Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (9): 2034-2045.doi: 10.12122/j.issn.1673-4254.2025.09.23
Jingjing ZHANG1( ), Song FENG2(
), Song FENG2( ), Dali ZHANG1, Jian XUE3, Chao ZHOU1, Pengcheng LIU1, Shuangnan FU1, Man GONG1, Hui FENG2(
), Dali ZHANG1, Jian XUE3, Chao ZHOU1, Pengcheng LIU1, Shuangnan FU1, Man GONG1, Hui FENG2( ), Ning ZHANG1(
), Ning ZHANG1( )
)
Received:2024-12-15
Online:2025-09-20
Published:2025-09-28
Contact:
Hui FENG, Ning ZHANG
E-mail:zt2230224@126.com;flying-1984@163.com;fenghui810@126.com;zhangning198191@sina.com
Jingjing ZHANG, Song FENG, Dali ZHANG, Jian XUE, Chao ZHOU, Pengcheng LIU, Shuangnan FU, Man GONG, Hui FENG, Ning ZHANG. Altered oral microbiome and metabolites are associated with improved lipid metabolism in HBV-infected patients with metabolic dysfunction-associated fatty liver disease[J]. Journal of Southern Medical University, 2025, 45(9): 2034-2045.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.09.23
| Indicator | MAFLD (n=48) | MAFLD+CHB (n=47) | Total (n=95) | P |
|---|---|---|---|---|
| Degree of steotosis | ||||
| Mild | 15 (31.3%) | 31 (66.0%) | 46 (48.4%) | 0.0179 |
| Medium | 26 (54.2%) | 14 (29.8%) | 40 (42.1%) | |
| Severe | 7 (14.6%) | 2 (4.3%) | 9 (9.5%) | |
| Age (years, Mean±SD) | 46.8±11.2 | 46.6±8.03 | 46.7±9.70 | 0.976 |
| Gender (Male) | 33 (68.8%) | 35 (74.5%) | 68 (71.6%) | 0.826 |
| BMI (Mean±SD) | 26.7±5.02 | 27.5±2.98 | 27.1±4.14 | 0.326 |
| Waist (cm) | 93.4±10.3 | 97.2±9.98 | 95.3±10.3 | 0.305 |
| Hypertension | 12 (25.0%) | 9 (19.1%) | 21 (22.1%) | 0.79 |
| T2DM | 10 (20.8%) | 3 (6.4%) | 13 (13.7%) | 0.123 |
| CHD | 3 (6.3%) | 2 (4.3%) | 5 (5.3%) | 0.910 |
| ALT (U/L) | 51.6±39.8 | 38.7±22.0 | 45.2±32.7 | 0.586 |
| AST (U/L) | 40.7±38.2 | 30.3±12.7 | 35.6±28.9 | 0.221 |
| ALP (U/L) | 90.2±26.0 | 78.7±20.8 | 84.5±24.2 | 0.0975 |
| GGT (U/L) | 64.8±52.9 | 35.2±16.9 | 50.1±42.0 | 0.00116 |
| Glucose (mmol/L) | 6.77±1.91 | 5.79±1.45 | 6.28±1.76 | <0.001 |
| TG (mmol/L) | 2.80±2.84 | 1.89±0.835 | 2.35±2.14 | 0.215 |
| TC (mmol/L) | 5.07±0.989 | 4.62±0.703 | 4.85±0.885 | 0.046 |
| HDL (mmol/L) | 1.18±0.246 | 1.12±0.235 | 1.15±0.242 | 0.541 |
| LDL (mmol/L) | 3.40±0.772 | 3.24±0.603 | 3.32±0.695 | 0.568 |
| Apo-A1 (g/L) | 1.23±0.206 | 1.18±0.191 | 1.21±0.199 | 0.419 |
| Apo-B (g/L) | 0.935±0.237 | 0.867±0.149 | 0.901±0.201 | 0.274 |
| Lp-a (mg/L) | 113±177 | 149±222 | 131±200 | 0.458 |
| APRI | 0.579±1.06 | 0.411±0.297 | 0.496±0.783 | 0.609 |
| FIB-4 | 1.37±1.47 | 1.37±0.972 | 1.37±1.24 | 0.862 |
Tab.1 Baseline demographic and clinical characteristics of the enrolled patients
| Indicator | MAFLD (n=48) | MAFLD+CHB (n=47) | Total (n=95) | P |
|---|---|---|---|---|
| Degree of steotosis | ||||
| Mild | 15 (31.3%) | 31 (66.0%) | 46 (48.4%) | 0.0179 |
| Medium | 26 (54.2%) | 14 (29.8%) | 40 (42.1%) | |
| Severe | 7 (14.6%) | 2 (4.3%) | 9 (9.5%) | |
| Age (years, Mean±SD) | 46.8±11.2 | 46.6±8.03 | 46.7±9.70 | 0.976 |
| Gender (Male) | 33 (68.8%) | 35 (74.5%) | 68 (71.6%) | 0.826 |
| BMI (Mean±SD) | 26.7±5.02 | 27.5±2.98 | 27.1±4.14 | 0.326 |
| Waist (cm) | 93.4±10.3 | 97.2±9.98 | 95.3±10.3 | 0.305 |
| Hypertension | 12 (25.0%) | 9 (19.1%) | 21 (22.1%) | 0.79 |
| T2DM | 10 (20.8%) | 3 (6.4%) | 13 (13.7%) | 0.123 |
| CHD | 3 (6.3%) | 2 (4.3%) | 5 (5.3%) | 0.910 |
| ALT (U/L) | 51.6±39.8 | 38.7±22.0 | 45.2±32.7 | 0.586 |
| AST (U/L) | 40.7±38.2 | 30.3±12.7 | 35.6±28.9 | 0.221 |
| ALP (U/L) | 90.2±26.0 | 78.7±20.8 | 84.5±24.2 | 0.0975 |
| GGT (U/L) | 64.8±52.9 | 35.2±16.9 | 50.1±42.0 | 0.00116 |
| Glucose (mmol/L) | 6.77±1.91 | 5.79±1.45 | 6.28±1.76 | <0.001 |
| TG (mmol/L) | 2.80±2.84 | 1.89±0.835 | 2.35±2.14 | 0.215 |
| TC (mmol/L) | 5.07±0.989 | 4.62±0.703 | 4.85±0.885 | 0.046 |
| HDL (mmol/L) | 1.18±0.246 | 1.12±0.235 | 1.15±0.242 | 0.541 |
| LDL (mmol/L) | 3.40±0.772 | 3.24±0.603 | 3.32±0.695 | 0.568 |
| Apo-A1 (g/L) | 1.23±0.206 | 1.18±0.191 | 1.21±0.199 | 0.419 |
| Apo-B (g/L) | 0.935±0.237 | 0.867±0.149 | 0.901±0.201 | 0.274 |
| Lp-a (mg/L) | 113±177 | 149±222 | 131±200 | 0.458 |
| APRI | 0.579±1.06 | 0.411±0.297 | 0.496±0.783 | 0.609 |
| FIB-4 | 1.37±1.47 | 1.37±0.972 | 1.37±1.24 | 0.862 |
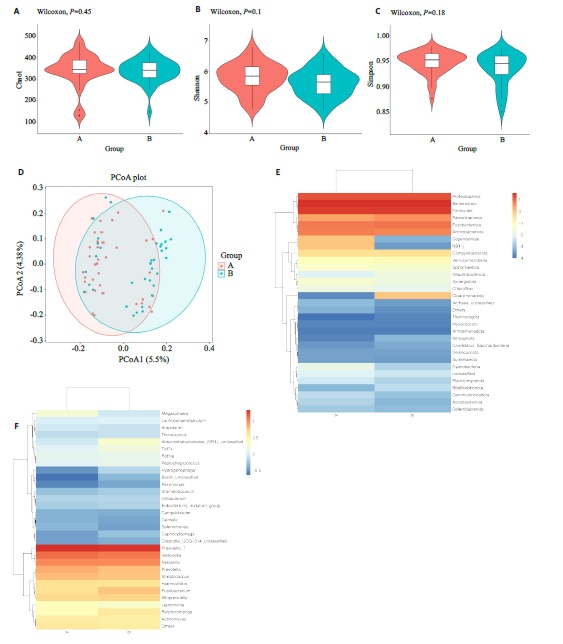
Fig.1 Differences in microbial flora between the two groups. A-C: Alpha diversity analysis usingthe Chao1, Shannon, and Simpson methods. D:Beta diversity analysis using the Principal Coordinates Analysis (PCoA) method. E, F:Heatmaps annotating species abundance at the phylum level, with a gradient from blue to red indicating a change in abundance from low to high, where bluer colors represent lower abundance and redder colors represent higher abundance.
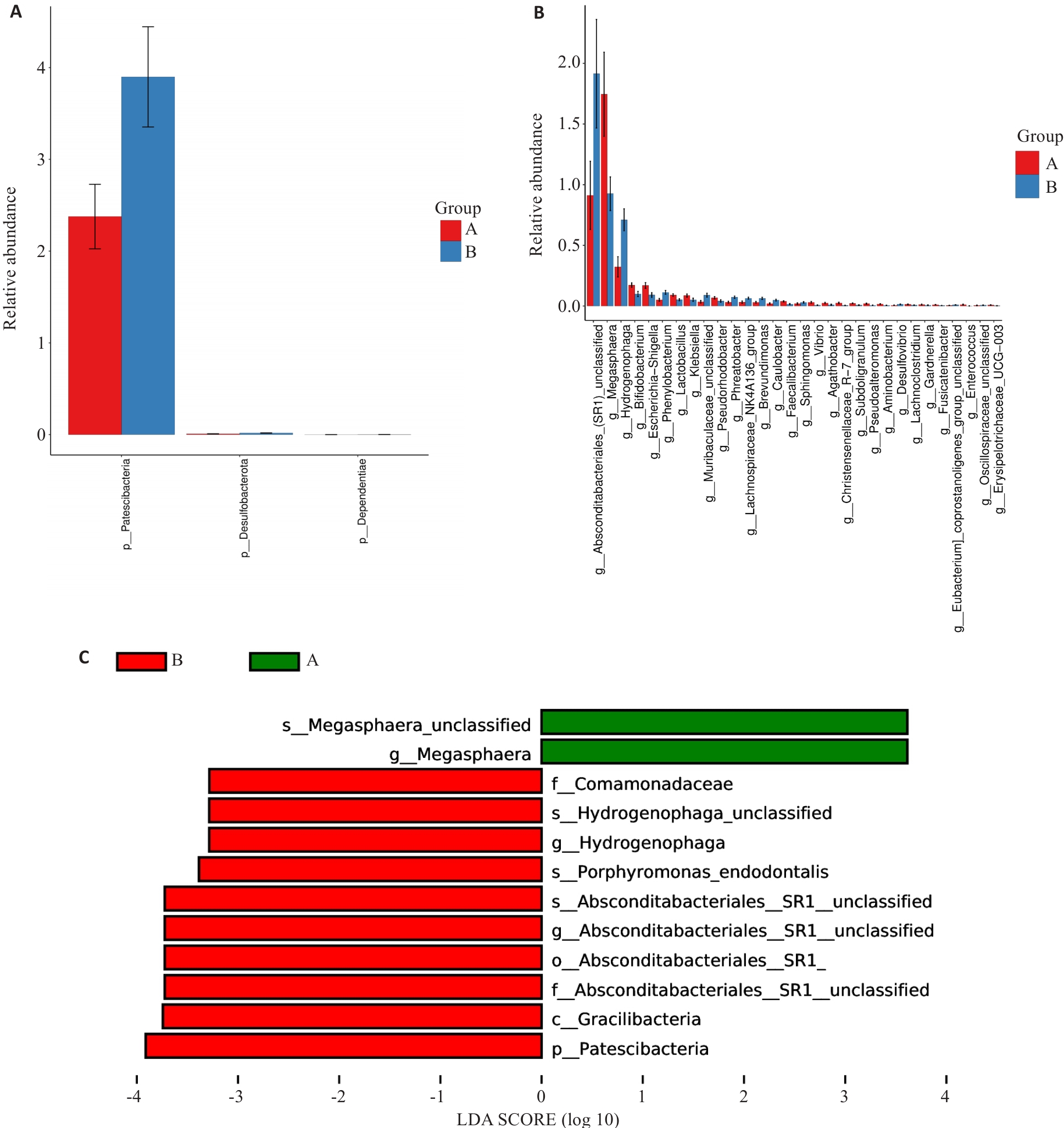
Fig.2 Analysis of variances. A: Differences at the phylum level. B: Differences at the genus level. C: LEfSe analysis at all levels. Group A: MAFLD; Group B: MAFLD+CHB.
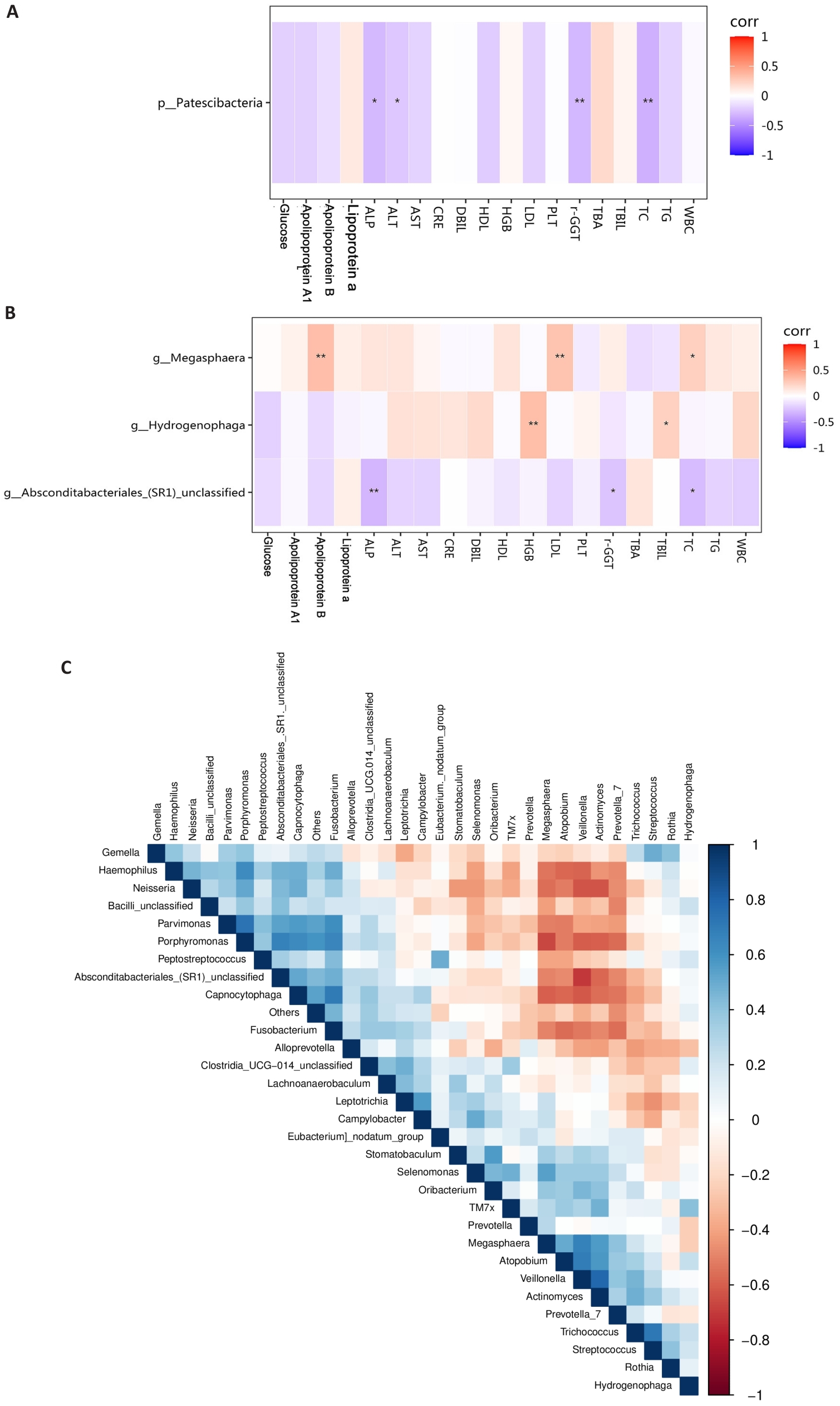
Fig.3 Correlation analysis of different bacterial groups. A: Correlation analysis between phylum-level bacteria and blood indicators. B: Correlation analysis between genus-level bacteria and biochemical indicators. C: Correlation analysis among differentially abundant genus-level bacteria. Group A: MAFLD; Group B: MAFLD+CHB. *P<0.05, **P<0.01.
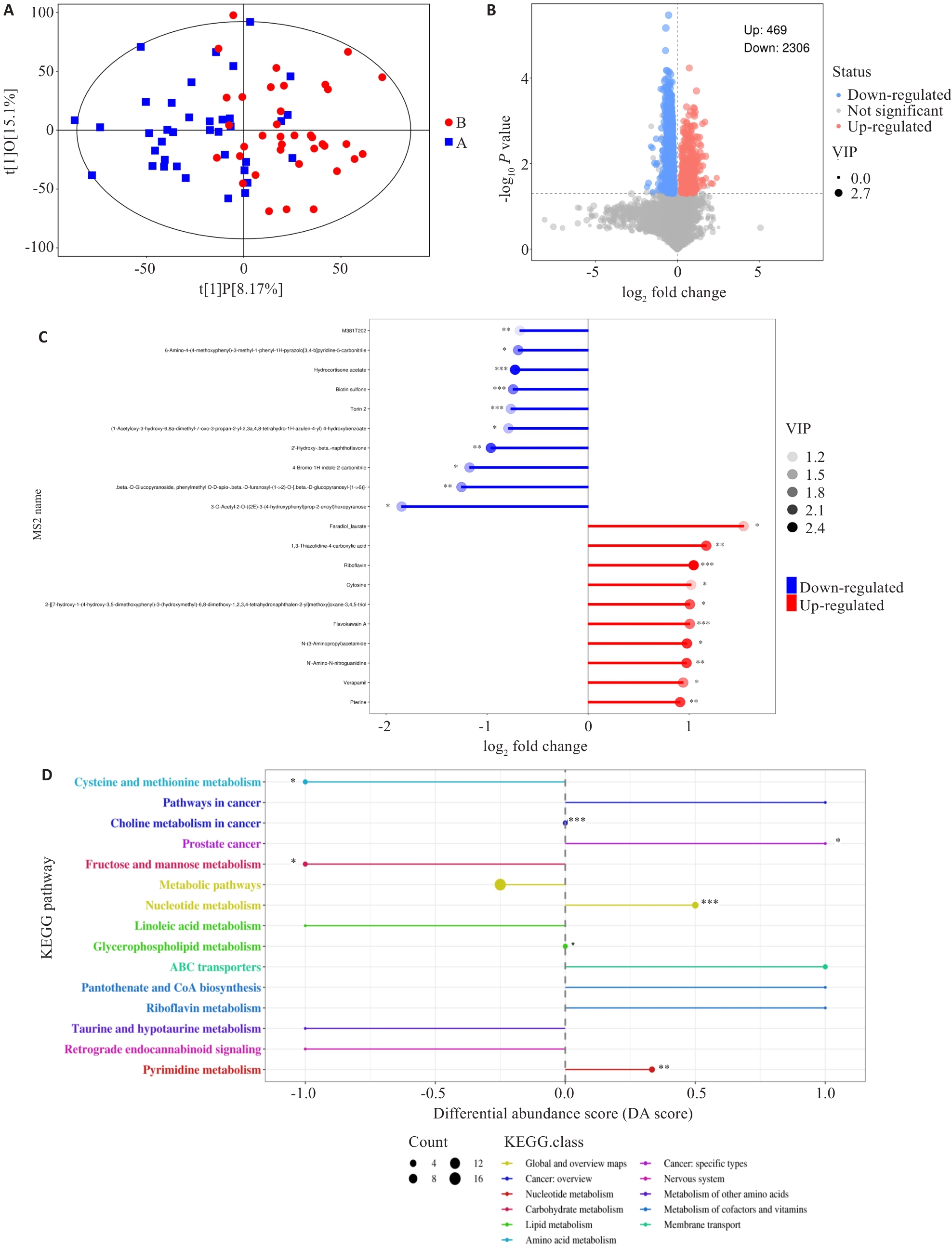
Fig.5 Distribution and pathway enrichment analysis of differential metabolites between the two groups. A: Scatter plot showing the differences between the sample groups (OPLS-DA model). B: Volcano plot showing the overall distribution of metabolite differences between the two groups. C: The top 10 metabolites with the highest upregulation and downregulation fold after logarithmic transformation among the differential metabolites. D: Differential abundance scores of KEGG enrichment for differential metabolites between the two groups. DA Score of 1 indicates an upregulation trend in the expression of all annotated differential metabolites within that pathway, while -1 indicates a downregulation trend. The larger the dot, the greater the number of differential metabolites in that pathway. *P<0.05, *P<0.01, ***P<0.001.
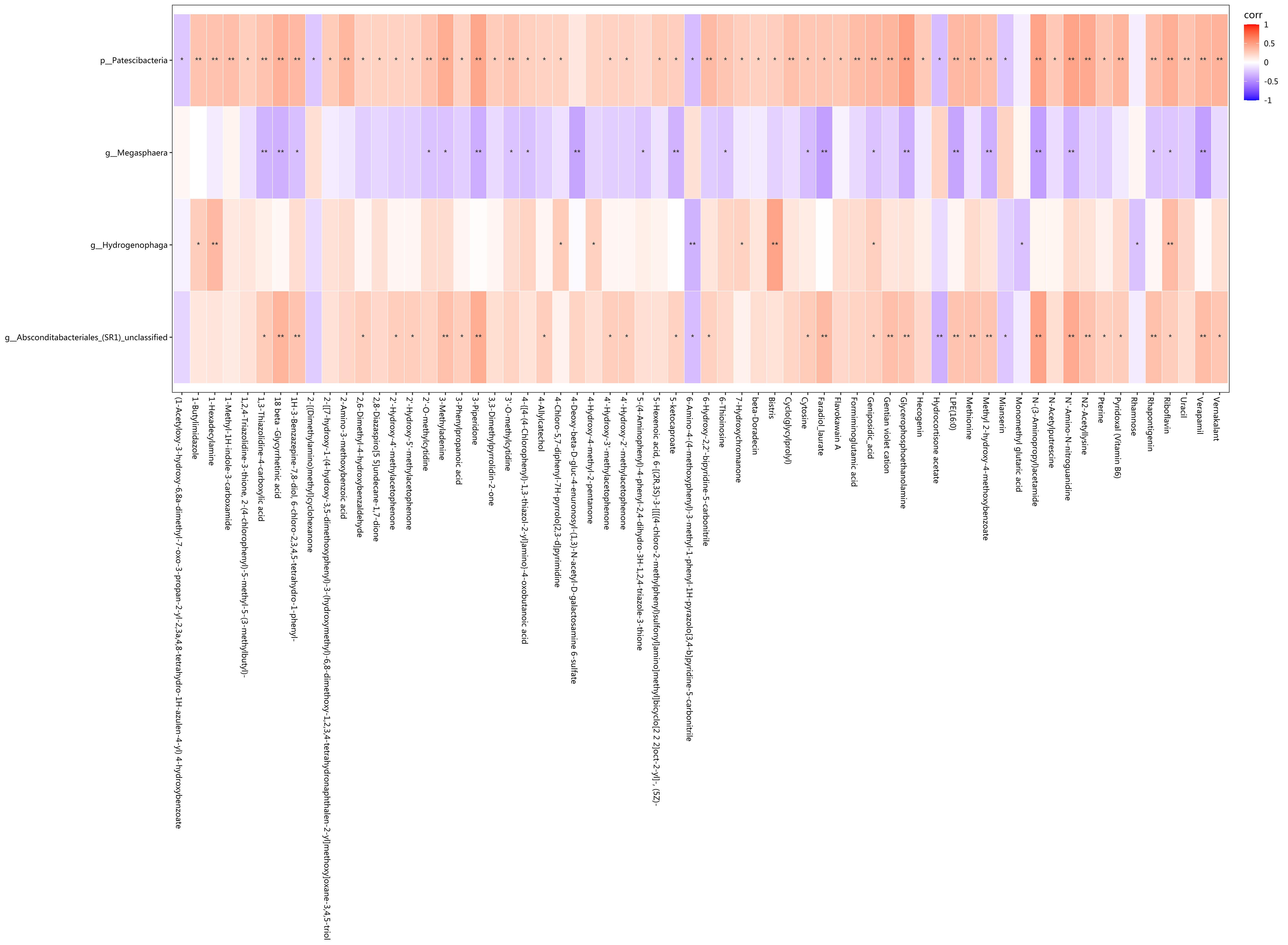
Fig.6 Correlation analysis showing statistical differences in the correlation between the differential bacteria and metabolites. Red represents positive correlation, blue represents negative correlation, and darker colors indicate stronger correlation. *P<0.05, **P<0.01.
| [1] | Younossi ZM, Wong G, Anstee QM, et al. The global burden of liver disease[J]. Clin Gastroenterol Hepatol, 2023, 21(8): 1978-91. doi:10.1016/j.cgh.2023.04.015 |
| [2] | European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD)[J]. J Hepatol, 2024, 81(3): 492-542. doi:10.1159/000539371 |
| [3] | Thomas JA, Kendall BJ, El-Serag HB, et al. Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease[J]. Lancet Gastroenterol Hepatol, 2024, 9(2): 159-69. doi:10.1016/s2468-1253(23)00275-3 |
| [4] | De A, Bhagat N, Mehta M, et al. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD[J]. J Hepatol, 2024, 80(2): e61-2. doi:10.1016/j.jhep.2023.07.031 |
| [5] | Yang A, Zhu X, Zhang L, et al. Transitioning from NAFLD to MAFLD and MASLD: consistent prevalence and risk factors in a Chinese cohort[J]. J Hepatol, 2024, 80(4): e154-5. doi:10.1016/j.jhep.2023.09.033 |
| [6] | Danpanichkul P, Duangsonk K, Chen VL, et al. Global burden of HBV-related liver disease: primary liver cancer due to chronic HBV infection increased in over one-third of countries globally from 2000 to 2021[J]. Hepatology, 2025, 29: 130945-58. doi:10.1097/hep.0000000000001260 |
| [7] | Wong RJ, Gish RG, Cheung R, et al. Low prevalence of vaccination or documented immunity to hepatitis a and hepatitis B viruses among individuals with chronic liver disease[J]. Am J Med, 2021, 134(7): 882-92. doi:10.1016/j.amjmed.2021.02.008 |
| [8] | Tourkochristou E, Assimakopoulos SF, Thomopoulos K, et al. NAFLD and HBV interplay-related mechanisms underlying liver disease progression[J]. Front Immunol, 2022, 13: 965548. doi:10.3389/fimmu.2022.965548 |
| [9] | Huang SC, Su TH, Tseng TC, et al. Metabolic dysfunction-associated steatotic liver disease facilitates hepatitis B surface antigen seroclearance and seroconversion[J]. Clin Gastroenterol Hepatol, 2024, 22(3): 581-90. e6. doi:10.1016/j.cgh.2023.09.040 |
| [10] | Kim MN, Han K, Yoo J, et al. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver[J]. Aliment Pharmacol Ther, 2022, 55(1): 97-107. doi:10.1111/apt.16706 |
| [11] | Vassilopoulos S, Kalligeros M, Vassilopoulos A, et al. Impact of prior HBV, HAV, and HEV infection on non-alcoholic fatty liver disease[J]. J Viral Hepat, 2023, 30(8): 685-93. doi:10.1111/jvh.13862 |
| [12] | Joo EJ, Cheong HS, Kwon MJ, et al. Relationship between gut microbiome diversity and hepatitis B viral load in patients with chronic hepatitis B[J]. Gut Pathog, 2021, 13(1): 65. doi:10.1186/s13099-021-00461-1 |
| [13] | Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications[J]. Cell Metab, 2022, 34(11): 1700-18. doi:10.1016/j.cmet.2022.09.017 |
| [14] | Lang S, Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future[J]. Cell Host Microbe, 2020, 28(2): 233-44. doi:10.1016/j.chom.2020.07.007 |
| [15] | Han W, Huang C, Ji Y, et al. Alterations in the gut microbiota and hepatitis-B-virus infection in southern Chinese patients with coexisting non-alcoholic fatty liver disease and type-2 diabetes mellitus[J]. Front Med: Lausanne, 2021, 8: 805029. doi:10.3389/fmed.2021.805029 |
| [16] | Lei Y, Li S, He M, et al. Oral pathogenic bacteria and the oral-gut-liver axis: a new understanding of chronic liver diseases[J]. Diagnostics: Basel, 2023, 13(21): 3324. doi:10.3390/diagnostics13213324 |
| [17] | Chen TP, Yu HC, Lin WY, et al. The role of microbiome in the pathogenesis of oral-gut-liver axis between periodontitis and nonalcoholic fatty liver disease[J]. J Dent Sci, 2023, 18(3): 972-5. doi:10.1016/j.jds.2023.03.012 |
| [18] | Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement[J]. J Hepatol, 2020, 73(1): 202-9. doi:10.1016/j.jhep.2020.07.045 |
| [19] | Eslam M, Fan JG, Yu ML, et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease[J]. Hepatol Int, 2025, 19(2): 261-301. |
| [20] | Liu G, Li T, Zhu X, et al. An independent evaluation in a CRC patient cohort of microbiome 16S rRNA sequence analysis methods: OTU clustering, DADA2, and Deblur[J]. Front Microbiol, 2023, 14: 1178744. doi:10.3389/fmicb.2023.1178744 |
| [21] | Cheng YM, Hsieh TH, Wang CC, et al. Impact of HBV infection on clinical outcomes in patients with metabolic dysfunction-associated fatty liver disease[J]. JHEP Rep, 2023, 5(9): 100836. doi:10.1016/j.jhepr.2023.100836 |
| [22] | Gu H, Tian Y, Xia J, et al. Li-Hong Tang alleviates dextran sodium sulfate-induced colitis by regulating NRF2/HO-1 signaling pathway and gut microbiota[J]. Front Pharmacol, 2024, 15: 1413666. doi:10.3389/fphar.2024.1413666 |
| [23] | Mukuda K, Inoue R, Takata M, et al. Temporal effects of lascufloxacin on human gut and salivary microbiota: Analysis using next-generation sequencing method[J]. J Infect Chemother, 2025, 31(1): 102483. doi:10.1016/j.jiac.2024.07.023 |
| [24] | Zhao YT, Liu S, Wang WZ, et al. Associations of indoor airborne microbiome with lung function: evidence from a randomized, double-blind, crossover study of microbial intervention[J]. Environ Sci: Processes Impacts, 2024, 26(11): 2020-35. doi:10.1039/d4em00392f |
| [25] | Yunusbayev B, Bogdanova A, Nadyrchenko N, et al. Gut dysbiosis narrative in psoriasis: matched-pair approach identifies only subtle shifts correlated with elevated fecal calprotectin[J]. Microbiol Spectr, 2025, 13(1): e0138224. doi:10.1128/spectrum.01382-24 |
| [26] | Du Y, Neng Q, Li Y, et al. Gastrointestinal autonomic neuropathy exacerbates gut microbiota dysbiosis in adult patients with type 2 diabetes mellitus[J]. Front Cell Infect Microbiol, 2021, 11: 804733. doi:10.3389/fcimb.2021.804733 |
| [27] | Badmus OO, Hillhouse SA, Anderson CD, et al. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways[J]. Clin Sci: Lond, 2022, 136(18): 1347-66. doi:10.1042/cs20220572 |
| [28] | Paulusma CC, Lamers WH, Broer S, et al. Amino acid metabolism, transport and signalling in the liver revisited[J]. Biochem Pharmacol, 2022, 201: 115074. doi:10.1016/j.bcp.2022.115074 |
| [29] | Li H, Xu QY, Xie Y, et al. Effects of chronic HBV infection on lipid metabolism in non-alcoholic fatty liver disease: a lipidomic analysis[J]. Ann Hepatol, 2021, 24: 100316. doi:10.1016/j.aohep.2021.100316 |
| [30] | Shen Y, Wu SD, Chen Y, et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response[J]. Gut Microbes, 2023, 15(1): 2155018. doi:10.1080/19490976.2022.2155018 |
| [31] | Li XX, Wu S, Du Y, et al. Entecavir therapy reverses gut microbiota dysbiosis induced by hepatitis B virus infection in a mouse model[J]. Int J Antimicrob Agents, 2020, 56(1): 106000. doi:10.1016/j.ijantimicag.2020.106000 |
| [32] | Albuquerque-Souza E, Sahingur SE. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis[J]. Periodontol 2000, 2022, 89(1): 125-41. doi:10.1111/prd.12427 |
| [1] | Guoyong LI, Renling LI, Yiting LIU, Hongxia KE, Jing LI, Xinhua WANG. Therapeutic mechanism of Arctium lappa extract for post-viral pneumonia pulmonary fibrosis: a metabolomics, network pharmacology analysis and experimental verification [J]. Journal of Southern Medical University, 2025, 45(6): 1185-1199. |
| [2] | Chao ZHOU, Jingjing ZHANG, Qiao TANG, Shuangnan FU, Ning ZHANG, Zhaoyun HE, Jin ZHANG, Tianyi ZHANG, Pengcheng LIU, Man GONG. Value of serum tryptophan in stratified management of 90-day mortality risk in patients with hepatitis B virus-related acute-on-chronic liver failure: a multicenter retrospective study [J]. Journal of Southern Medical University, 2025, 45(1): 59-64. |
| [3] | Guanzheng YU, Weiqiang CHENG, Xing TU, Man ZHANG, Hong LI, Juan NIE. Therapeutic mechanism of Cynanchum wilfordii for ulcerative colitis: an analysis using UPLC-QE-MS, network pharmacology and metabolomics [J]. Journal of Southern Medical University, 2024, 44(8): 1485-1496. |
| [4] | Pengcheng LIU, Lijuan LOU, Xia LIU, Jian WANG, Ying JIANG. A risk scoring model based on M2 macrophage-related genes for predicting prognosis of HBV-related hepatocellular carcinoma [J]. Journal of Southern Medical University, 2024, 44(5): 827-840. |
| [5] | HU Sigan, CHENG Zengwei, LI Min, GAO Shiyi, GAO Dasheng, KANG Pinfang. Correlation between insulin resistance and coronary collateral circulation in patients with chronic total coronary occlusion [J]. Journal of Southern Medical University, 2024, 44(4): 780-786. |
| [6] | Chengling CUI, Yuzhen XU, Chaoqun TANG, Jiaying JIANG, Ying HU, Jie SHUANG. Molecular mechanism of high-altitude hypoxia-induced lipid metabolism disorder in mouse spleen tissue [J]. Journal of Southern Medical University, 2024, 44(10): 2024-2032. |
| [7] | XU Longfei, HAN Jing, YANG Zhe, YANG Yanping, CHEN Jinhui, WU Xijun, WANG Qi, HONG Yan. LRG1 inhibits hepatic macrophage activation by enhancing TGF-β1 signaling to alleviate MAFLD in mice [J]. Journal of Southern Medical University, 2023, 43(7): 1164-1171. |
| [8] | WANG Xuancheng, ZHU Yifan, ZHOU Hailin, HUANG Zongsheng, CHEN Hongwei, ZHANG Jiahao, YANG Shanyi, CHEN Guanghui, ZHANG Qisong. Integrated analysis of serum untargeted metabolomics and targeted bile acid metabolomics for identification of diagnostic biomarkers for colorectal cancer [J]. Journal of Southern Medical University, 2023, 43(3): 443-453. |
| [9] | GAO Yinan, WANG Peijun, LU Sumei, MA Wanshan. METTL3 inhibitor STM2457 improves metabolic dysfunction-associated fatty liver disease by regulating mitochondrial function in mice [J]. Journal of Southern Medical University, 2023, 43(10): 1689-1696. |
| [10] | LIU Wenhu, TANG Jiancai, CHANG Jinxia. RUNX3 regulates trastuzumab resistance of gastric cancer cells: a metabolomic analysis based on UPLC-Q Exactive Focus Orbitrap mass spectrometry [J]. Journal of Southern Medical University, 2022, 42(4): 498-508. |
| [11] | WANG Jiayuan, YUAN Yiyi, ZHANG Kun, SUN Xiang, BU Xin, DONG Jian, WU Yousheng, TIAN Hongying, SHEN Lan. NDRG2 inhibits tumorigenesis of hepatocellular carcinoma by regulating metabolism of phospholipids and triglyceride: a metabonomic analysis [J]. Journal of Southern Medical University, 2022, 42(12): 1765-1773. |
| [12] | LI Yujie, WU Dengqiang, WEI Changhong, YANG Xuejia, ZHOU Sufang. CDK1, CCNB1 and NDC80 are associated with prognosis and progression of hepatitis B virus-associated hepatocellular carcinoma: a bioinformatic analysis [J]. Journal of Southern Medical University, 2021, 41(10): 1509-1518. |
| [13] | . Generation of a novel HBeAg transgenic mice using CRISPR/Cas9 technique [J]. Journal of Southern Medical University, 2019, 39(09): 1017-. |
| [14] | . High-performance liquid chromatography-mass spectrometry-based serum metabolic profiling in patients with HBV-related hepatocellular carcinoma [J]. Journal of Southern Medical University, 2019, 39(01): 49-. |
| [15] | . Red blood cell distribution width is a independent prognostic indicator for mortality in patients with HBV related acute-on-chronic liver failure [J]. Journal of Southern Medical University, 2018, 38(11): 1354-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||