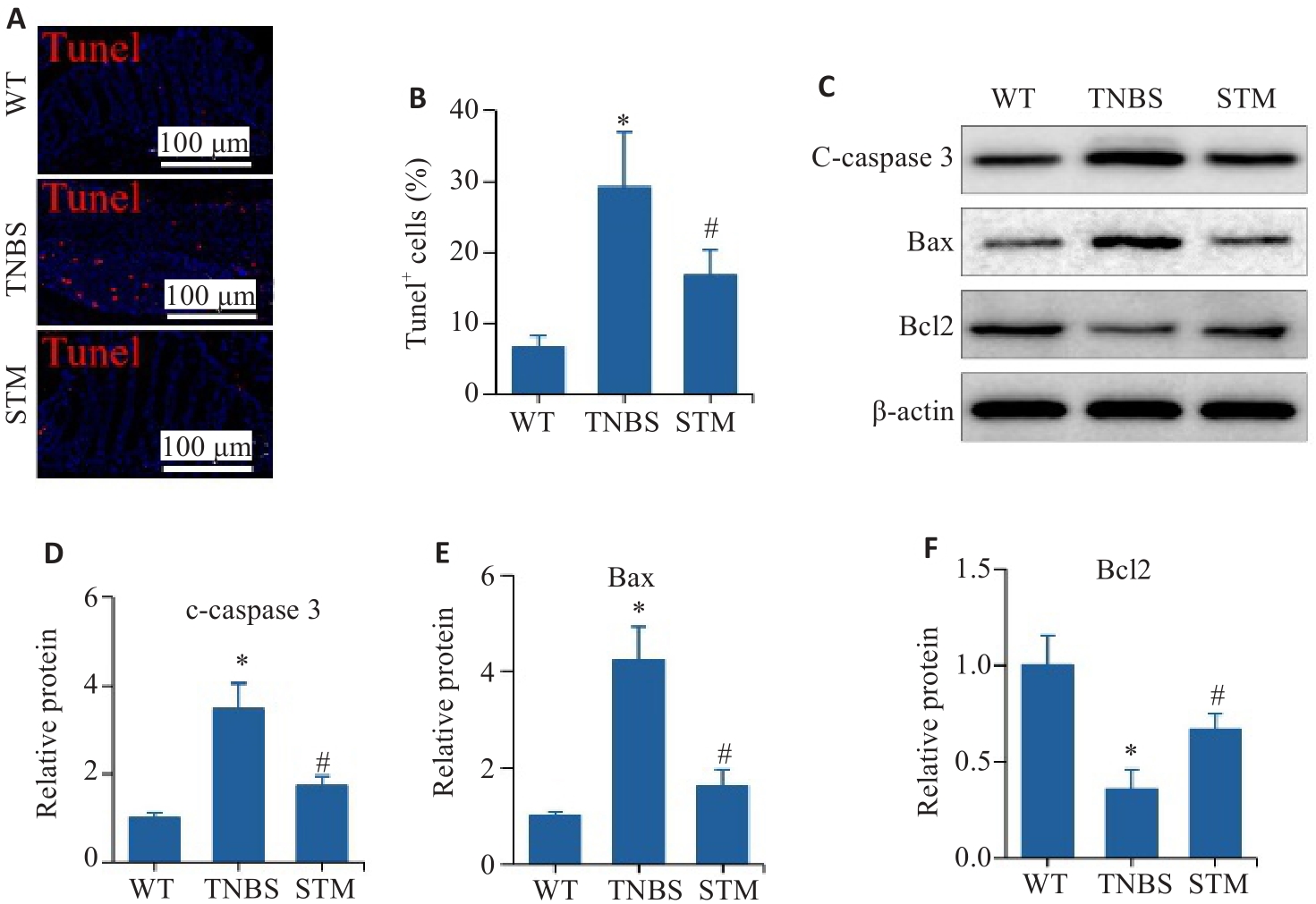
Fig. 5 Effect of STM on apoptosis of intestinal epithelial cells in TNBS-induced mice. A, B: Representative image of TUNEL staining and statistical analysis of the positively stained intestinal epithelial cells. C-F: Levels of c-caspase 3, Bax and Bcl2 detected by Western blotting. *P<0.05 vs WT group. #P<0.05 vs TNBS group.
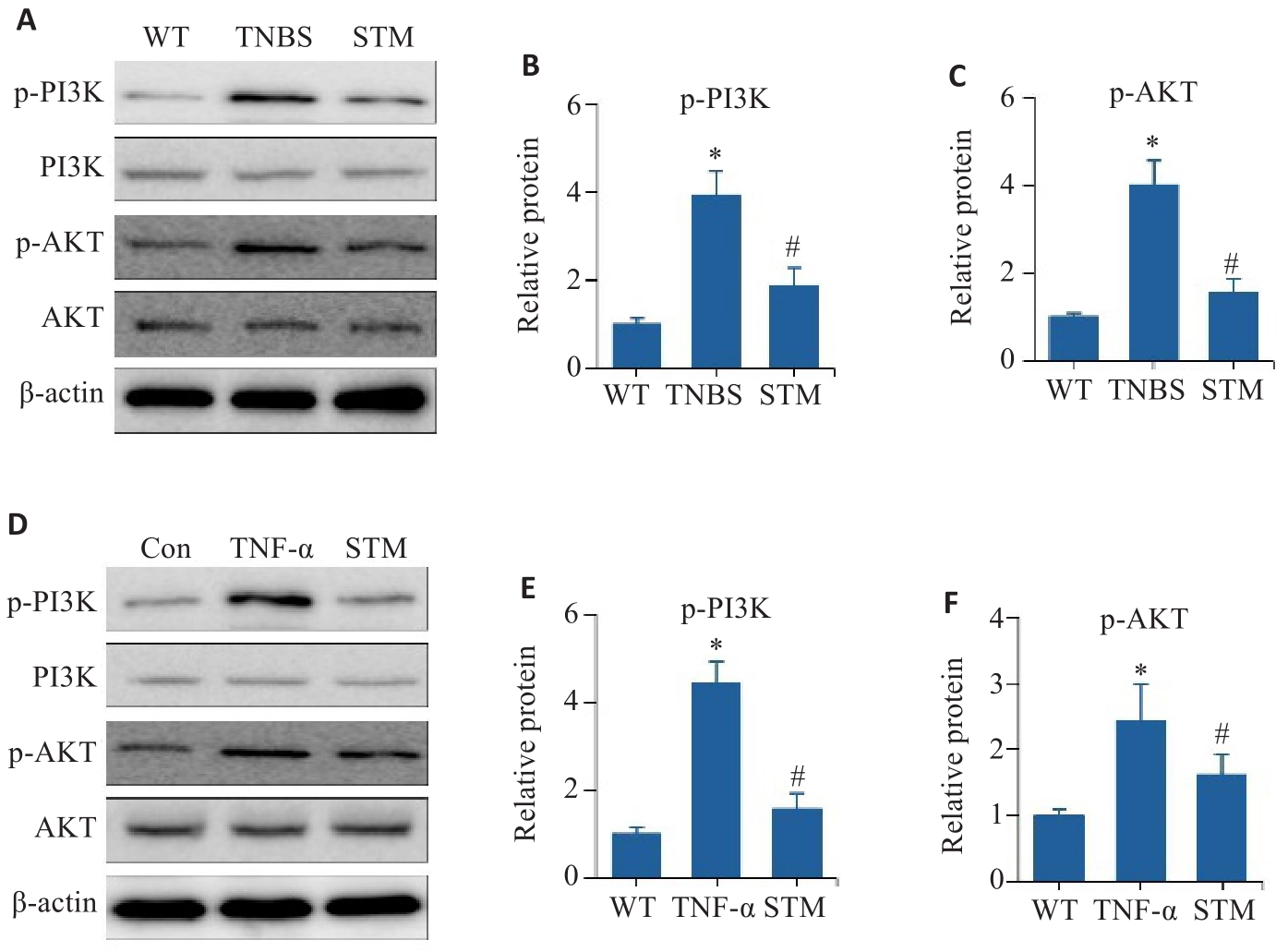
Fig. 6 In vitro and in vivo experiments for validating the effect of STM on the PI3K/AKT pathway. A-C: Levels of p-PI3K, PI3K, p-AKT and AKT detected in TNF-α-induced Caco-2 cells by Western blotting. D-F: Western blotting for detecting expression levels of p-PI3K, PI3K, p-AKT and AKT in the intestinal mucosal tissues of TNBS-induced mice. *P<0.05 vs WT or Con group. #P<0.05 vs TNBS or TNF-α group.
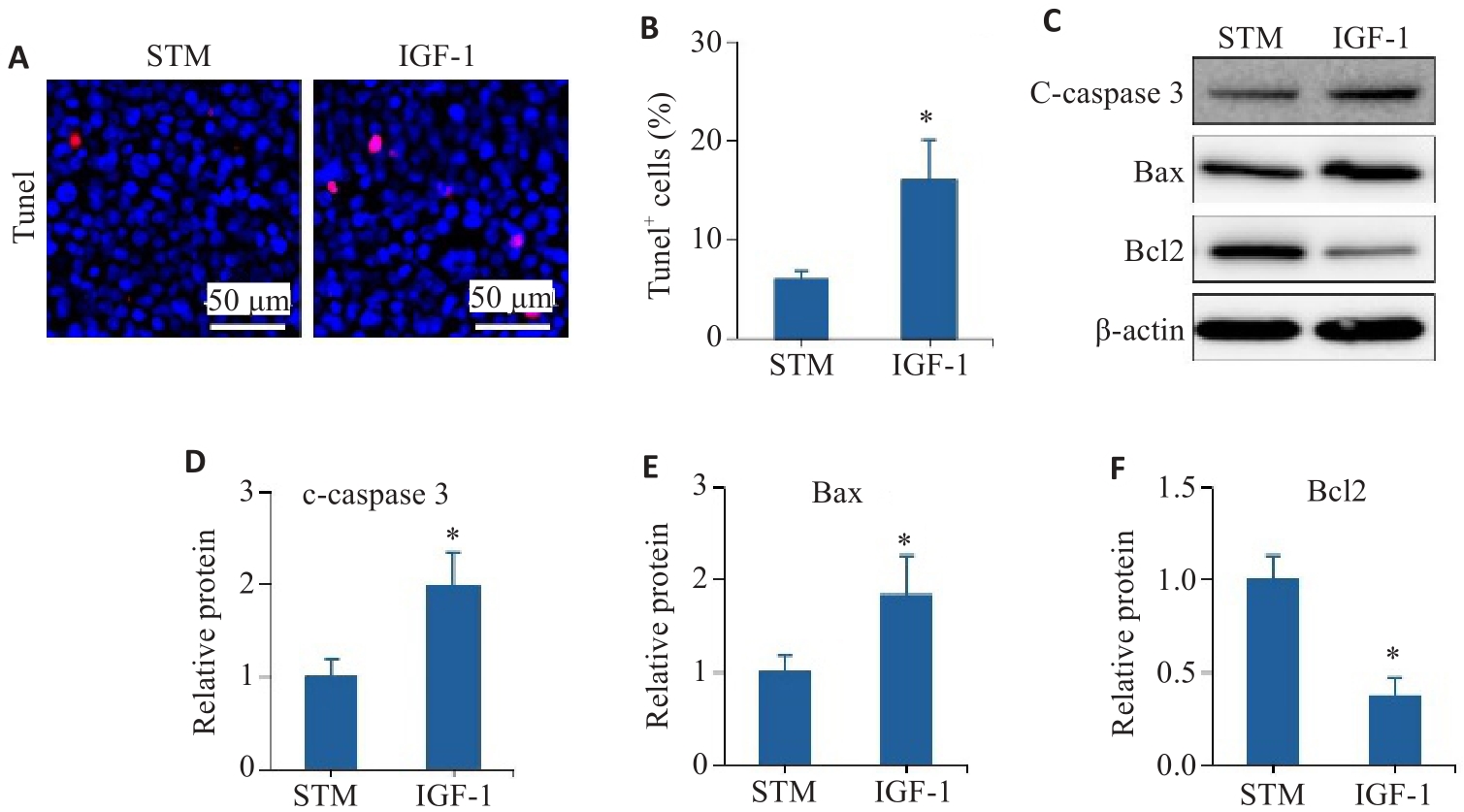
Fig.7 The PI3K/AKT pathway is involved in STM-mediated inhibition of intestinal epithelial cell apoptosis. A, B: TUNEL staining and statistical analysis of the positively stained intestinal epithelial cells. C-F: Levels of c-caspase 3, Bax and Bcl2 detected by Western blotting. *P<0.05 vs STM group.
| 1 | Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease[J]. Nat Rev Dis Primers, 2020, 6(1): 22. |
| 2 | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(1): 39-49. |
| 3 | Lo BC, Shin SB, Canals Hernaez D, et al. IL-22 preserves gut epithelial integrity and promotes disease remission during chronic Salmonella infection[J]. J Immunol, 2019, 202(3): 956-65. |
| 4 | Deng FH, Peng L, Li ZJ, et al. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury[J]. Cell Death Dis, 2018, 9(2): 153. |
| 5 | Stremmel W, Staffer S, Schneider MJ, et al. Genetic mouse models with intestinal-specific tight junction deletion resemble an ulcerative colitis phenotype[J]. J Crohns Colitis, 2017, 11(10): 1247-57. |
| 6 | Woznicki JA, Saini, Flood P, et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells[J]. Cell Death Dis, 2021, 12(10): 864. |
| 7 | Dotti I, Mora-Buch R, Ferrer-Picón E, et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis[J]. Gut, 2017, 66(12): 2069-79. |
| 8 | Zhang J, Cen L, Zhang XF, et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT[J]. Redox Biol, 2022, 56: 102469. |
| 9 | Lei H, Yang L, Xu HZ, et al. Ubiquitin-specific protease 47 regulates intestinal inflammation through deubiquitination of TRAF6 in epithelial cells[J]. Sci China Life Sci, 2022, 65(8): 1624-35. |
| 10 | Zhao S, Dejanovic D, Yao P, et al. Selective deletion of MyD88 signaling in α‑SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation[J]. Mucosal Immunol, 2020, 13(4): 665-78. |
| 11 | Mao R, Doyon G, Gordon IO, et al. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn's disease[J]. Gut, 2022, 71(1): 55-67. |
| 12 | Zhang QR, Chen K, Wu T, et al. Swertiamarin ameliorates carbon tetrachloride-induced hepatic apoptosis via blocking the PI3K/Akt pathway in rats[J]. Korean J Physiol Pharmacol, 2019, 23(1): 21-8. |
| 13 | Saravanan S, Islam VI, Thirugnanasambantham K, et al. Swertiamarin ameliorates inflammation and osteoclastogenesis intermediates in IL-1β induced rat fibroblast-like synoviocytes[J]. Inflamm Res, 2014, 63(6): 451-62. |
| 14 | Yang WJ, Han FH, Gu YP, et al. TGR5 agonist inhibits intestinal epithelial cell apoptosis via cAMP/PKA/c-FLIP/JNK signaling pathway and ameliorates dextran sulfate sodium-induced ulcerative colitis[J]. Acta Pharmacol Sin, 2023, 44(8): 1649-64. |
| 15 | Li R, Shi CR, Wei CT, et al. Fufang Shenhua Tablet inhibits renal fibrosis by inhibiting PI3K/AKT[J]. Phytomedicine, 2023, 116: 154873. |
| 16 | Zuo LG, Geng ZJ, Song X, et al. Browning of mesenteric white adipose tissue in Crohn's disease: a new pathological change and therapeutic target[J]. J Crohns Colitis, 2023, 17(8): 1179-92. |
| 17 | Huang LY, Qian WW, Xu YH, et al. Mesenteric adipose tissue contributes to intestinal fibrosis in Crohn's disease through the ATX-LPA axis[J]. J Crohns Colitis, 2022, 16(7): 1124-39. |
| 18 | Shi YJ, Sheng WJ, Xue MT, et al. Effect of morroniside on the transcriptome profiles of rat in injured spinal cords[J]. Gene, 2022, 823: 146338. |
| 19 | Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease[J]. J Crohns Colitis, 2021, 15(6): 879-913. |
| 20 | Wang T, Wu SB, Ibrahim IAA, et al. Cardioprotective role of swertiamarin, a plant glycoside against experimentally induced myocardial infarction via antioxidant and anti-inflammatory functions[J]. Appl Biochem Biotechnol, 2023, 195(9): 5394-408. |
| 21 | Wang GW, Quan J, Su NR, et al. Proteomic analysis of swertiamarin-treated BV-2 cells and possible implications in neuroinflammation[J]. J Oleo Sci, 2022, 71(3): 395-400. |
| 22 | Patel N, Zinzuvadia A, Prajapati M, et al. Swertiamarin-mediated immune modulation/adaptation confers protection against Plasmodium berghei [J]. Future Microbiol, 2022, 17: 931-41. |
| 23 | Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice[J]. Gastroenterology, 1990, 98(3): 694-702. |
| 24 | Gäbele E, Dostert K, Hofmann C, et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH[J]. J Hepatol, 2011, 55(6): 1391-9. |
| 25 | Dong LJ, Xie JW, Wang YY, et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity[J]. Nat Commun, 2022, 13(1): 4804. |
| 26 | Kinchen J, Chen HH, Parikh K, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease[J]. Cell, 2018, 175(2): 372-86.e17. |
| 27 | Varani J, McClintock SD, Aslam MN. Cell-matrix interactions contribute to barrier function in human colon organoids[J]. Front Med, 2022, 9: 838975. |
| 28 | Jelinsky SA, Derksen M, Bauman E, et al. Molecular and functional characterization of human intestinal organoids and monolayers for modeling epithelial barrier[J]. Inflamm Bowel Dis, 2023, 29(2): 195-206. |
| 29 | Chen SB, Liu HB, Li ZJ, et al. Epithelial PBLD attenuates intestinal inflammatory response and improves intestinal barrier function by inhibiting NF-κB signaling[J]. Cell Death Dis, 2021, 12(6): 563. |
| 30 | Kuo WT, Shen L, Zuo L, et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression[J]. Gastroenterology, 2019, 157(5): 1323-37. |
| 31 | Li CC, Gong LQ, Jiang Y, et al. Sanguisorba officinalis ethyl acetate extract attenuates ulcerative colitis through inhibiting PI3K-AKT/NF‑κB/STAT3 pathway uncovered by single-cell RNA sequencing[J]. Phytomedicine, 2023, 120: 155052. |
| 32 | Kim SM, Park S, Hwang SH, et al. Secreted Akkermansia muciniphila threonyl-tRNA synthetase functions to monitor and modulate immune homeostasis[J]. Cell Host Microbe, 2023, 31(6): 1021-37.e10. |
| 33 | Ma WJ, Long JL, Dong LJ, et al. Uncovering the key pharmacodynamic material basis and possible molecular mechanism of Xiaoke formulation improve insulin resistant through a comprehensive investigation[J]. J Ethnopharmacol, 2024, 323: 117752. |
| 34 | Stein J, Aksan A, Farrag K, et al. Management of inflammatory bowel disease-related anemia and iron deficiency with specific reference to the role of intravenous iron in current practice[J]. Expert Opin Pharmacother, 2017, 18(16): 1721-37. |
| 35 | Guo BJ, Bian ZX, Qiu HC, et al. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease[J]. Ann N Y Acad Sci, 2017, 1401(1): 37-48. |
| 36 | Wan P, Zhu XD, Liu XP, et al. Inhibition of neddylation ameliorates DSS-induced colitis[J]. Cell Mol Immunol, 2018, 15(6): 649-50. |
| 37 | Mizuta Y, Isomoto H, Takahashi T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium[J]. Gastroenterology, 2000, 118(4): 714-23. |
| [1] | Minzhu NIU, Lixia YIN, Tong QIAO, Lin YIN, Keni ZHANG, Jianguo HU, Chuanwang SONG, Zhijun GENG, Jing LI. Ecliptasaponin A ameliorates DSS-induced colitis in mice by suppressing M1 macrophage polarization via inhibiting the JAK2/STAT3 pathway [J]. Journal of Southern Medical University, 2025, 45(6): 1297-1306. |
| [2] | Lixia YIN, Minzhu NIU, Keni ZHANG, Zhijun GENG, Jianguo HU, Jiangyan LI, Jing LI. Cimifugin ameliorates Crohn's disease-like colitis in mice by modulating Th-cell immune balance via inhibiting the MAPK pathway [J]. Journal of Southern Medical University, 2025, 45(3): 595-602. |
| [3] | Xueli ZHOU, Hua LI, Qingyu CHEN, Meina JIN, Haibo LI, Wei BAI, Chuxuan JIA, Cuiying WEI. Effects of chronic intermittent hypoxia and reoxygenation on insulin resistance and skeletal muscle miR-27a-3p/PPARγ/IRS1/PI3K/AKT expressions in rats [J]. Journal of Southern Medical University, 2024, 44(9): 1729-1737. |
| [4] | Jincun FANG, Liwei LIU, Junhao LIN, Fengsheng CHEN. Overexpression of CDHR2 inhibits proliferation of breast cancer cells by inhibiting the PI3K/Akt pathway [J]. Journal of Southern Medical University, 2024, 44(6): 1117-1125. |
| [5] | Yan WANG, Yuqing RUAN, Can CUI, Xiu WANG. Jiaotaiwan improves brain glucose metabolism in a mouse model of Alzheimer's disease by activating the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 894-903. |
| [6] | Minzhu NIU, Lixia YIN, Ting DUAN, Ju HUANG, Jing LI, Zhijun GENG, Jianguo HU, Chuanwang SONG. Asperosaponin VI alleviates TNBS-induced Crohn's disease-like colitis in mice by reducing intestinal epithelial cell apoptosis via inhibiting the PI3K/AKT/NF-κB signaling pathway [J]. Journal of Southern Medical University, 2024, 44(12): 2335-2346. |
| [7] | SHAO Rongrong, YANG Zi, ZHANG Wenjing, ZHANG Nuo, ZHAO Yajing, ZHANG Xiaofeng, ZUO Lugen, GE Sitang. Pachymic acid protects against Crohn's disease-like intestinal barrier injury and colitis in mice by suppressing intestinal epithelial cell apoptosisviainhibiting PI3K/AKT signaling [J]. Journal of Southern Medical University, 2023, 43(6): 935-942. |
| [8] | XU Wenqin, YE Jingjing, WANG Fei, CHEN Tianbing. Piroctone olamine disrupts mitochondrial dynamics in glioma cells through the PI3K/AKT pathway [J]. Journal of Southern Medical University, 2023, 43(5): 764-771. |
| [9] | . Changes of guanylate cyclase C in colon tissues of rats with intestinal injury associated with severe acute pancreatitis [J]. Journal of Southern Medical University, 2021, 41(3): 376-383. |
| [10] | YAO Yu-chuan1, CHEN Cun-long1, WANG Ji-de1, ZHANG Ya-li1, JIANG Bo1, XIONG De-xin2. Intestinal permeability of patients with advanced digestive tract malignant tumors [J]. Journal of Southern Medical University, 2004, 24(05): 569-571. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||