Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (9): 1704-1711.doi: 10.12122/j.issn.1673-4254.2024.09.10
Previous Articles Next Articles
Na ZHONG1( ), Huijie WANG1, Wenying ZHAO1, Zhengui SUN2, Biao GENG2(
), Huijie WANG1, Wenying ZHAO1, Zhengui SUN2, Biao GENG2( )
)
Received:2024-03-25
Online:2024-09-20
Published:2024-09-30
Contact:
Biao GENG
E-mail:20229198@stu.wnmc.edu.cn;wnyxy1@163.com
Na ZHONG, Huijie WANG, Wenying ZHAO, Zhengui SUN, Biao GENG. High RNF7 expression enhances PD-1 resistance of non-small cell lung cancer cells by promoting CXCL1 expression and myeloid-derived suppressor cell recruitment via activating NF-κB signaling[J]. Journal of Southern Medical University, 2024, 44(9): 1704-1711.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.09.10
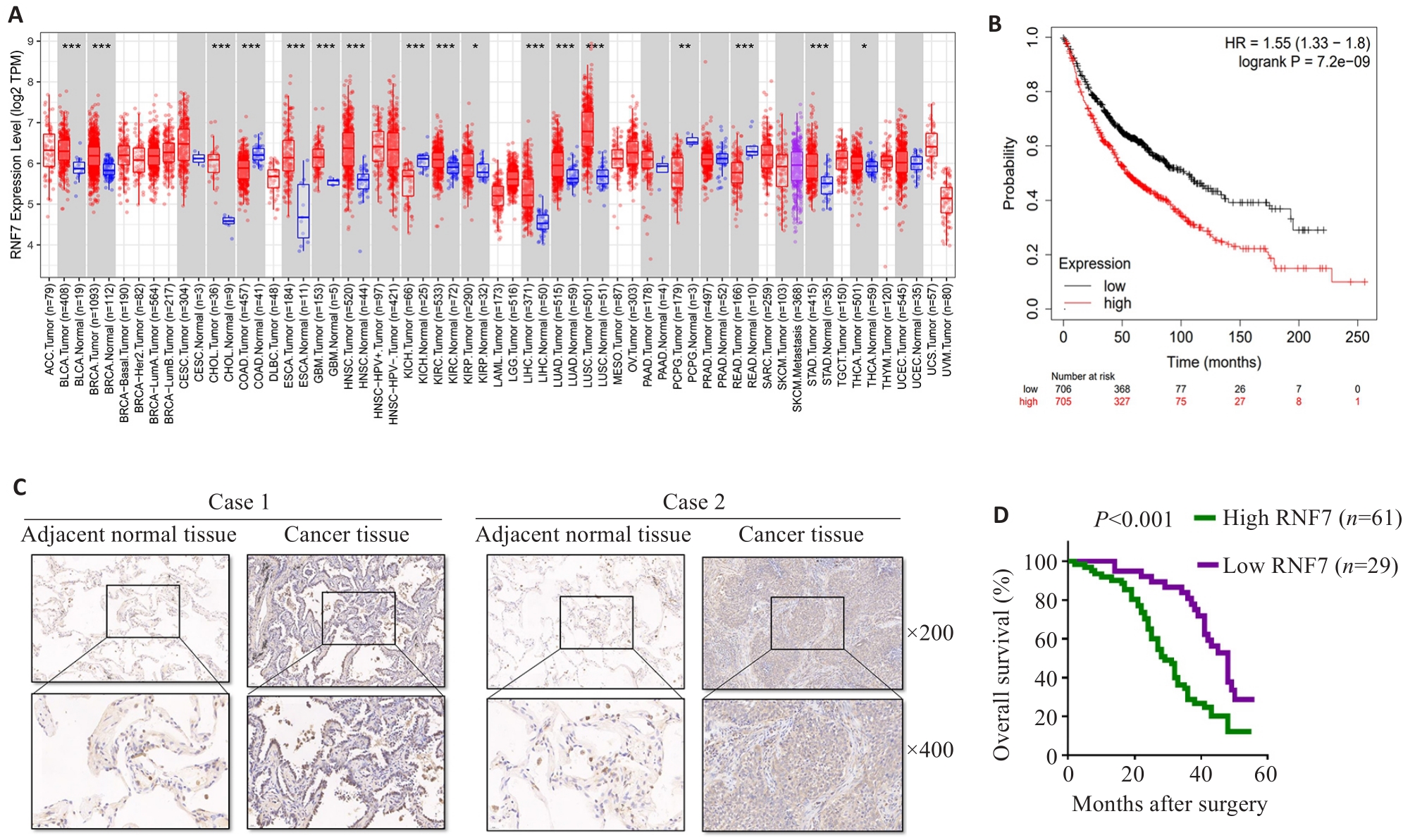
Fig. 1 RNF7 promotes lung adenocarcinoma progression. A: TIMER2.0 database analysis showing increased RNF7 mRNA expressions in multiple cancer tissues compared with the adjacent tissues. B: Survival analysis showing a close correlation between high RNF7 expression level and poor prognosis of lung cancer patients. C: RNF7 expression in lung adenocarcinoma tissues and adjacent tissues determined by immunohistochemistry. D: Kaplan-Meier survival analysis showing that patients with high RNF7 expression had shorter overall survival than those with low RNF7 expression (n=90). *P<0.05, **P<0.01, ***P<0.001.
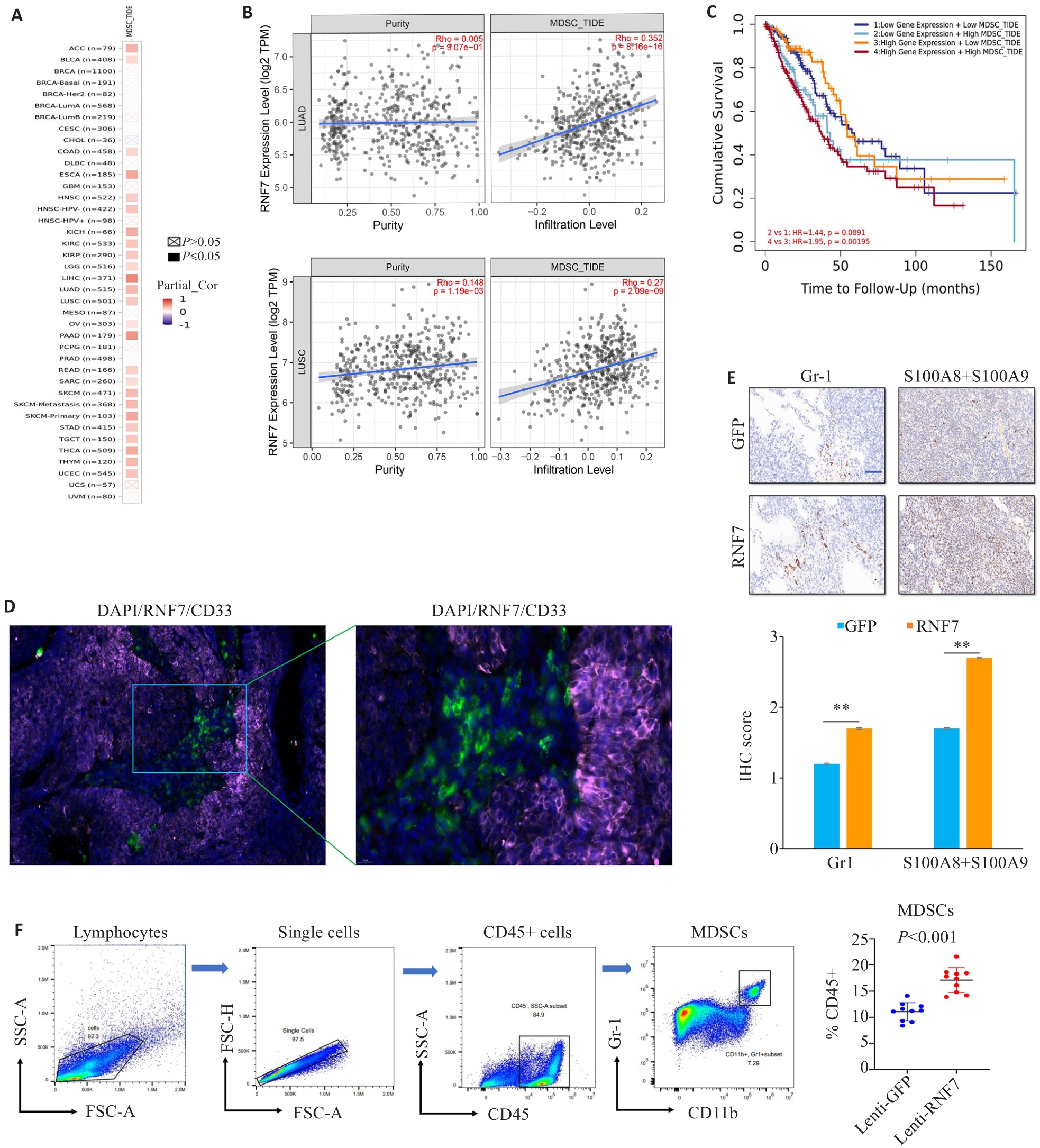
Fig.2 RNF7 expression is positively correlated with MDSC infiltration in NSCLC. A: Correlation of RNF7 expression with MDSCs infiltration in pan-cancers predicted by TIMER2.0 database. B: Correlation of RNF7 expression with MDSCs infiltration in lung adenocarcinoma and squamous lung cancer. C: Cox regression analyses using data from TCGA indicating that high MDSC infiltration was significantly associated with poorer prognosis of NSCLC patients. D: Immunofluorescence staining of RNF7 and CD33 in lung adenocarcinoma tissue (DAPI, blue; RNF7, violet; CD33, green). E: Immunohistochemical staining of immune cell markers Gr1 and S100A8+A9 in the tissues (scale bar=50 μm). F: Infiltration of MDSCs assessed by flow cytometry in the tumors. **P<0.01.
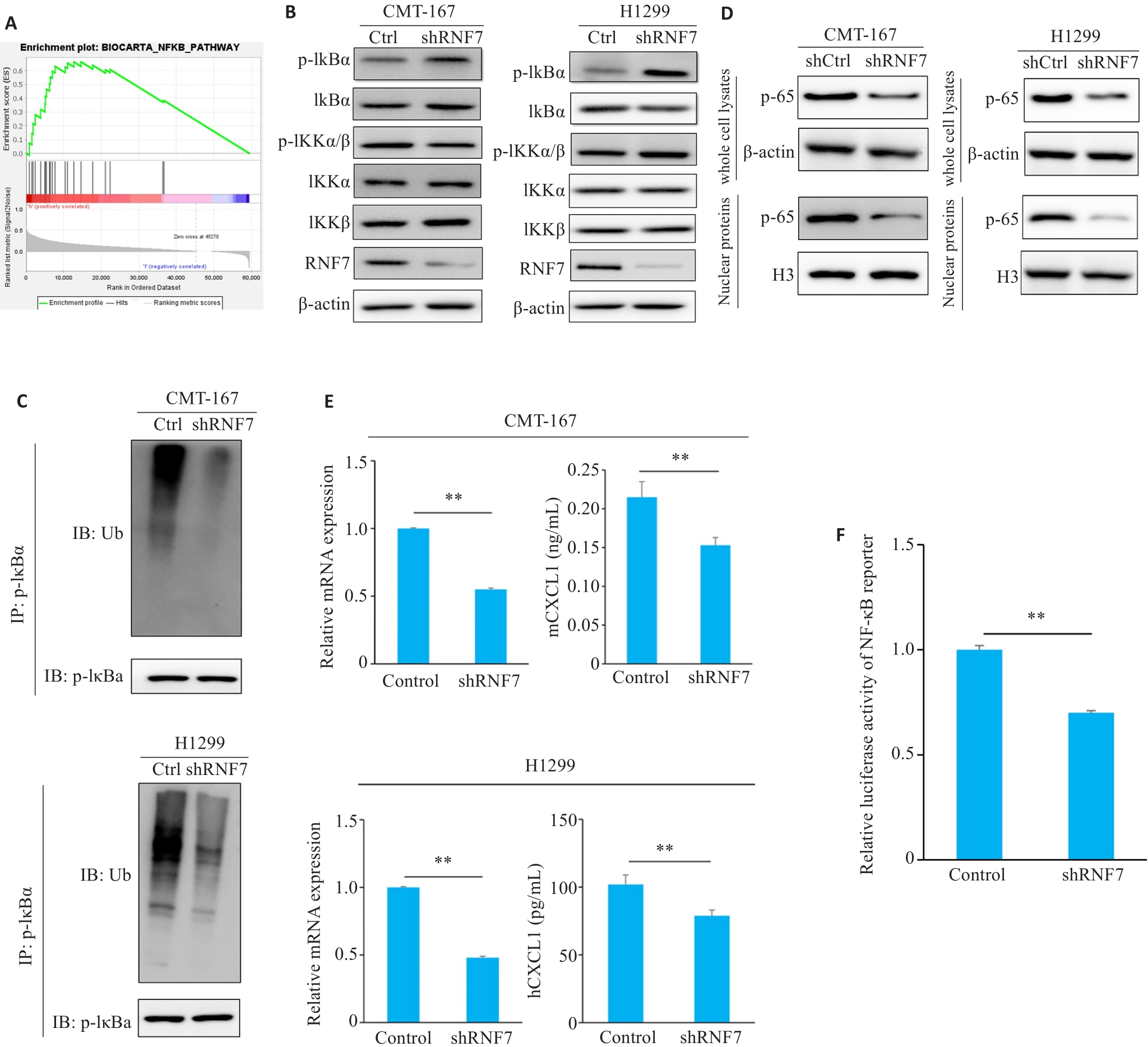
Fig.3 RNF7 induces MDSCs recruitment by promoting NF-κB signaling pathway activation. A: GSEA analysis showing that the high expression of RNF7 in NSCLC was significantly enriched in the NF-κB signaling pathway. B: Western blotting of the key effectors in the NF-κB signaling pathway in cells with RNF7 knockdown. C: Immunoprecipitation of p-IκBα and analysis of its ubiquitination. D: Detection of protein expressions of p-65 and H3 by Western blotting. E: ELISA validation of CXCL1 levels in cell culture supernatants and RT-qPCR of CXCL1 mRNA expression in CMT-167 or H1299 cells. F: Luciferase reporter-based NF-κB activity assay. **P<0.01.
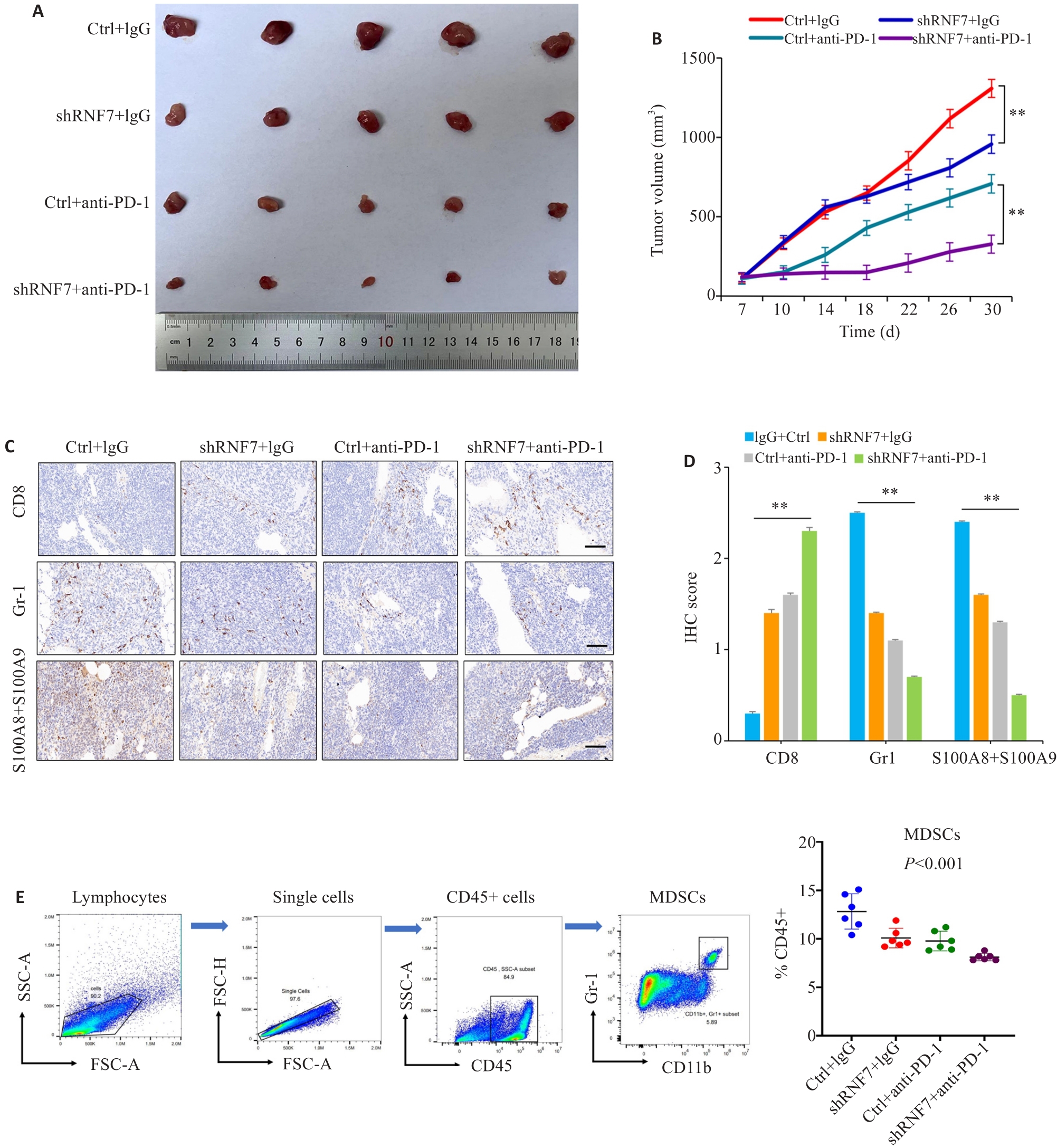
Fig. 4 RNF7 is a potential therapeutic target for immunotherapy of non-small cell lung cancer. A: Dissected tumors from each group of the mice. CMT167 Ctrl or RNF7 knockdown cells were implanted in C57BL/6 (n=5). Isotype control (IgG) or anti-PD-1 were given at 200 µg/mouse on days 7 after cell injection. B: Comparison of tumor volume among the groups. C, D: Immunohistochemical staining of immune cell markers (CD8, Gr1 and S100A8+A9) in different groups (scale bar=50 μm). E: Flow cytometry gating strategy of immune cells and the proportion of MDSCs. **P<0.01.
| 1 | Schabath MB, Cote ML. Cancer progress and priorities: lung cancer[J]. Cancer Epidemiol Biomarkers Prev, 2019, 28(10): 1563-79. |
| 2 | Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J, 2021, 134(7): 783-91. |
| 3 | Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer[J]. BMJ, 2021, 375: n2363. |
| 4 | Sun Y, Li H. Functional characterization of SAG/RBX2/ROC2/RNF7, an antioxidant protein and an E3 ubiquitin ligase[J]. Protein Cell, 2013, 4(2): 103-16. |
| 5 | Sampson C, Wang QP, Otkur W, et al. The roles of E3 ubiquitin ligases in cancer progression and targeted therapy[J]. Clin Transl Med, 2023, 13(3): e1204. |
| 6 | Huang TT, Li JW, Liu XL, et al. An integrative pan-cancer analysis revealing the difference in small ring finger family of SCF E3 ubiquitin ligases[J]. Front Immunol, 2022, 13: 968777. |
| 7 | Gu QY, Bowden GT, Normolle D, et al. SAG/ROC2 E3 ligase regulates skin carcinogenesis by stage-dependent targeting of c-Jun/AP1 and IkappaB-alpha/NF-kappaB[J]. J Cell Biol, 2007, 178(6): 1009-23. |
| 8 | Goenka A, Khan F, Verma B, et al. Tumor microenvironment signaling and therapeutics in cancer progression[J]. Cancer Commun, 2023, 43(5): 525-61. |
| 9 | Sui HS, Dongye SY, Liu XC, et al. Immunotherapy of targeting MDSCs in tumor microenvironment[J]. Front Immunol, 2022, 13: 990463. |
| 10 | Hegde S, Leader AM, Merad M. MDSC: markers, development, states, and unaddressed complexity[J]. Immunity, 2021, 54(5): 875-84. |
| 11 | Ma J, Xu HX, Wang SJ. Immunosuppressive role of myeloid-derived suppressor cells and therapeutic targeting in lung cancer[J]. J Immunol Res, 2018, 2018: 6319649. |
| 12 | Li MSC, Mok KKS, Mok TSK. Developments in targeted therapy & immunotherapy-how non-small cell lung cancer management will change in the next decade: a narrative review[J]. Ann Transl Med, 2023, 11(10): 358. |
| 13 | Tao YR, Dai LR, Liang WL, et al. Advancements and perspectives of RBX2 as a molecular hallmark in cancer[J]. Gene, 2024, 892: 147864. |
| 14 | Li K, Shi HH, Zhang BX, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer[J]. Signal Transduct Target Ther, 2021, 6(1): 362. |
| 15 | Li YN, Qiao KL, Zhang XY, et al. Erratum: targeting myeloid-derived suppressor cells to attenuate vasculogenic mimicry and synergistically enhance the anti-tumor effect of PD-1 inhibitor[J]. iScience, 2022, 25(10): 105281. |
| 16 | Papadaki MA, Aggouraki D, Vetsika EK, et al. Epithelial-to-mesenchymal transition heterogeneity of circulating tumor cells and their correlation with MDSCs and tregs in HER2-negative metastatic breast cancer patients[J]. Anticancer Res, 2021, 41(2): 661-70. |
| 17 | Adah D, Hussain M, Qin LM, et al. Implications of MDSCs-targeting in lung cancer chemo-immunotherapeutics[J]. Pharmacol Res, 2016, 110: 25-34. |
| 18 | Xiong XF, Mathewson ND, Li H, et al. SAG/RBX2 E3 ubiquitin ligase differentially regulates inflammatory responses of myeloid cell subsets[J]. Front Immunol, 2018, 9: 2882. |
| 19 | Su L, Zhang F, Liu MX, et al. The Tian-Men-Dong Decoction suppresses the tumour-infiltrating G-MDSCs via IL-1β-mediated signalling in lung cancer[J]. J Ethnopharmacol, 2023, 313: 116491. |
| 20 | Li H, Tan MJ, Jia LJ, et al. Inactivation of SAG/RBX2 E3 ubiquitin ligase suppresses KrasG12D-driven lung tumorigenesis[J]. J Clin Invest, 2014, 124(2): 835-46. |
| 21 | Inui T, Watanabe M, Nakamoto K, et al. Bronchial epithelial cells produce CXCL1 in response to LPS and TNFα: a potential role in the pathogenesis of COPD[J]. Exp Lung Res, 2018, 44(7): 323-31. |
| 22 | Hoesel B, Schmid JA. The complexity of NF‑κB signaling in inflammation and cancer[J]. Mol Cancer, 2013, 12: 86. |
| 23 | Zhang LL, Ludden CM, Cullen AJ, et al. Nuclear factor kappa B expression in non-small cell lung cancer[J]. Biomed Pharmacother, 2023, 167: 115459. |
| 24 | Tang HC, Li H, Sun ZJ. Targeting myeloid-derived suppressor cells for cancer therapy[J]. Cancer Biol Med, 2021, 18(4): 992-1009. |
| 25 | Kumar V, Patel S, Tcyganov E, et al. The nature of myeloid-derived suppressor cells in the tumor microenvironment[J]. Trends Immunol, 2016, 37(3): 208-20. |
| 26 | Highfill SL, Cui YZ, Giles AJ, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy[J]. Sci Transl Med, 2014, 6(237): 237ra67. |
| 27 | Barry ST, Gabrilovich DI, Sansom OJ, et al. Therapeutic targeting of tumour myeloid cells[J]. Nat Rev Cancer, 2023, 23(4): 216-37. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||