Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (6): 1226-1239.doi: 10.12122/j.issn.1673-4254.2025.06.12
Previous Articles Next Articles
Xinrui HOU1,2( ), Zhendong ZHANG1,2, Mingyuan CAO1,2, Yuxin DU1,2, Xiaoping WANG1(
), Zhendong ZHANG1,2, Mingyuan CAO1,2, Yuxin DU1,2, Xiaoping WANG1( )
)
Received:2025-03-21
Online:2025-06-20
Published:2025-06-27
Contact:
Xiaoping WANG
E-mail:362380328@qq.com;xpwang@xzmz.edu.cn
Supported by:Xinrui HOU, Zhendong ZHANG, Mingyuan CAO, Yuxin DU, Xiaoping WANG. Salidroside inhibits proliferation of gastric cancer cells by regulating the miR-1343-3p-OGDHL/PDHB glucose metabolic axis[J]. Journal of Southern Medical University, 2025, 45(6): 1226-1239.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.06.12
| Primer name | Direction | Sequence (5'→3') |
|---|---|---|
| GAPDH | Forward | TGACATCAAGAAGGTGGTGAAGCAG |
| Reverse | GTGTCGCTGTTGAAGTCAGAGGAG | |
| U6 | Forward | GCTTCGGCAGCACATATACTAAAAT |
| Reverse | CGCTTCACGAATTTGCGTGTCAT | |
| miR-1343-3p | Forward | CGCGGCCTTAATGCTAATTGTGA |
| Reverse | AGTGCAGGGTCCGAGGTATT | |
| OGDHL | Forward | ATCACTCTGTCGCTGGTTGCC |
| Reverse | CGCTCAGGTGGAAGGTCTCATATAC | |
| PDHB | Forward | TTCTCCATGCAAGCCATTGACCAG |
| Reverse | CTACACCTGCTGAGGCACCATTG | |
| miR-1343-3p mimic | Sense | CUCCUGGGGCCCGCACUCUCGCUU |
| Antisense | AAGCGAGAGUGCGGGCCCAGGAG | |
| NC mimic | Sense | UCACAACCUCCUAGAAAGAGUAGA |
| Antisense | UCUACUCUUUCUAGGAGGUUGUGA | |
| miR-1343-3p inhibitor | Antisense | AAGCGAGAGUGCGGGCCCCAGGAG |
| NC inhibitor | Antisense | UCUACUCUUUCUAGGAGGUUGUGA |
| miR1343-3p agomir | sense | CUCCUGGGGCCCGCACUCUCGCUU |
| antisense | AAGCGAGAGUGCGGGCCCAGGAG | |
| si-OGDHL | Sense | CCCUCAGACUUGAUCACAAdTdT |
| Antisense | UUGUGAUCAAGUCUGAGGGdTdT | |
| si-NC | Sense | UUCUCCGAACGUGUCACGUdTdT |
| Antisense | ACGUGACACGUUCGGAGAAdTdT |
Tab.1 Primer and oligonucleotide sequences
| Primer name | Direction | Sequence (5'→3') |
|---|---|---|
| GAPDH | Forward | TGACATCAAGAAGGTGGTGAAGCAG |
| Reverse | GTGTCGCTGTTGAAGTCAGAGGAG | |
| U6 | Forward | GCTTCGGCAGCACATATACTAAAAT |
| Reverse | CGCTTCACGAATTTGCGTGTCAT | |
| miR-1343-3p | Forward | CGCGGCCTTAATGCTAATTGTGA |
| Reverse | AGTGCAGGGTCCGAGGTATT | |
| OGDHL | Forward | ATCACTCTGTCGCTGGTTGCC |
| Reverse | CGCTCAGGTGGAAGGTCTCATATAC | |
| PDHB | Forward | TTCTCCATGCAAGCCATTGACCAG |
| Reverse | CTACACCTGCTGAGGCACCATTG | |
| miR-1343-3p mimic | Sense | CUCCUGGGGCCCGCACUCUCGCUU |
| Antisense | AAGCGAGAGUGCGGGCCCAGGAG | |
| NC mimic | Sense | UCACAACCUCCUAGAAAGAGUAGA |
| Antisense | UCUACUCUUUCUAGGAGGUUGUGA | |
| miR-1343-3p inhibitor | Antisense | AAGCGAGAGUGCGGGCCCCAGGAG |
| NC inhibitor | Antisense | UCUACUCUUUCUAGGAGGUUGUGA |
| miR1343-3p agomir | sense | CUCCUGGGGCCCGCACUCUCGCUU |
| antisense | AAGCGAGAGUGCGGGCCCAGGAG | |
| si-OGDHL | Sense | CCCUCAGACUUGAUCACAAdTdT |
| Antisense | UUGUGAUCAAGUCUGAGGGdTdT | |
| si-NC | Sense | UUCUCCGAACGUGUCACGUdTdT |
| Antisense | ACGUGACACGUUCGGAGAAdTdT |
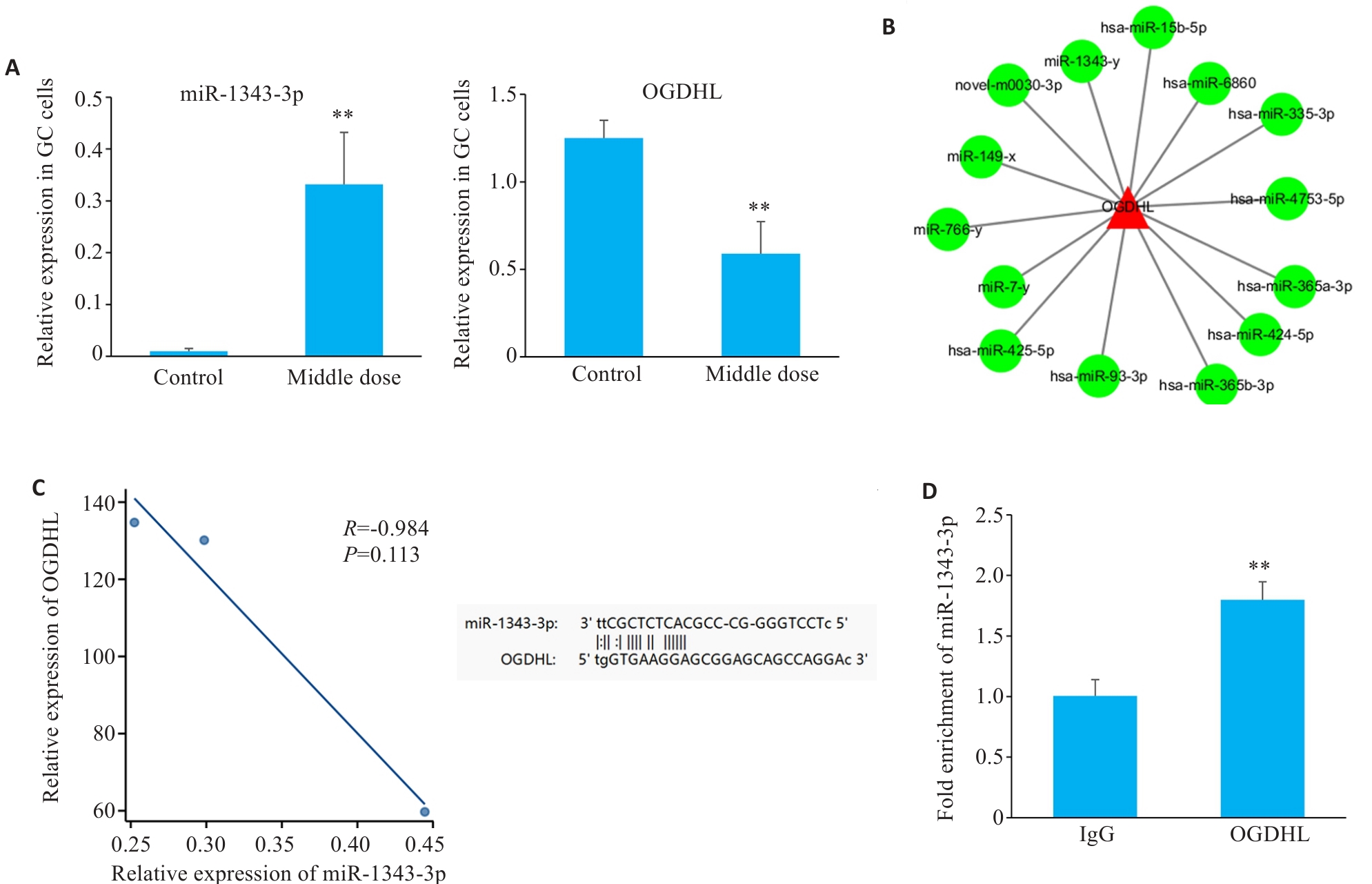
Fig.1 High-throughput sequencing combined with bioinformatics analysis for interaction between miR-1343-3p and OGDHL. A: Changes in miR-1343-3p and OGDHL in human gastric cancer cells after salidroside treatment detected by high-throughput sequencing. B: miRNA network and target gene analysis of the interaction between OGDHL and miR-1343-3p. C: Pearson correlation and genomic locus analysis of the association of OGDHL with miR-1343-3p in human gastric cancer cells. D: RNA immunoprecipitation assay for validating the interaction between OGDHL and miR-1343-3p. **P<0.01 vs Control/Ig G group (n=3).
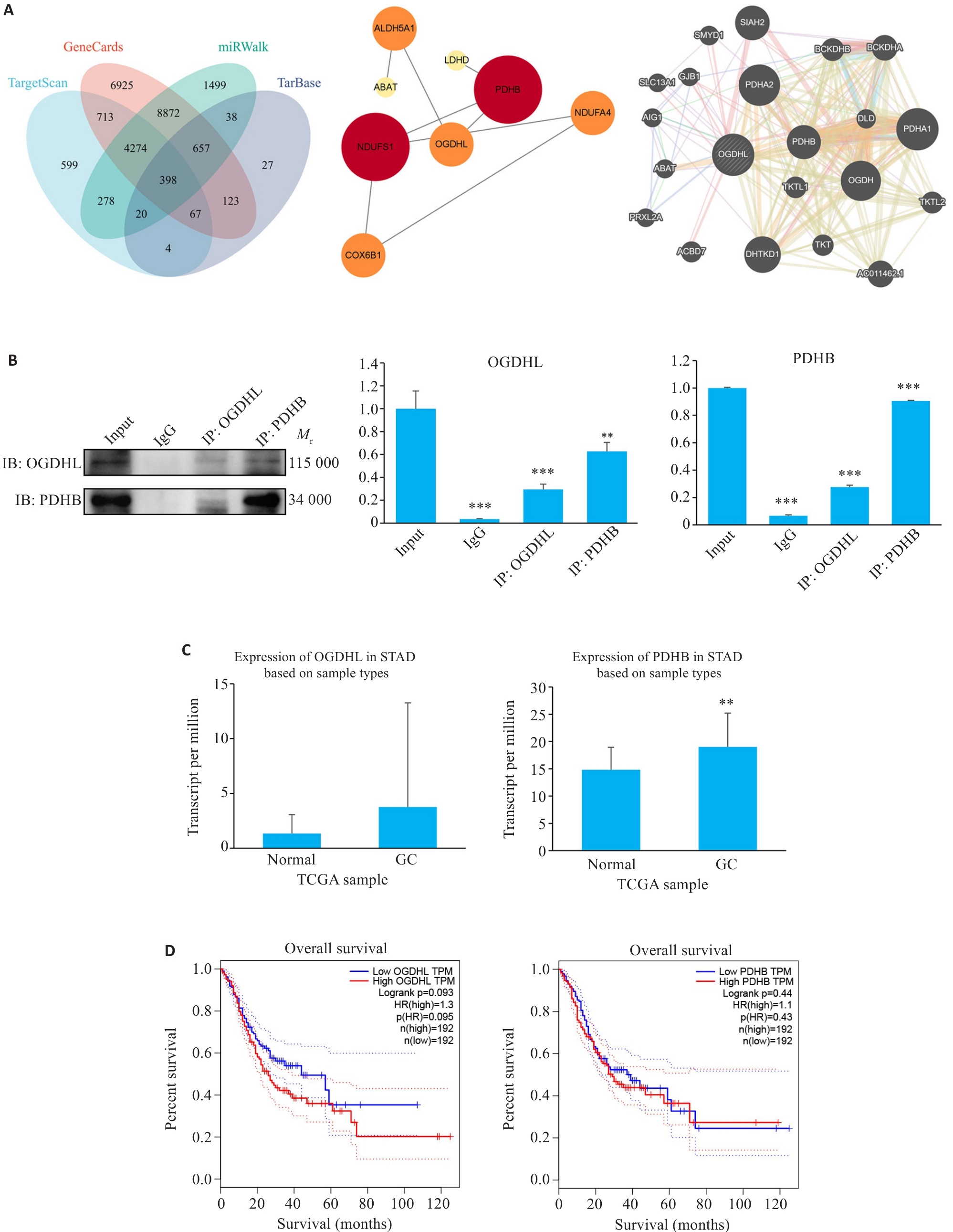
Fig.2 Interaction between OGDHL and PDHB proteins. A: Bioinformatics analysis of the OGDHL-PDHB interaction network. B: Co-immunoprecipitation assay for confirming their binding (**P<0.01, ***P<0.001 vs Input; n=3). C: Differential expression of OGDHL and PDHB in gastric cancer (GC) tissues from The Cancer Genome Atlas (TCGA) (**P<0.01 vs Normal; n=3). D: Gene Expression Profiling Interactive Analysis (GEPIA) showing a negative correlation between high OGDHL/PDHB expression and clinical outcomes of GC patients, highlighting a significantly shorter overall survival (OS) of patients in high-expression groups.
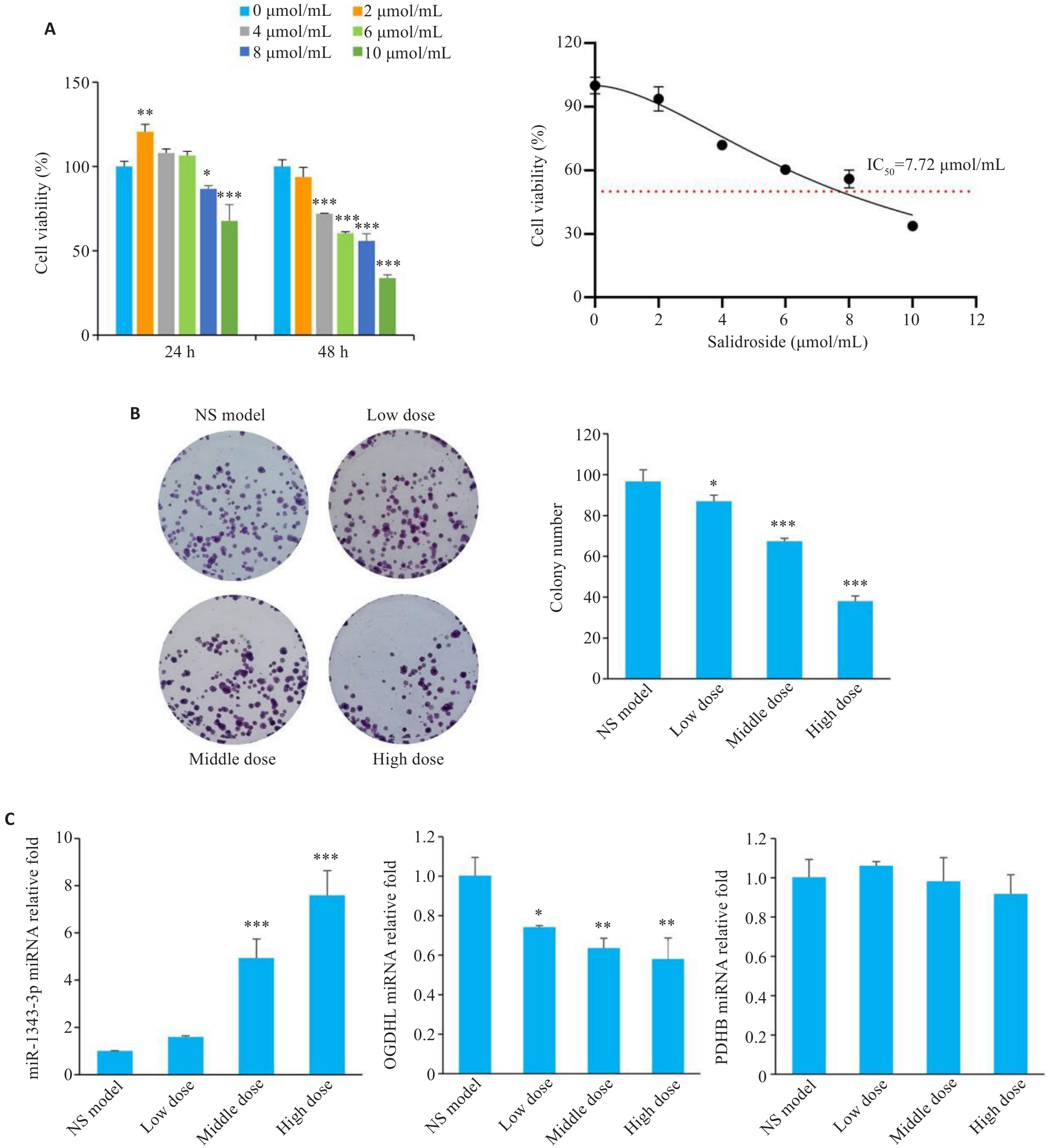
Fig.3 Salidroside inhibits proliferation of gastric cancer cells through the miR-1343-3p-OGDHL/PDHB axis. A: CCK-8 assay for assessing viability of MGC-803 cells treated with different concentrations of salidroside for 24 h/48 h. B: Colony formation assay of GC cells after salidroside treatment. C: qRT-PCR analysis showing changes in miR-1343-3p, OGDHL, and PDHB in GC cells after salidroside treatment. *P<0.05, **P<0.01, ***P<0.001 vs 0 μmol/mL or NS model (n=3).
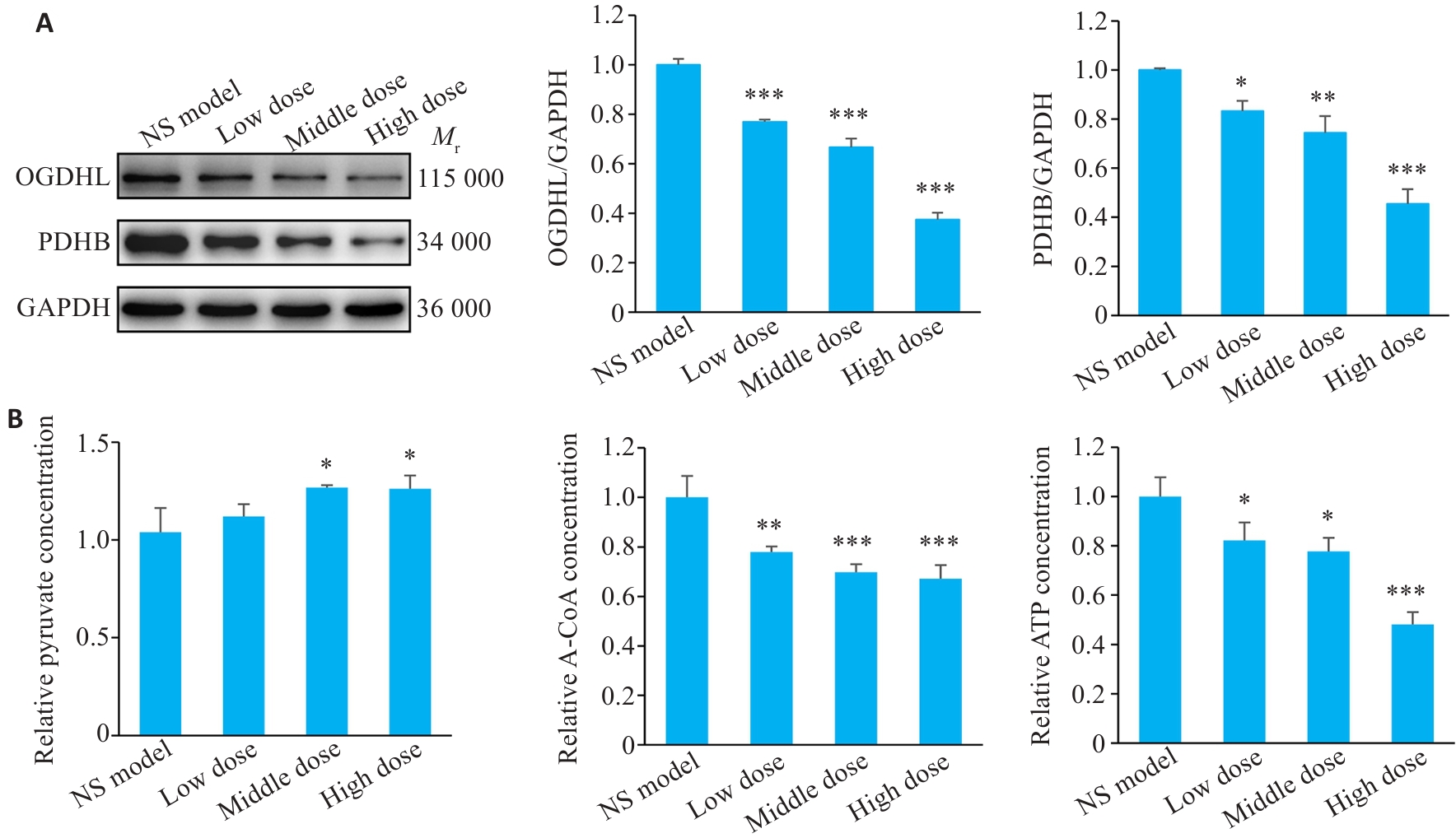
Fig.4 Expression of downstream protein molecules and energy-related metabolites post-salidroside treatment. A: Western blotting results. B: Detection results of pyruvate, acetyl-CoA, and ATP in salidroside-treated GC cells using biochemical assay kits. *P<0.05, **P<0.01, ***P<0.001 vs NS model (n=3).
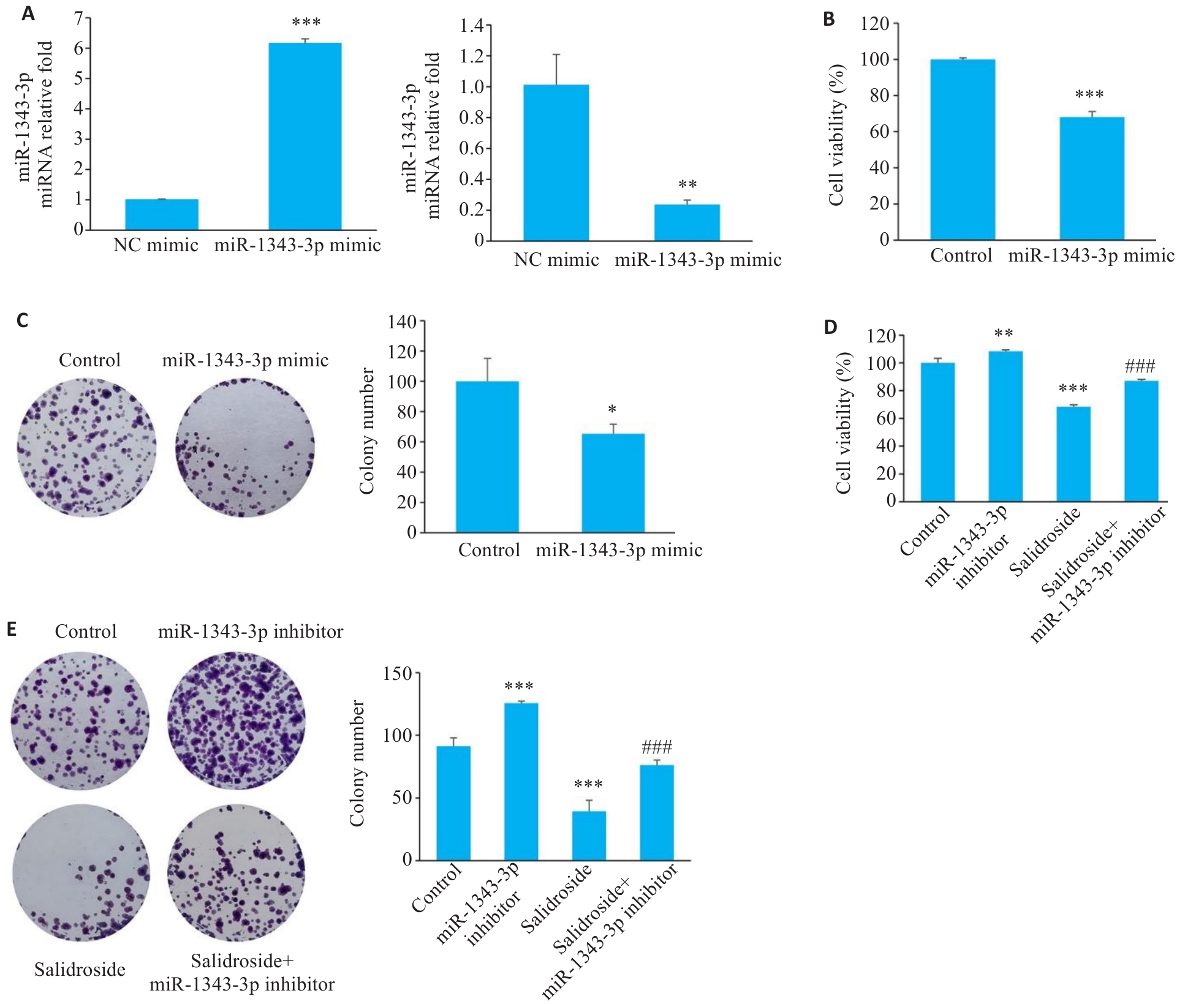
Fig.5 Transfection efficiency and functional changes of miR-1343-3p mimic/inhibitor in human GC cells. A: Successful transfection of miR-1343-3p mimic or inhibitor into human GC cells (***P<0.01 vs NC mimic, **P<0.001 vs NC inhibitor; n=3). B-E: Effects of miR-1343-3p mimic/inhibitor on cell viability and clonogenic ability of GC cells (*P<0.05, **P<0.01, ***P<0.01 vs Control; ###P<0.001 vs Salidroside; n=3).
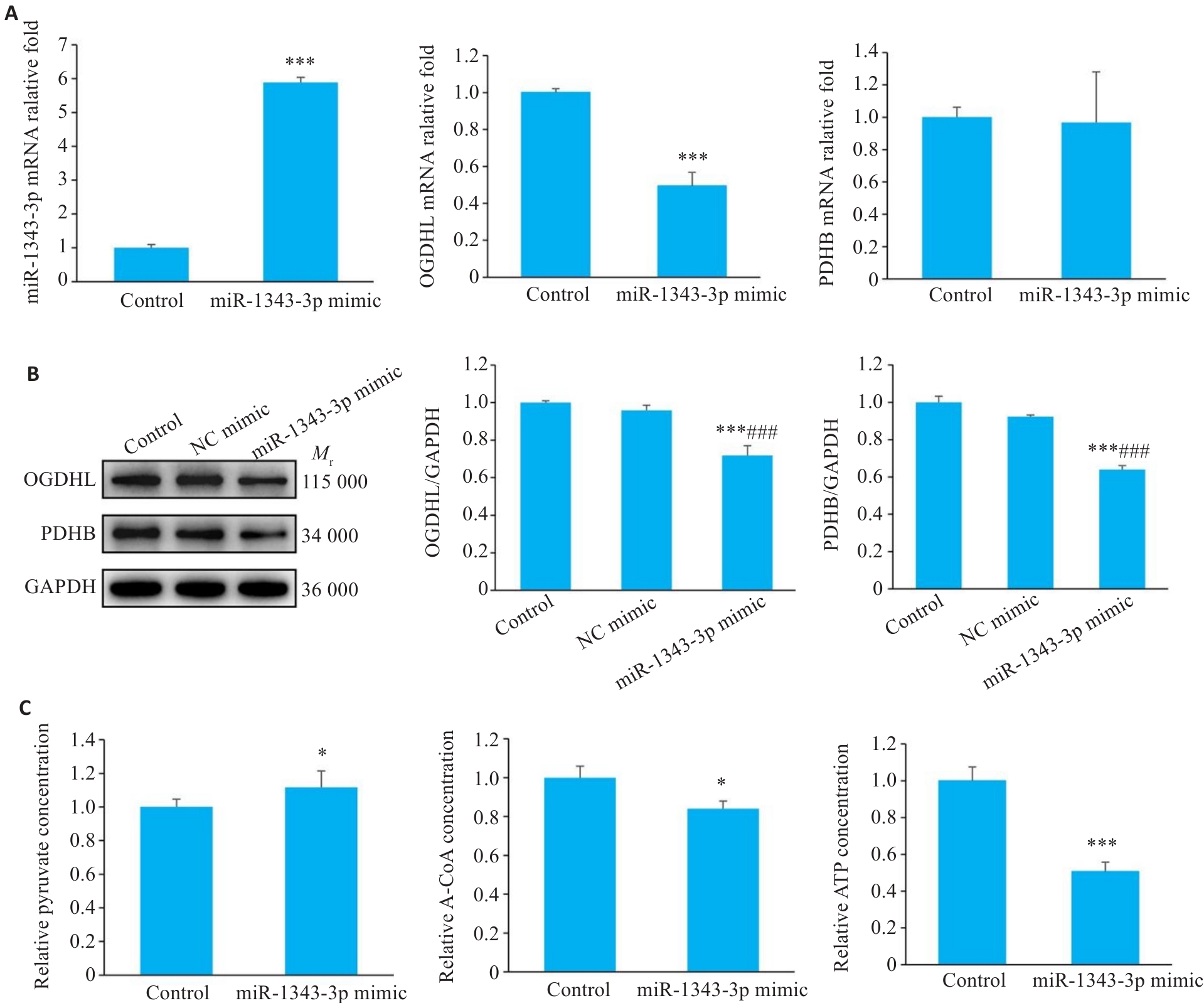
Fig.6 Synergistic regulation of GC cell growth by miR-1343-3p mimics and salidroside. A-C: Results of qRT-PCR, Western blotting, and biochemical assays for assessing changes in miR-1343-3p, OGDHL, PDHB, pyruvate, acetyl-CoA, and ATP levels in GC cells treated with miR-1343-3p mimic or inhibitors. *P<0.05, ***P<0.001 vs Control; ###P<0.001 vs NC mimic (n=3).
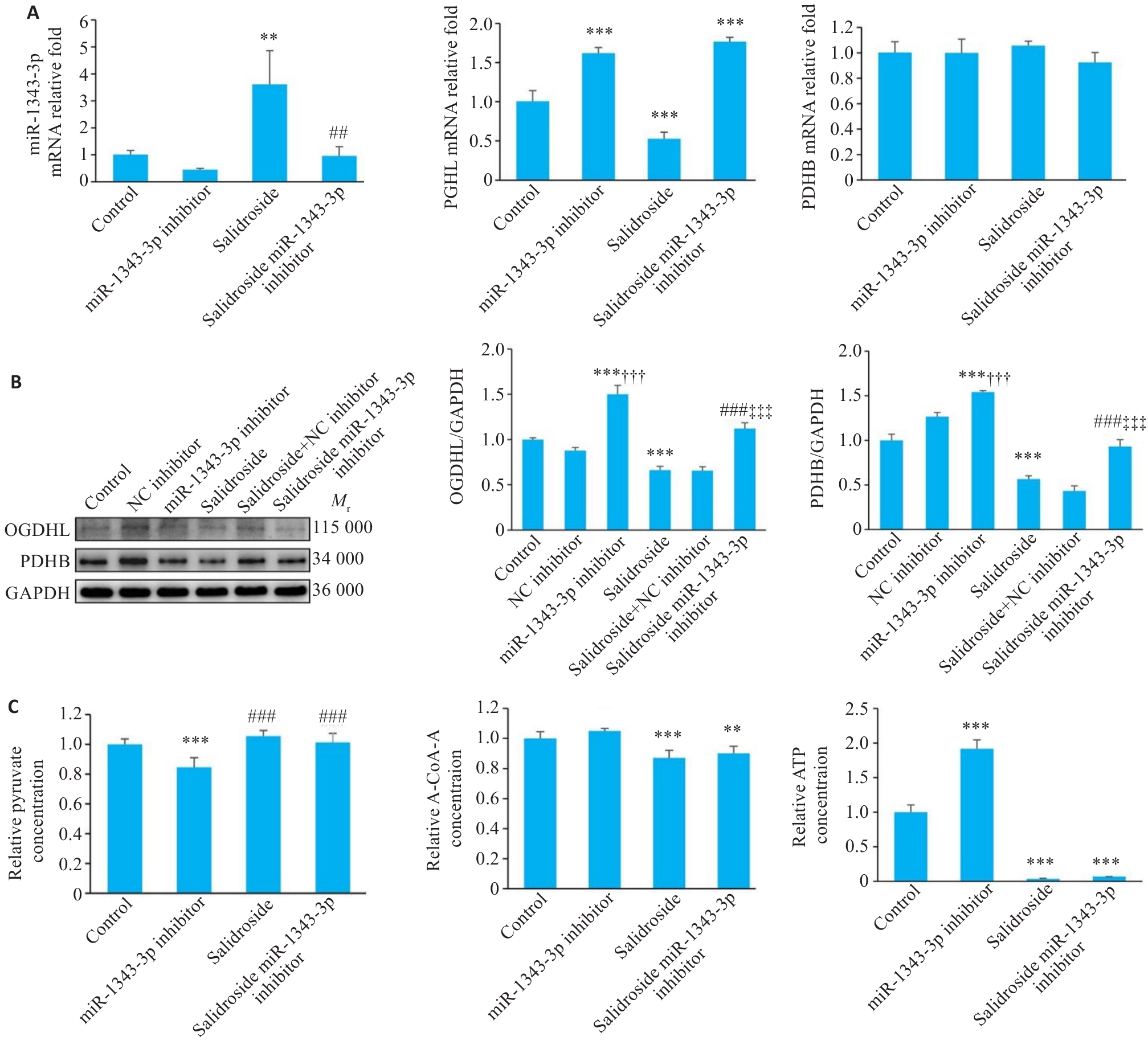
Fig.7 miR-1343-3p inhibitor reverses salidroside-mediated suppression of GC cell growth. A-C: Results of qRT-PCR, Western blotting, and biochemical detection miR-1343-3p, OGDHL, PDHB, pyruvate, acetyl-CoA, and ATP levels in GC cells following miR-1343-3p inhibitor treatment. **P<0.01, ***P<0.001 vs Control, ##P<0.01, ###P<0.001 vs Salidroside; †††P<0.001 vs NC inhibitor, ‡‡‡P<0.001 vs Salidroside+NC inhibitor; C: ###P<0.001 vs miR-1343-3p inhibitor (n=3).
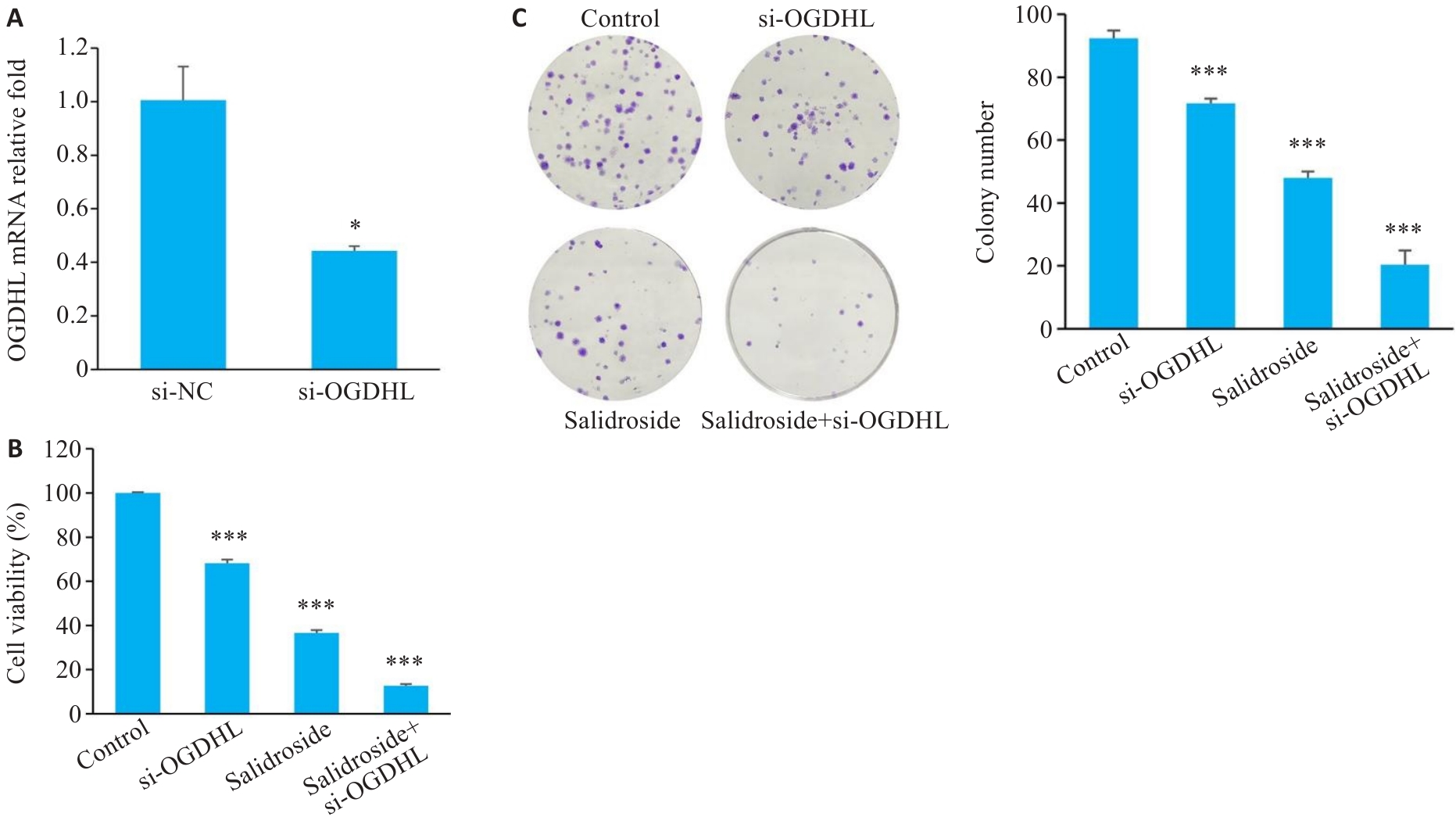
Fig.8 Transfection efficiency and functional changes of si-OGDHL in human GC cells. A: Successful transfection of si-OGDHL into GC cells. B, C: Effects of si-OGDHL on cell viability and clonogenic ability in GC cells. *P<0.05, ***P<0.001 vs Control (n=3).
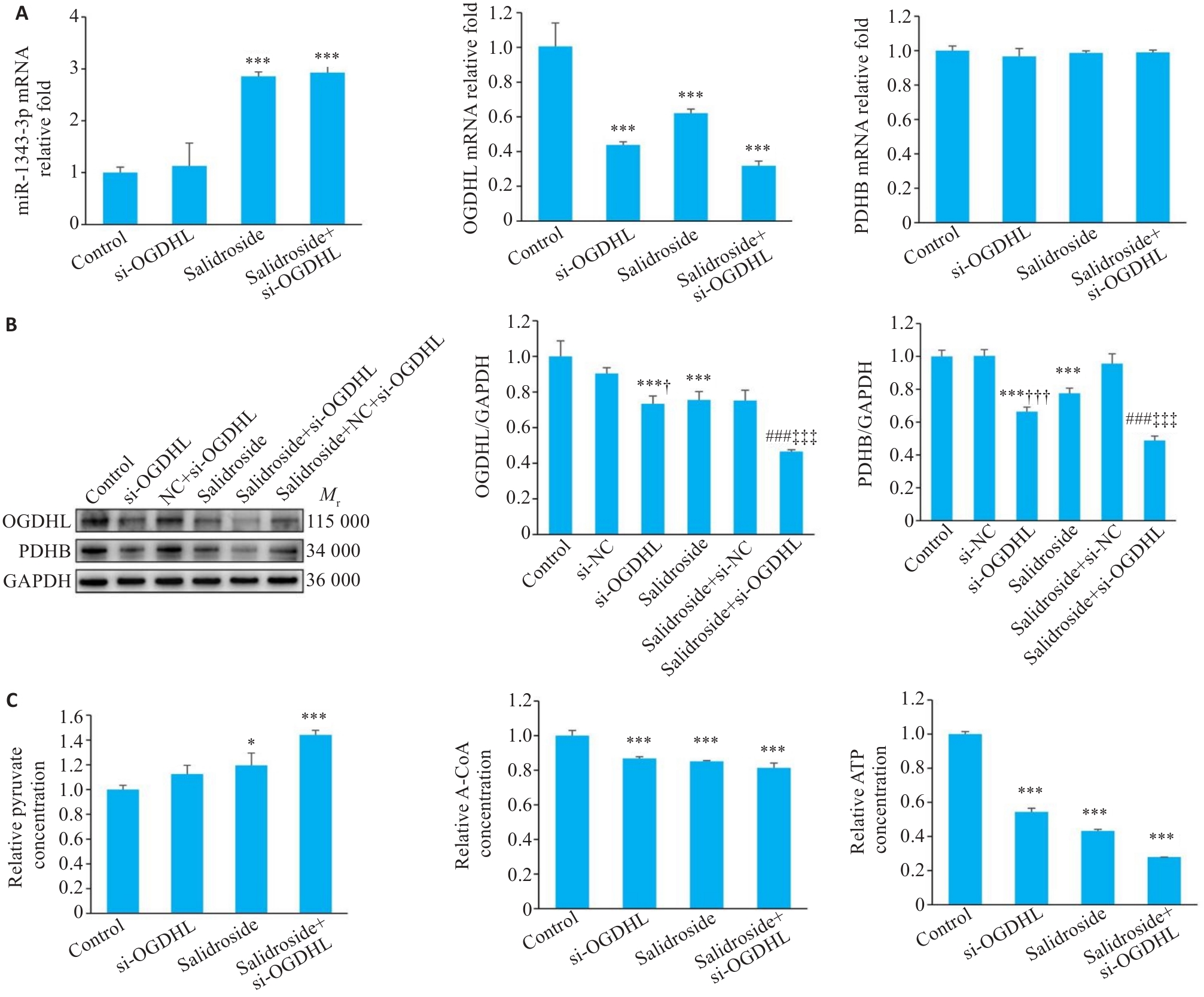
Fig.9 Knockdown of OGDHL synergizes with salidroside to inhibit GC cell growth. A-C: Results of qRT-PCR, Western blotting, and assay kits for miR-1343-3p, OGDHL, PDHB, pyruvate, acetyl-CoA, and ATP levels in GC cells treated with si-OGDHL. *P<0.05, ***P<0.001 vs Control; ###P<0.001 vs Salidroside; †P<0.05, †††P<0.001 vs si-NC, ‡‡‡P<0.001 vs Salidroside+si-NC (n=3).
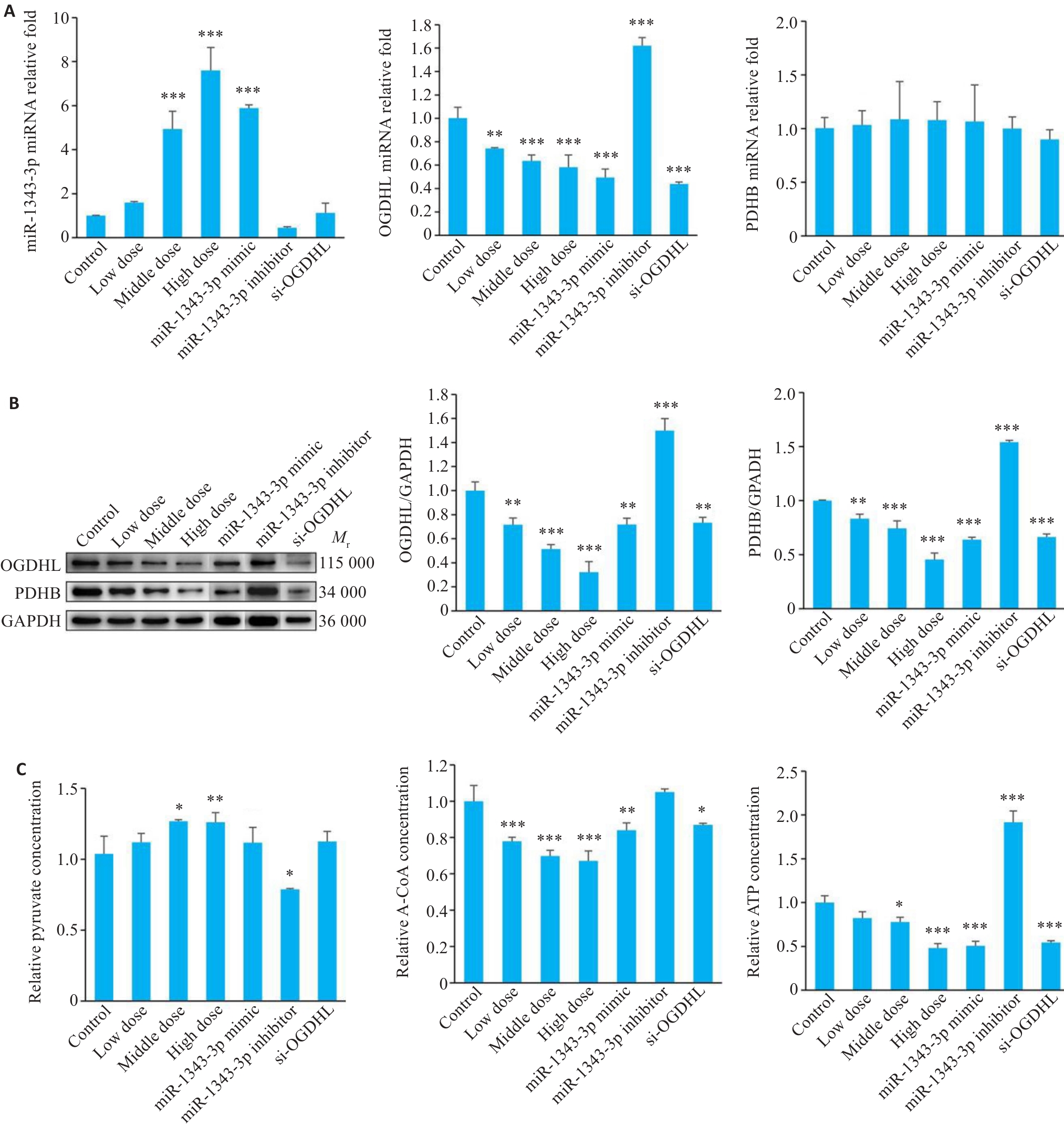
Fig.10 Changes in miR-1343-3p, OGDHL, PDHB, pyruvate, acetyl-CoA, and ATP levels in GC cells following different treatments. A-C:Results of qRT-PCR, Western blotting and biochemical assay kits for detecting miR-1343-3p, OGDHL, PDHB, pyruvate, acetyl-CoA and ATP levels in GC cells treated with salidroside, miR-1343-3p mimic, miR-1343-3p inhibitor and si-OGDHL. *P<0.05, **P<0.01, ***P<0.001 vs Control (n=3).
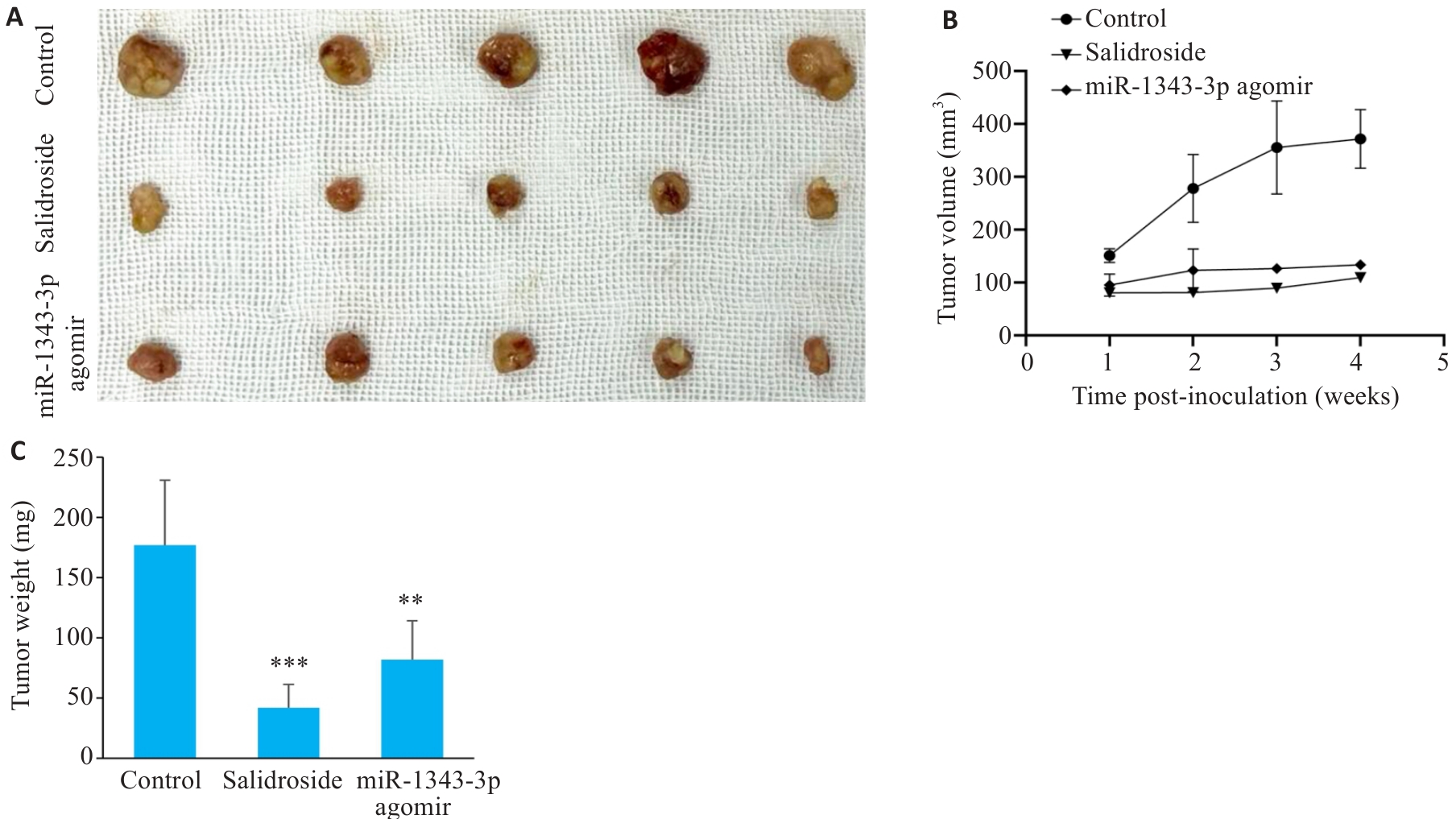
Fig.11 Observation of morphological changes in tumor masses after xenografting of GC cells in nude mice. A-C: Gross appearance and comparison of volume/weight in tumor masses treated with salidroside and miR-1343-3p overexpression. **P<0.01, ***P<0.001 vs Control.
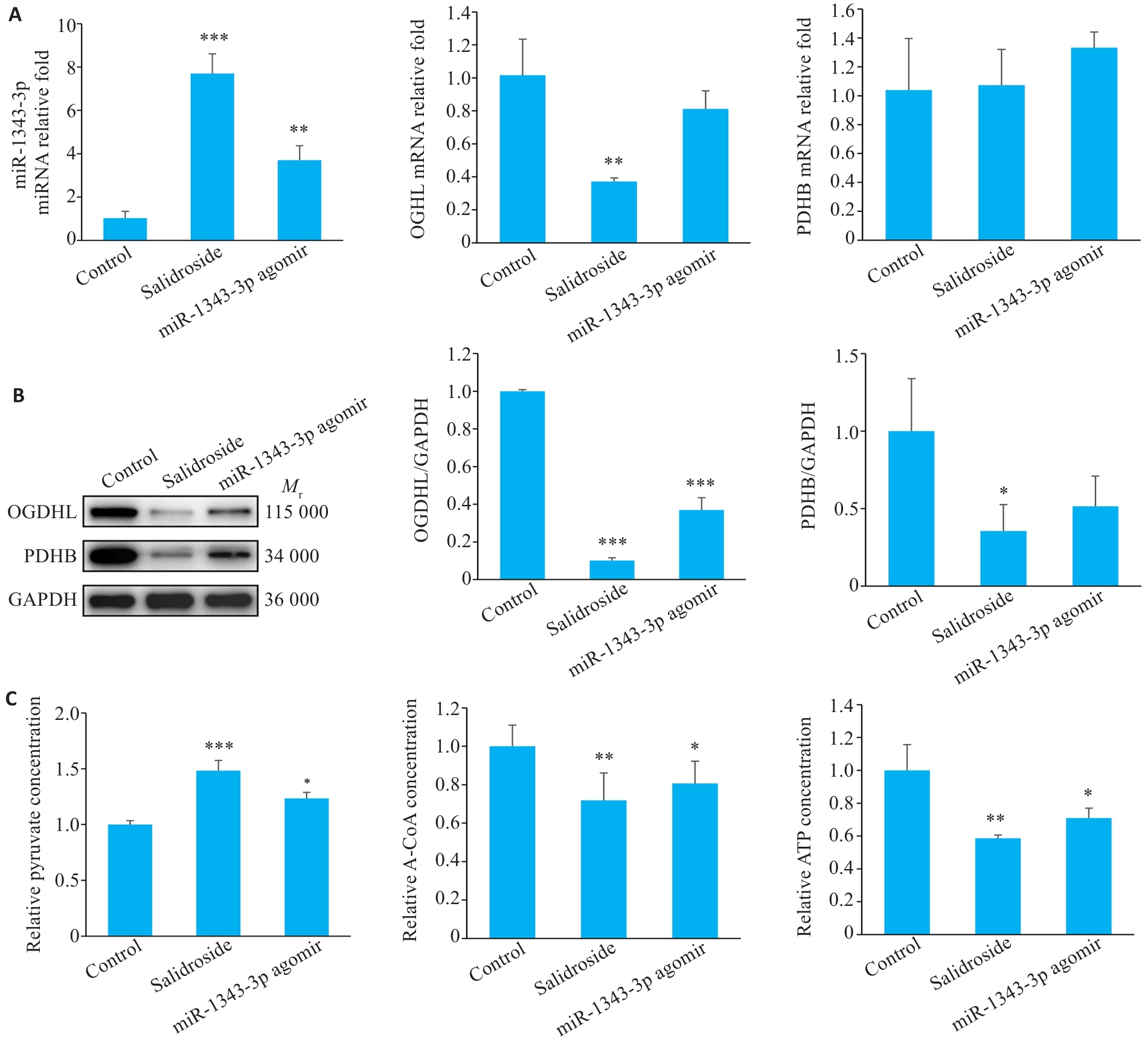
Fig.12 Salidroside and miR-1343-3p inhibit gastric cancer tumor growth in nude mice. A-C: Changes in miR-1343-3p levels, OGDHL and PDHB expression, and concentrations of pyruvate, acetyl-CoA, and ATP in tumor tissues analyzed by qRT-PCR, Western blotty, and biochemical kits. *P<0.05, **P<0.01, ***P<0.001 vs Control.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. doi:10.3322/caac.21660 |
| 2 | Han BF, Zheng RS, Zeng HM, et al. Cancer incidence and mortality in China, 2022[J]. J Natl Cancer Cent, 2024, 4(1): 47-53. doi:10.1016/j.jncc.2024.01.006 |
| 3 | He SY, Xia CF, Li H, et al. Cancer profiles in China and comparisons with the USA: a comprehensive analysis in the incidence, mortality, survival, staging, and attribution to risk factors[J]. Sci China Life Sci, 2024, 67(1): 122-31. doi:10.1007/s11427-023-2423-1 |
| 4 | Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications[J]. Semin Cancer Biol, 2022, 86(Pt 2): 851-9. doi:10.1016/j.semcancer.2022.02.002 |
| 5 | Jiang B, Feng LF, Yang T, et al. Combination of chloroquine diphosphate and salidroside induces human liver cell apoptosis via regulation of mitochondrial dysfunction and autophagy[J]. Mol Med Rep, 2023, 27(2): 37. doi:10.3892/mmr.2022.12924 |
| 6 | Rong L, Li ZD, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomed Pharmacother, 2020, 122: 109726. doi:10.1016/j.biopha.2019.109726 |
| 7 | Kang DY, Sp N, Kim DH, et al. Salidroside inhibits migration, invasion and angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2[J]. Int J Oncol, 2018, 53(2): 877-85. doi:10.3892/ijo.2018.4430 |
| 8 | Warburg O. On the origin of cancer cells[J]. Science, 1956, 123(3191): 309-14. doi:10.1126/science.123.3191.309 |
| 9 | Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding[J]. J Physiol, 2021, 599(6): 1745-57. doi:10.1113/JP278810 |
| 10 | Liu XL, Ma RX, Yi B, et al. microRNAs are involved in the development and progression of gastric cancer[J]. Acta Pharmacol Sin, 2021, 42(7): 1018-26. doi:10.1038/s41401-020-00540-0 |
| 11 | Shen XJ, Zhu XY, Hu PX, et al. Knockdown circZNF131 inhibits cell progression and glycolysis in gastric cancer through miR-186-5p/PFKFB2 axis[J]. Biochem Genet, 2022, 60(5): 1567-84. doi:10.1007/s10528-021-10165-5 |
| 12 | Wang XP, Zhang ZD, Cao XL. Salidroside inhibited the proliferation of gastric cancer cells through up-regulating tumor suppressor miR-1343-3p and down-regulating MAP3K6/MMP24 signal molecules[J]. Cancer Biol Ther, 2024, 25(1): 2322206. doi:10.1080/15384047.2024.2322206 |
| 13 | Sumegi B, Srere PA. Complex I binds several mitochondrial NAD-coupled dehydrogenases[J]. J Biol Chem, 1984, 259(24): 15040-5. doi:10.1016/s0021-9258(17)42511-7 |
| 14 | Liu G, Wu XY, Chen J. Identification and validation of a glycolysis-related gene signature for depicting clinical characteristics and its relationship with tumor immunity in patients with colon cancer[J]. Aging, 2022, 14(21): 8700-18. doi:10.18632/aging.204226 |
| 15 | Hoque MO, Kim MS, Ostrow KL, et al. Genome-wide promoter analysis uncovers portions of the cancer methylome[J]. Cancer Res, 2008, 68(8): 2661-70. doi:10.1158/0008-5472.CAN-07-5913 |
| 16 | Khalaj-Kondori M, Hosseinnejad M, Hosseinzadeh A, et al. Aberrant hypermethylation of OGDHL gene promoter in sporadic colorectal cancer[J]. Curr Probl Cancer, 2020, 44(1): 100471. doi:10.1016/j.currproblcancer.2019.03.001 |
| 17 | Liu YT, Leng P, Liu Y, et al. Crosstalk between methylation and ncRNAs in breast cancer: therapeutic and diagnostic implications[J]. Int J Mol Sci, 2022, 23(24): 15759. doi:10.3390/ijms232415759 |
| 18 | Patel MS, Nemeria NS, Furey W, et al. The pyruvate dehydrogenase complexes: structure-based function and regulation[J]. J Biol Chem, 2014, 289(24): 16615-23. doi:10.1074/jbc.R114.563148 |
| 19 | Perham RN. Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein[J]. Biochemistry, 1991, 30(35): 8501-12. doi:10.1021/bi00099a001 |
| 20 | Yonashiro R, Eguchi K, Wake M, et al. Pyruvate dehydrogenase PDH-E1β controls tumor progression by altering the metabolic status of cancer cells[J]. Cancer Res, 2018, 78(7): 1592-603. doi:10.1158/0008-5472.can-17-1751 |
| 21 | Wang YH, Patti GJ. The Warburg effect: a signature of mitochondrial overload[J]. Trends Cell Biol, 2023, 33(12): 1014-20. doi:10.1016/j.tcb.2023.03.013 |
| 22 | Yang SX, Wang LS, Zeng Y, et al. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice[J]. Phytomedicine, 2023, 114: 154762. doi:10.1016/j.phymed.2023.154762 |
| 23 | Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets[J]. Cells, 2020, 9(10): 2308. doi:10.3390/cells9102308 |
| 24 | Zhao JY, Feng KR, Wang F, et al. A retrospective overview of PHGDH and its inhibitors for regulating cancer metabolism[J]. Eur J Med Chem, 2021, 217: 113379. doi:10.1016/j.ejmech.2021.113379 |
| 25 | Min HY, Lee HY. Oncogene-driven metabolic alterations in cancer[J]. Biomol Ther, 2018, 26(1): 45-56. doi:10.4062/biomolther.2017.211 |
| 26 | Hu J, Huang LJ, Ding Q, et al. Long noncoding RNA HAGLR sponges miR-338-3p to promote 5-Fu resistance in gastric cancer through targeting the LDHA-glycolysis pathway[J]. Cell Biol Int, 2022, 46(2): 173-84. doi:10.1002/cbin.11714 |
| 27 | Liu HP, Chen K, Wang L, et al. miR-613 inhibits Warburg effect in gastric cancer by targeting PFKFB2[J]. Biochem Biophys Res Commun, 2019, 515(1): 37-43. doi:10.1016/j.bbrc.2019.05.001 |
| 28 | Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma[J]. Cell Cycle, 2009, 8(23): 3984-4001. doi:10.4161/cc.8.23.10238 |
| 29 | Mookerjee SA, Gerencser AA, Nicholls DG, et al. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements[J]. J Biol Chem, 2017, 292(17): 7189-207. doi:10.1074/jbc.M116.774471 |
| 30 | Annison EF, Lindsay DB, White RR. Metabolic interrelations of glucose and lactate in sheep[J]. Biochem J, 1963, 88(2): 243-8. doi:10.1042/bj0880243 |
| 31 | Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate[J]. Nature, 2017, 551(7678): 115-8. doi:10.1038/nature24057 |
| 32 | Sharif T, Martell E, Dai C, et al. Phosphoglycerate dehydrogenase inhibition induces p-mTOR-independent autophagy and promotes multilineage differentiation in embryonal carcinoma stem-like cells[J]. Cell Death Dis, 2018, 9(10): 990. doi:10.1038/s41419-018-0997-8 |
| 33 | Shen LL, Zhang JF, Zheng ZT, et al. PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder cancer[J]. Int J Biol Sci, 2022, 18(14): 5459-74. doi:10.7150/ijbs.74546 |
| 34 | Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment[J]. Nat Med, 2014, 20(11): 1242-53. doi:10.1038/nm.3739 |
| 35 | Cockram PE, Kist M, Prakash S, et al. Ubiquitination in the regulation of inflammatory cell death and cancer[J]. Cell Death Differ, 2021, 28(2): 591-605. doi:10.1038/s41418-020-00708-5 |
| [1] | Xinyuan CHEN, Chengting WU, Ruidi LI, Xueqin PAN, Yaodan ZHANG, Junyu TAO, Caizhi LIN. Shuangshu Decoction inhibits growth of gastric cancer cell xenografts by promoting cell ferroptosis via the P53/SLC7A11/GPX4 axis [J]. Journal of Southern Medical University, 2025, 45(7): 1363-1371. |
| [2] | Xuan WU, Jiamin FANG, Weiwei HAN, Lin CHEN, Jing SUN, Qili JIN. High PRELID1 expression promotes epithelial-mesenchymal transition in gastric cancer cells and is associated with poor prognosis [J]. Journal of Southern Medical University, 2025, 45(7): 1535-1542. |
| [3] | Yi ZHANG, Yu SHEN, Zhiqiang WAN, Song TAO, Yakui LIU, Shuanhu WANG. High expression of CDKN3 promotes migration and invasion of gastric cancer cells by regulating the p53/NF-κB signaling pathway and inhibiting cell apoptosis [J]. Journal of Southern Medical University, 2025, 45(4): 853-861. |
| [4] | Qingqing HUANG, Wenjing ZHANG, Xiaofeng ZHANG, Lian WANG, Xue SONG, Zhijun GENG, Lugen ZUO, Yueyue WANG, Jing LI, Jianguo HU. High MYO1B expression promotes proliferation, migration and invasion of gastric cancer cells and is associated with poor patient prognosis [J]. Journal of Southern Medical University, 2025, 45(3): 622-631. |
| [5] | Xue SONG, Yue CHEN, Min ZHANG, Nuo ZHANG, Lugen ZUO, Jing LI, Zhijun GENG, Xiaofeng ZHANG, Yueyue WANG, Lian WANG, Jianguo HU. GPSM2 is highly expressed in gastric cancer to affect patient prognosis by promoting tumor cell proliferation [J]. Journal of Southern Medical University, 2025, 45(2): 229-238. |
| [6] | Xiaohua CHEN, Hui LU, Ziliang WANG, Lian WANG, Yongsheng XIA, Zhijun GENG, Xiaofeng ZHANG, Xue SONG, Yueyue WANG, Jing LI, Jianguo HU, Lugen ZUO. Role of Abelson interactor 2 in progression and prognosis of gastric cancer and its regulatory mechanisms [J]. Journal of Southern Medical University, 2024, 44(9): 1653-1661. |
| [7] | Mengnan YE, Hongmei WU, Yan MEI, Qingling ZHANG. High expression of CREM is associated with poor prognosis in gastric cancer patients [J]. Journal of Southern Medical University, 2024, 44(9): 1776-1782. |
| [8] | Zhijun GENG, Jingjing YANG, Minzhu NIU, Xinyue LIU, Jinran SHI, Yike LIU, Xinyu YAO, Yulu ZHANG, Xiaofeng ZHANG, Jianguo HU. Kuwanon G inhibits growth, migration and invasion of gastric cancer cells by regulating the PI3K/AKT/mTOR pathway [J]. Journal of Southern Medical University, 2024, 44(8): 1476-1484. |
| [9] | Yidan PANG, Ya LIU, Siai CHEN, Jinglei ZHANG, Jin ZENG, Yuanming PAN, Juan AN. Biological role of SPAG5 in the malignant proliferation of gastric cancer cells [J]. Journal of Southern Medical University, 2024, 44(8): 1497-1507. |
| [10] | Yongsheng XIA, Lian WANG, Xiaohua CHEN, Yulu ZHANG, Aofei SUN, Deli CHEN. TSR2 overexpression inhibits proliferation and invasion of gastric cancer cells by downregulating the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 913-919. |
| [11] | LIANG Yihao, LAI Yingjun, YUAN Yanwen, YUAN Wei, ZHANG Xibo, ZHANG Bashan, LU Zhifeng. Screening of differentially expressed genes in gastric cancer based on GEO database and function and pathway enrichment analysis [J]. Journal of Southern Medical University, 2024, 44(3): 605-616. |
| [12] | SHEN Mengdi, ZHAO Na, DENG Xiaojing, DENG Min. High expression of COX6B2 in gastric cancer is associated with poor long-term prognosis and promotes cell proliferation and cell cycle progression by inhibiting p53 signaling [J]. Journal of Southern Medical University, 2024, 44(2): 289-297. |
| [13] | ZHANG Nuo, ZHANG Zhen, ZHANG Yulu, SONG Xue, ZHANG Xiaofeng, LI Jing, ZUO Lugen, HU Jianguo. PCID2 is highly expressed in gastric cancer and affects the prognosis by regulating cancer cell cycle and proliferation [J]. Journal of Southern Medical University, 2024, 44(2): 324-332. |
| [14] | ZHANG Wenjing, ZHANG Nuo, YANG Zi, ZHANG Xiaofeng, SUN Aofei, WANG Lian, SONG Xue, GENG Zhijun, LI Jing, HU Jianguo. Overexpression of BZW1 promotes invasion and metastasis of gastric cancer cells by regulating Wnt/β-catenin signaling and promoting epithelial-mesenchymal transition [J]. Journal of Southern Medical University, 2024, 44(2): 354-362. |
| [15] | Fuxing ZHANG, Guoqing LIU, Rui DONG, Lei GAO, Weichen LU, Lianxia GAO, Zhongkuo ZHAO, Fei LU, Mulin LIU. High expression of CRTAC1 promotes proliferation, migration and immune cell infiltration of gastric cancer by regulating the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(12): 2421-2433. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||