Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (6): 1131-1142.doi: 10.12122/j.issn.1673-4254.2025.06.03
Previous Articles Next Articles
Bing XIA1( ), Jin PENG1,2, Jiuyang DING1, Jie WANG1, Guowei TANG3, Guojie LIU4, Yun WANG4, Changwu WAN1(
), Jin PENG1,2, Jiuyang DING1, Jie WANG1, Guowei TANG3, Guojie LIU4, Yun WANG4, Changwu WAN1( ), Cuiyun LE1(
), Cuiyun LE1( )
)
Received:2025-01-05
Online:2025-06-20
Published:2025-06-27
Contact:
Changwu WAN, Cuiyun LE
E-mail:372732293@qq.com;wcw005@sina.com;675493755@qq.com
Supported by:Bing XIA, Jin PENG, Jiuyang DING, Jie WANG, Guowei TANG, Guojie LIU, Yun WANG, Changwu WAN, Cuiyun LE. ATF3 regulates inflammatory response in atherosclerotic plaques in mice through the NF-κB signaling pathway[J]. Journal of Southern Medical University, 2025, 45(6): 1131-1142.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.06.03
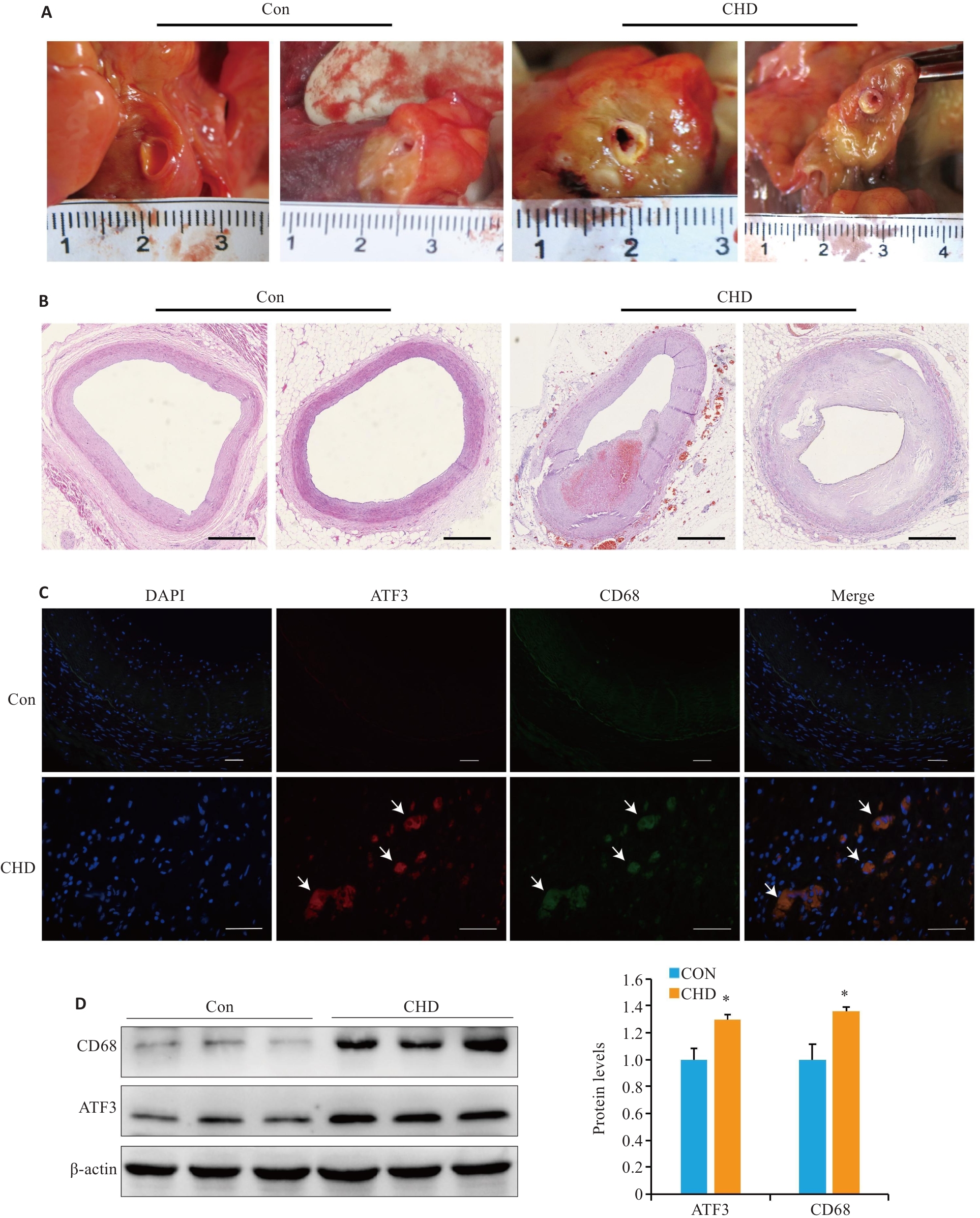
Fig.1 Examinations of human coronary arteries. A: Human coronary artery tissue specimens. B: Microscopic observation of coronary atherosclerosis (AS; HE staining, scale bar=1 mm). C: Partial co-localization of ATF3 (red) and CD68 (green) in the plaques (arrow,scale bar=50 μm). D: Western blotting for detecting ATF3 and CD68 expressions in the plaques. All experiments were repeated at least 3 times, and data are presented as Mean±SD (n=20). *P<0.05 vs Con group.
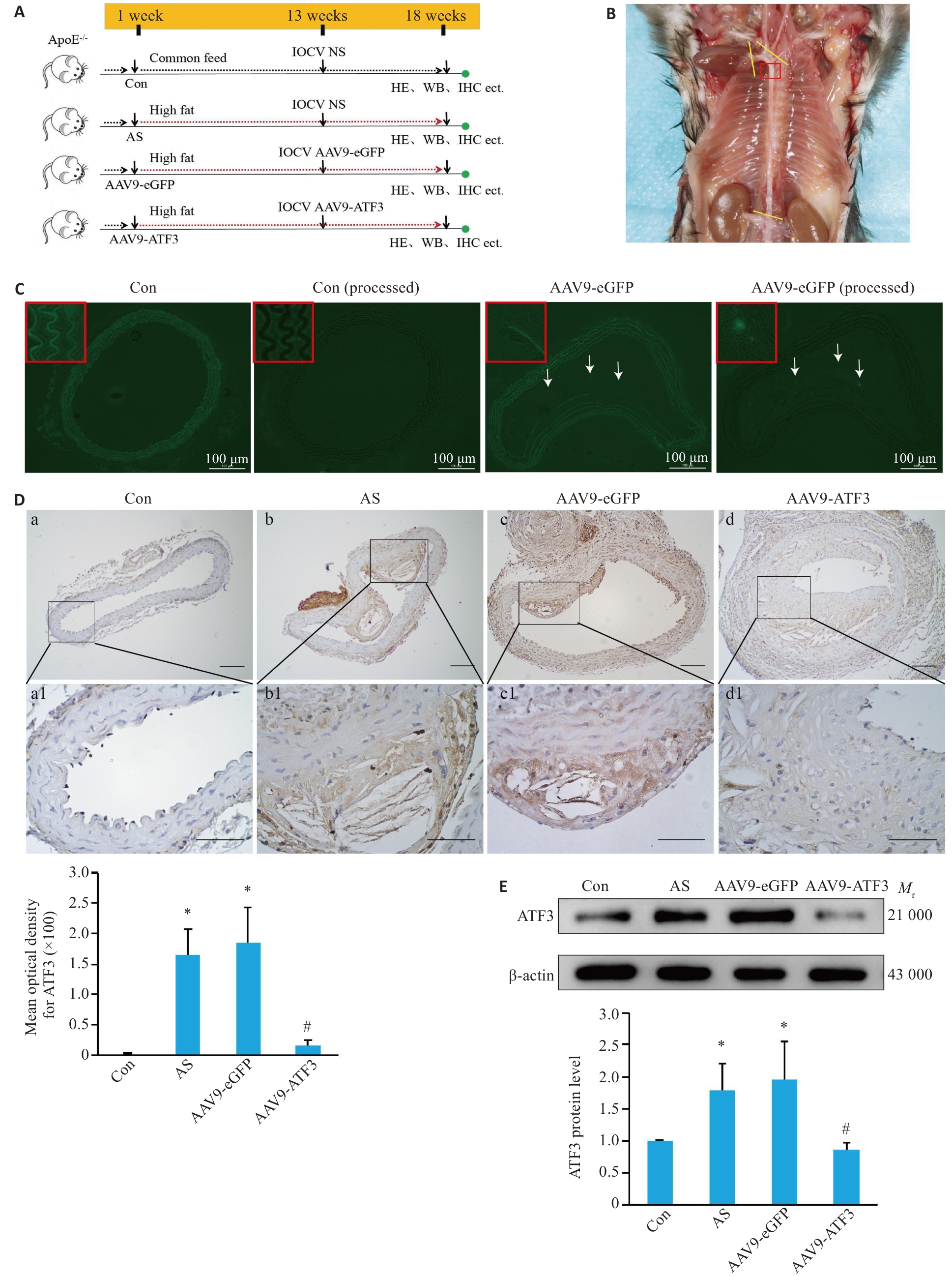
Fig.2 Verification of ATF3 gene knockout in ApoE-/- mice. A: Schematic diagram of mouse grouping and treatment. B: Observation of a mouse aorta. The yellow lines indicate the sampling site, and the red box indicates the tissues used for preparing frozen sections or paraffin sections. C: Expression of eGFP in the aortic vessels. After masking autofluorescence in the mouse aortas with pontamine sky blue, no fluorescence signals were detected in the vessels in the control group, but eGFP expression (arrows) was observed in the aortic plaques in AAV9-eGFP group. D: Immunohistochemical staining for detecting distribution of ATF3 expression in the aorta (a1-d1 are enlarged images of the boxed areas in a-d. Scale bar=50 μm, n=6). E: Western blotting for detecting aortic ATF3 protein content (n=6). Data are presented as Mean±SD. *P<0.05 vs Con group; #P<0.05 vs AS or AAV9-eGFP group.
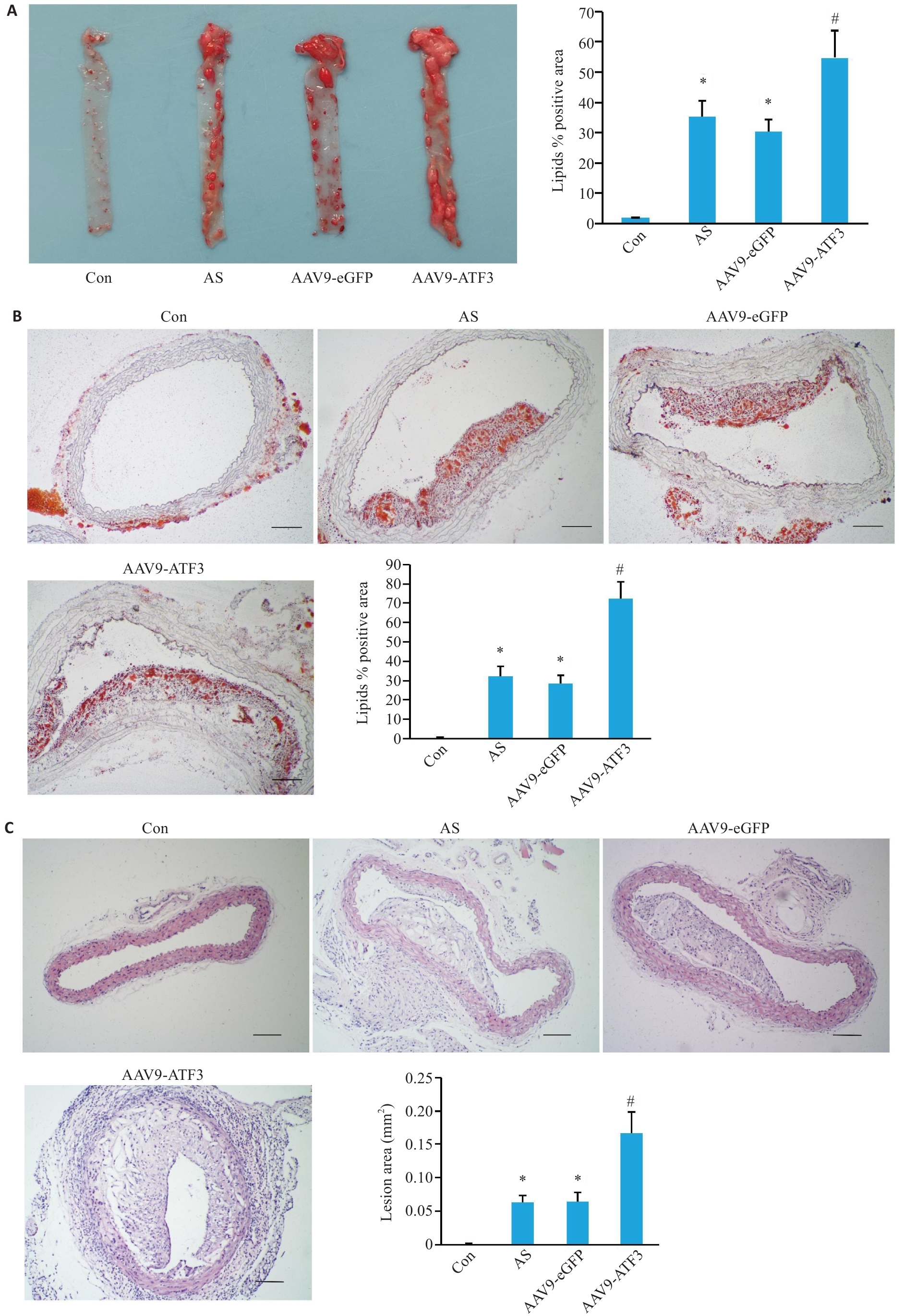
Fig.3 Changes in mouse aortic plaques after ATF3 gene knockout. A: Gross oil red O staining of the aorta showing lipid deposition in the total vascular area (n=6). B: Oil red O staining of cross-sections within the plaque area showing lipid deposition in the aortic lumen (n=3). C: HE staining of the aortic plaque area (n=6). Scale bar=50 μm. Data are presented as Mean±SD. *P<0.05 vs Con group, #P<0.05 vs AS or AAV9-eGFP group.
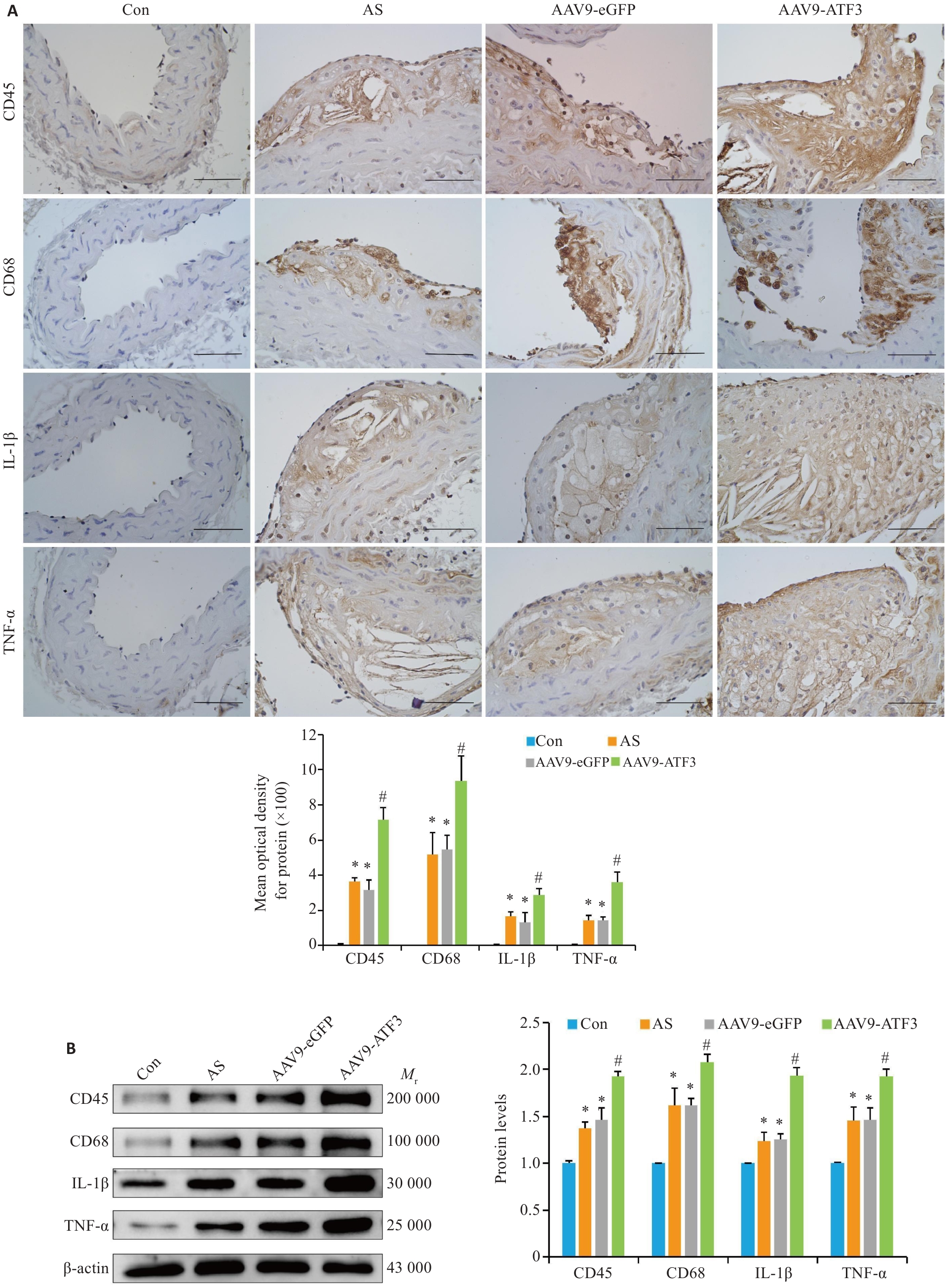
Fig.4 ATF3 gene knockout increases expressions of inflammatory factors in aortic plaques of the mice. A: Immunohistochemical staining for detecting aortic CD45, CD68, IL-1β and TNF-α protein expressions and MOD value statistics (scale bar=50 μm, n=6). B: Western blotting of aortic CD45, CD68, IL-1β and TNF-α protein expressions (n=6). Data are presented as Mean±SD. *P<0.05 vs Con group, #P<0.05 vs Con, AS or AAV9-eGFP group.
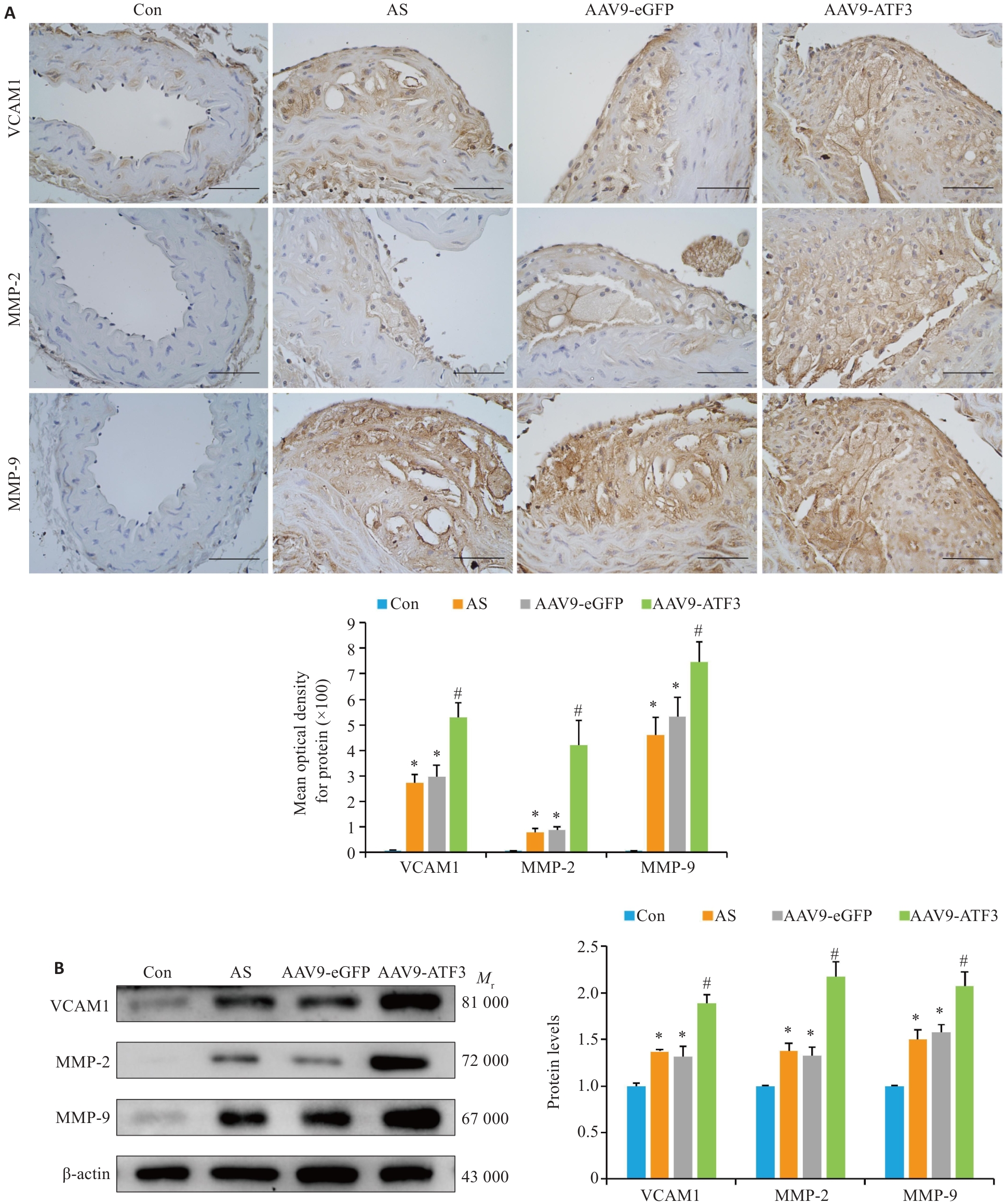
Fig. 5 ATF3 knockout increases expressions of VCAM1, MMP-2 and MMP-9 in aortic plaques of the mice. A: Immunohistochemical staining for detecting aortic VCAM1, MMP-2 and MMP-9 protein expressions and MOD value statistics (scale bar=50 μm, n=6). B: Western blotting for detecting aortic VCAM1, MMP-2 and MMP-9 protein expressions (n=6). Data are presented as Mean±SD. *P<0.05 vs Con group, #P<0.05 vs AS or AAV9-eGFP group.
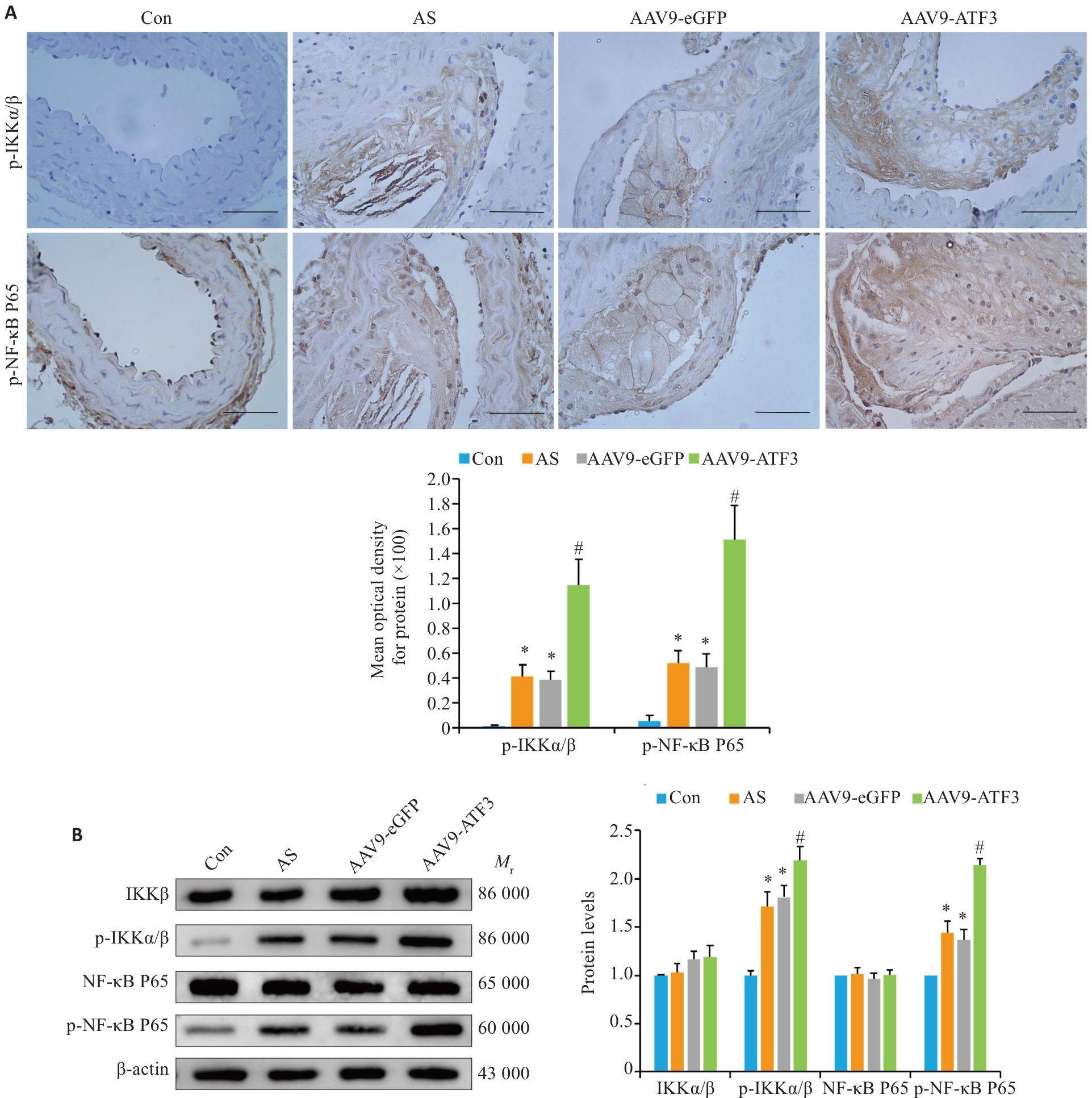
Fig.6 ATF3 knockout activates the NF-κB signaling pathway in aortic plaques of the mice. A: Immunohistochemical staining for detecting aortic p-IKKα/β and p-NF-κB P65 protein expressions and MOD value statistics (scale bar=50 μm, n=6). B: Western blotting for detecting aortic IKKα/β, p-IKKα/β, NF-κB P65 and p-NF-κB P65 expressions (n=6). Data are presented as Mean±SD. *P<0.05 vs Con group, #P<0.05 vs AS or AAV9-eGFP group.

Fig.7 Cell experiment results. A: THP-1 cells, macrophages and foam cells stained with oil red O to identify lipid deposition (scale bar=50 μm). B: Western blotting for detecting ATF3 silencing efficiency and IKKα/β, p-IKKα/β, NF-κB P65, and p-NF-κB P65 protein expressions. C: Western blotting for detecting ATF3 overexpression efficiency and IKKα/β, p-IKKα/β, NF-κB P65, and p-NF-κB P65 protein expressions. Data are presented as Mean±SD. *P<0.05 vs PMA group, #P<0.05 vs PMA+Ox-LDL, PMA+Ox-LDL+Lip3000 or PMA+Ox-LDL+OE-vector group.
| 1 | Grandhi GR, Mszar R, Vahidy F, et al. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States[J]. JAMA Cardiol, 2021, 6(1): 87-91. |
| 2 | Döring Y, van der Vorst EPC, Weber C. Targeting immune cell recruitment in atherosclerosis[J]. Nat Rev Cardiol, 2024, 21(11): 824-40. doi:10.1038/s41569-024-01023-z |
| 3 | Akazawa H. Mechanisms of cardiovascular homeostasis and pathophysiology: from gene expression, signal transduction to cellular communication[J]. Circ J, 2015, 79(12): 2529-36. doi:10.1253/circj.cj-15-0818 |
| 4 | Ghigo A, Laffargue M, Li MC, et al. PI3K and calcium signaling in cardiovascular disease[J]. Circ Res, 2017, 121(3): 282-92. doi:10.1161/circresaha.117.310183 |
| 5 | Wang XW, Yan KY, Wen CF, et al. Simvastatin combined with resistance training improves outcomes in patients with chronic heart failure by modulating mitochondrial membrane potential and the Janus kinase/signal transducer and activator of transcription 3 signaling pathways[J]. Cardiovasc Ther, 2022, 2022: 8430733. doi:10.1155/2022/8430733 |
| 6 | Bauer AJ, Martin KA. Coordinating regulation of gene expression in cardiovascular disease: interactions between chromatin modifiers and transcription factors[J]. Front Cardiovasc Med, 2017, 4: 19. doi:10.3389/fcvm.2017.00019 |
| 7 | Hai T, Wolfgang CD, Marsee DK, et al. ATF3 and stress responses[J]. Gene Expr, 1999, 7(4-6): 321–35. |
| 8 | Thompson MR, Xu DK, Williams BRG. ATF3 transcription factor and its emerging roles in immunity and cancer[J]. J Mol Med (Berl), 2009, 87(11): 1053-60. doi:10.1007/s00109-009-0520-x |
| 9 | Zhou H, Li N, Yuan Y, et al. Activating transcription factor 3 in cardiovascular diseases: a potential therapeutic target[J]. Basic Res Cardiol, 2018, 113(5): 37. doi:10.1007/s00395-018-0698-6 |
| 10 | Peng J, Le CY, Xia B, et al. Research on the correlation between activating transcription factor 3 expression in the human coronary artery and atherosclerotic plaque stability[J]. BMC Cardiovasc Disord, 2021, 21(1): 356. doi:10.21203/rs.3.rs-155718/v1 |
| 11 | Kwon JW, Kwon HK, Shin HJ, et al. Activating transcription factor 3 represses inflammatory responses by binding to the p65 subunit of NF-κB[J]. Sci Rep, 2015, 5: 14470. doi:10.1038/srep14470 |
| 12 | Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis[J]. Front Immunol, 2019, 10: 85. doi:10.3389/fimmu.2019.00085 |
| 13 | Zhao YL, Shao CY, Zhou HF, et al. Salvianolic acid B inhibits atherosclerosis and TNF-α-induced inflammation by regulating NF-κB/NLRP3 signaling pathway[J]. Phytomedicine, 2023, 119: 155002. doi:10.1016/j.phymed.2023.155002 |
| 14 | Sun Y, Guan J, Hou YF, et al. Silencing of junctional adhesion molecule-like protein attenuates atherogenesis and enhances plaque stability in ApoE-/- mice[J]. Clin Sci (Lond), 2019, 133(11): 1215-28. doi:10.1042/cs20180561 |
| 15 | Chen QJ, Zhai H, Li XM, et al. Recombinant adeno-associated virus serotype 9 in a mouse model of atherosclerosis: Determination of the optimal expression time in vivo [J]. Mol Med Rep, 2017, 15(4): 2090-6. doi:10.3892/mmr.2017.6235 |
| 16 | Wen YC, Ahmad F, Mohri Z, et al. Cysteamine inhibits lysosomal oxidation of low density lipoprotein in human macrophages and reduces atherosclerosis in mice[J]. Atherosclerosis, 2019, 291: 9-18. doi:10.1016/j.atherosclerosis.2019.09.019 |
| 17 | Li HY, Sun K, Zhao RP, et al. Inflammatory biomarkers of coronary heart disease[J]. Front Biosci (Schol Ed), 2018, 10(1): 185-96. doi:10.2741/s508 |
| 18 | Ma CY, Xu ZY, Wang SP, et al. Change of inflammatory factors in patients with acute coronary syndrome[J]. Chin Med J (Engl), 2018, 131(12): 1444-9. doi:10.4103/0366-6999.233953 |
| 19 | Hou PB, Fang JK, Liu ZH, et al. Macrophage polarization and metabolism in atherosclerosis[J]. Cell Death Dis, 2023, 14(10): 691. doi:10.1038/s41419-023-06206-z |
| 20 | Radecke CE, Warrick AE, Singh GD, et al. Coronary artery endo-thelial cells and microparticles increase expression of VCAM-1 in myocardial infarction[J]. Thromb Haemost, 2015, 113(3): 605-16. doi:10.1160/th14-02-0151 |
| 21 | Woudstra L, Biesbroek PS, Emmens RW, et al. CD45 is a more sensitive marker than CD3 to diagnose lymphocytic myocarditis in the endomyocardium[J]. Hum Pathol, 2017, 62: 83-90. doi:10.1016/j.humpath.2016.11.006 |
| 22 | Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond[J]. J Am Coll Cardiol, 2017, 70(18): 2278-89. doi:10.1016/j.jacc.2017.09.028 |
| 23 | Williams JW, Huang LH, Randolph GJ. Cytokine circuits in cardiovascular disease[J]. Immunity, 2019, 50(4): 941-54. doi:10.1016/j.immuni.2019.03.007 |
| 24 | Xia F, Zeng QR, Chen J. Circulating brain-derived neurotrophic factor dysregulation and its linkage with lipid level, stenosis degree, and inflammatory cytokines in coronary heart disease[J]. J Clin Lab Anal, 2022, 36(7): e24546. doi:10.1002/jcla.24546 |
| 25 | Wågsäter D, Zhu CY, Björkegren J, et al. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr (-/-) Apob(100/100) mouse[J]. Int J Mol Med, 2011, 28(2): 247-53. doi:10.3892/ijmm.2011.693 |
| 26 | Volkov AM, Murashov IS, Polonskaya YV, et al. Changes of content of matrix metalloproteinases and their tissue expression in various types of atherosclerotic plaques[J]. Kardiologiia, 2018(10): 12-8. doi:10.18087/cardio.2018.10.10180 |
| 27 | Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component[J]? Gene Expr, 2010, 15(1): 1-11. doi:10.3727/105221610x12819686555015 |
| 28 | Udayakumar TS, Stoyanova R, Shareef MM, et al. Edelfosine promotes apoptosis in androgen-deprived prostate tumors by increasing ATF3 and inhibiting androgen receptor activity[J]. Mol Cancer Ther, 2016, 15(6): 1353-63. doi:10.1158/1535-7163.mct-15-0332 |
| 29 | Zhao J, Li XY, Guo MX, et al. The common stress responsive transcription factor ATF3 binds genomic sites enriched with p300 and H3K27ac for transcriptional regulation[J]. BMC Genomics, 2016, 17: 335. doi:10.1186/s12864-016-2664-8 |
| 30 | Jeong BC, Kim JH, Kim K, et al. ATF3 modulates calcium signaling in osteoclast differentiation and activity by associating with c-Fos and NFATc1 proteins[J]. Bone, 2017, 95: 33-40. doi:10.1016/j.bone.2016.11.005 |
| 31 | Qin WW, Yang HY, Liu GZ, et al. Activating transcription factor 3 is a potential target and a new biomarker for the prognosis of atherosclerosis[J]. Hum Cell, 2021, 34(1): 49-59. doi:10.1007/s13577-020-00432-9 |
| 32 | Gold ES, Ramsey SA, Sartain MJ, et al. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation[J]. J Exp Med, 2012, 209(4): 807-17. doi:10.1084/jem.20111202 |
| 33 | Clément M, Basatemur G, Masters L, et al. Necrotic cell sensor Clec4e promotes a proatherogenic macrophage phenotype through activation of the unfolded protein response[J]. Circulation, 2016, 134(14): 1039-51. doi:10.1161/circulationaha.116.022668 |
| 34 | Ren JC, Han YS, Ren TM, et al. AEBP1 promotes the occurrence and development of abdominal aortic aneurysm by modulating inflammation via the NF-κB pathway[J]. J Atheroscler Thromb, 2020, 27(3): 255-70. doi:10.5551/jat.49106 |
| 35 | Gilchrist M, Thorsson V, Li B, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4[J]. Nature, 2006, 441(7090): 173-8. doi:10.1038/nature04768 |
| 36 | Li HF, Cheng CF, Liao WJ, et al. ATF3-mediated epigenetic regulation protects against acute kidney injury[J]. J Am Soc Nephrol, 2010, 21(6): 1003-13. doi:10.1681/asn.2009070690 |
| 37 | Wang L, Deng S, Lu Y, et al. Increased inflammation and brain injury after transient focal cerebral ischemia in activating tran-scription factor 3 knockout mice[J]. Neuroscience, 2012, 220: 100-8. doi:10.1016/j.neuroscience.2012.06.010 |
| [1] | Na ZHONG, Huijie WANG, Wenying ZHAO, Zhengui SUN, Biao GENG. High RNF7 expression enhances PD-1 resistance of non-small cell lung cancer cells by promoting CXCL1 expression and myeloid-derived suppressor cell recruitment via activating NF-κB signaling [J]. Journal of Southern Medical University, 2024, 44(9): 1704-1711. |
| [2] | Hanjun ZUO, Zhaoda DUAN, Zhao WANG, Tao GUO, Jinsha SHI, Haolong SHI, Juanjuan LI. Gastrodin improves microglia-mediated inflammatory response after hypoxic-ischemic brain damage in neonatal rats via PI3K/AKT pathway [J]. Journal of Southern Medical University, 2024, 44(9): 1712-1719. |
| [3] | Na ZHAO, Mengdi SHEN, Rui ZHAO, Di AO, Zetan LUO, Yinliang ZHANG, Zhidong XU, Fangtian FAN, Hailun ZHENG. column:Sanguinarine alleviates ulcerative colitis in mice by regulating the Nrf2/NF-κB pathway [J]. Journal of Southern Medical University, 2024, 44(8): 1467-1475. |
| [4] | Shanyuan ZHANG, Qiaoyan CAI, Jianghan QI, Kaixin YIN, Chenchen HE, Zhuye GAO, Ling ZHANG, Jianfeng CHU. Pharmacodynamics of Qingxin Jieyu Granules for treatment of atherosclerosis and its regulatory mechanism for lipid metabolism [J]. Journal of Southern Medical University, 2024, 44(8): 1518-1528. |
| [5] | Lingjun LU, Xiaodi YANG, Huaping ZHANG, Yuan LIANG, Xiulan SHI, Xin ZHOU. Recombinant Schistosoma japonicum cystatin alleviates acute liver injury in mice by inhibiting endoplasmic reticulum stress, inflammation and hepatocyte apoptosis [J]. Journal of Southern Medical University, 2024, 44(6): 1126-1134. |
| [6] | HE Cheng, CHEN Wei, ZHANG Nianzhi, LUAN Jun, WANG Sanfeng, ZHANG You. Shenqi Chongcao Formula ameliorates inflammatory response in rats with pulmonary fibrosis by activating the ASS1/src/STAT3 signaling pathway [J]. Journal of Southern Medical University, 2024, 44(4): 644-651. |
| [7] | BAO Hansheng, WANG Sutong, LÜ Mujie, WANG Yongcheng, JIANG Ping, LI Xiao. Activation of α7 nAChR improves white fat homeostasis and promotes beige adipogenesis and thermogenesis in obese mice [J]. Journal of Southern Medical University, 2024, 44(3): 499-506. |
| [8] | Xiuqi SUN, Jing CAI, Anbang ZHANG, Bo PANG, Chunyan CHENG, Qiqi CHA, Fei QUAN, Tao YE. Electroacupuncture pretreatment alleviates post-stroke spasticity in rats by inhibiting NF‑κB/NLRP3 signaling pathway-mediated inflammation and neuronal apoptosis [J]. Journal of Southern Medical University, 2024, 44(11): 2102-2109. |
| [9] | Hong YAO, Kedi LIU, Chengzhao LIU, Weihong LI, Qi DAI, Shi ZHAO, Ziheng DING, Hefei WANG, Xiaojing GE, Peifeng WEI, Jialin DUAN, Miaomiao XI. Maggot alleviates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting immune stress and complement activation [J]. Journal of Southern Medical University, 2024, 44(11): 2121-2130. |
| [10] | Mengmeng CHENG, Xinguang LIU, Yanxin WEI, Xiaoxiang XING, Lan LIU, Nan XIN, Peng ZHAO. Tongsai Granules inhibit autophagy and macrophage-mediated inflammatory response to improve acute exacerbations of chronic obstructive pulmonary disease in rats [J]. Journal of Southern Medical University, 2024, 44(10): 1995-2003. |
| [11] | LI Shai, LI Li, MIN Simin, LIU Saisai, QIN Zhiwen, XIONG Zhishang, XU Jianguo, WANG Bowen, DING Dushan, ZHAO Shidi. Soybean isoflavones alleviate cerebral ischemia/reperfusion injury in rats by inhibiting ferroptosis and inflammatory cascade reaction [J]. Journal of Southern Medical University, 2023, 43(2): 323-330. |
| [12] | CAO Jing, LIU Haibo, AN Qi, HAN Feng. Metformin alleviates pathologic pain in mice with radiation dermatitis by inhibiting p38MAPK/NF-κB signaling pathway [J]. Journal of Southern Medical University, 2023, 43(10): 1815-1820. |
| [13] | LU Qi, BAO Yingchun, CHEN Xujiao, WANG Chunming. Effect of local unstable atherosclerotic plaque on plaque formation in the carotid artery and abdominal aorta of rabbits [J]. Journal of Southern Medical University, 2023, 43(1): 117-121. |
| [14] | LI Wei, SHI Yongkang, GUO Yuhua, TIAN Shengwang. Nur77 promotes invasion and migration of gastric cancer cells through the NF-κB/IL-6 pathway [J]. Journal of Southern Medical University, 2022, 42(9): 1410-1417. |
| [15] | WANG Shaoxin, CUI Lihong, LIU Xinyao, LUO Zhe, LI Hui, PU Jiang. WDSUB1 knockdown alleviates dextran sulfate sodium-induced colitis in mice by inhibiting nuclear factor-κB signaling pathway [J]. Journal of Southern Medical University, 2022, 42(8): 1119-1125. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||