Journal of Southern Medical University ›› 2026, Vol. 46 ›› Issue (1): 150-158.doi: 10.12122/j.issn.1673-4254.2026.01.16
Jiayi XU( ), Di YANG(
), Di YANG( ), Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI(
), Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI( )
)
Received:2025-05-22
Online:2026-01-20
Published:2026-01-16
Contact:
Ning LI
E-mail:2624309950@qq.com;13792651232@163.com;lining@qdu.edu.cn
Supported by:Jiayi XU, Di YANG, Kailai ZANG, Mengen CHU, Qingyao ZHAO, Qing LI, Sen LU, Xiuli CHEN, Ning LI. EVA1A overexpression improves non-alcoholic fatty liver disease in mice by regulating lipid metabolism and promoting lipophagy[J]. Journal of Southern Medical University, 2026, 46(1): 150-158.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2026.01.16
| Gene | Forward primer (5'-3') Reverse primer (5'-3') |
|---|---|
Mus Eva1a Homo EVA1A | CCTTGGCCGCCTTGGTGATGAG TACCATCCTCGCTGTCGCTGCT AGATGGCTTTGCTCAGCAACA GATGCACACGCCAGAAACAA |
| Mus ACC1 | TCGGATCGGTTCCTTTGGGCCT TGTTCGCTGCCACGTAGATGCG |
| Mus CD36 | TGACGTGGCAAAGAACAGCAGCA AGACACAGTGTGGTCCTCGGGG |
| Mus DGAT2 | TCCCAGCAGCTGTGGCCTTACT GCACCACAGGTTGACATCCCGG |
Mus ATGL Mus β-actin Homo β-actin | TCCAAGGGGTGCGCTATGTGGA GTGGAGCTGTCCTGAGGGCAGA CCCGGGCTGTATTCCCCTCCAT CCTCTCTTGCTCTGGGCCTCGT AGGATTCCTATGTGGGCGAC ATAGCACAGCCTGGATAGCAA |
Tab.1 Primers sequences for RT-qPCR
| Gene | Forward primer (5'-3') Reverse primer (5'-3') |
|---|---|
Mus Eva1a Homo EVA1A | CCTTGGCCGCCTTGGTGATGAG TACCATCCTCGCTGTCGCTGCT AGATGGCTTTGCTCAGCAACA GATGCACACGCCAGAAACAA |
| Mus ACC1 | TCGGATCGGTTCCTTTGGGCCT TGTTCGCTGCCACGTAGATGCG |
| Mus CD36 | TGACGTGGCAAAGAACAGCAGCA AGACACAGTGTGGTCCTCGGGG |
| Mus DGAT2 | TCCCAGCAGCTGTGGCCTTACT GCACCACAGGTTGACATCCCGG |
Mus ATGL Mus β-actin Homo β-actin | TCCAAGGGGTGCGCTATGTGGA GTGGAGCTGTCCTGAGGGCAGA CCCGGGCTGTATTCCCCTCCAT CCTCTCTTGCTCTGGGCCTCGT AGGATTCCTATGTGGGCGAC ATAGCACAGCCTGGATAGCAA |
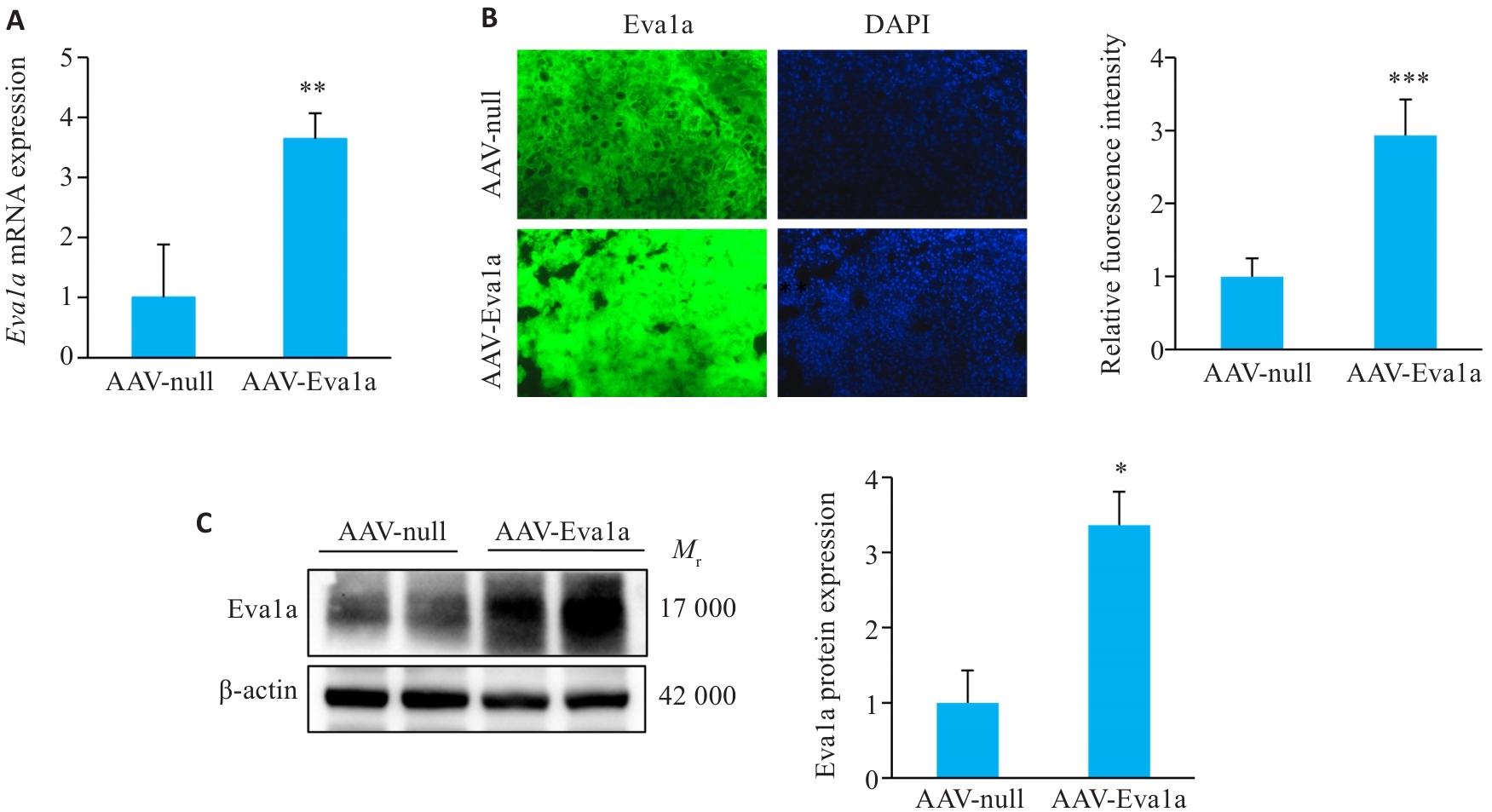
Fig.1 Overexpression of Eva1a in ob/ob mouse liver. A: Relative hepatic Eva1a mRNA levels. B: Immunofluorescence staining for detecting Eva1a expression in mouse liver tissues (Original magnification: ×200). C: Hepatic Eva1a protein expression levels detected with Western blotting. AAV-Eva1a: Mice overexpressing Eva1a; AAV-null: Control mice. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group.
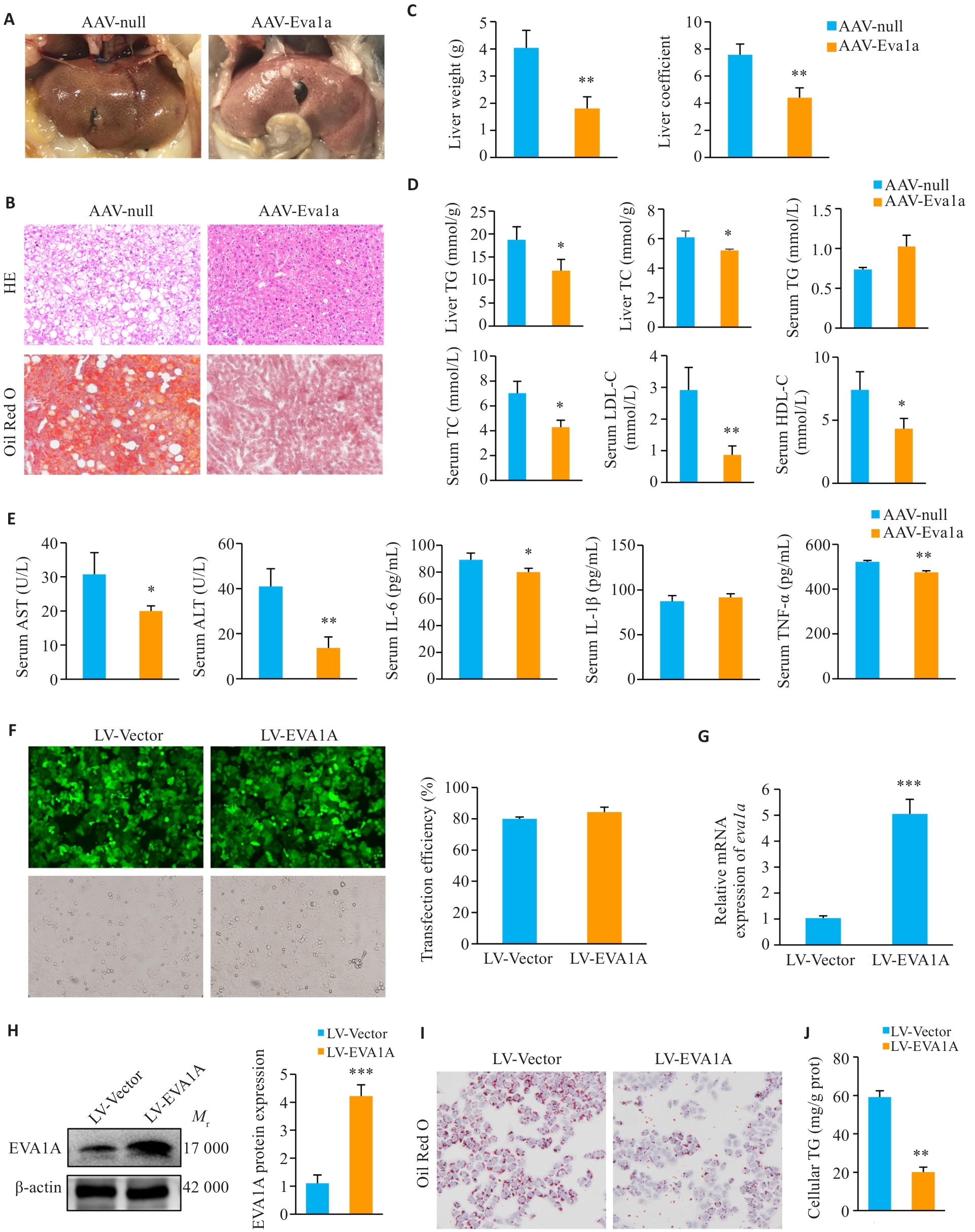
Fig.2 Effect of EVA1A overexpression on steatosis and inflammation in ob/ob mice and on lipid deposition in OA-induced HepG2 cells. A: Observation of mouse livers. B: Liver tissue HE staining and Oil Red O (ORO) staining (×200). C: Liver weight and liver coefficients of the mice. D: Triglyceride (TG) and total cholesterol (TC) levels in mouse livers, and TG, TC, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels in mouse serum. E: Serum levels of AST, ALT, IL-6, IL-1β, and TNF‑α of the mice. F: Infection efficiency of LV-EVA1A or LV-Vector in HepG2 cells determined by fluorescence microscopy (×200). G: RT-qPCR analysis of Eva1a mRNA levels in HepG2 cells infected with LV-EVA1A or LV-Vector. H: Western blotting of EVA1A protein levels in HepG2 cells infected with LV-EVA1A or LV-Vector. I: ORO staining for lipid droplets in the cells in LV-Vector and LV-EVA1A groups treated with 400 μmol/L OA for 12 h (×400). J: Quantitative analysis of cellular TG contents. AAV-Eva1a: Mice overexpressing Eva1a; AAV-null: Control mice. LV-EVA1A: HepG2 cells overexpressing EVA1A; LV-Vector: Control cells. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
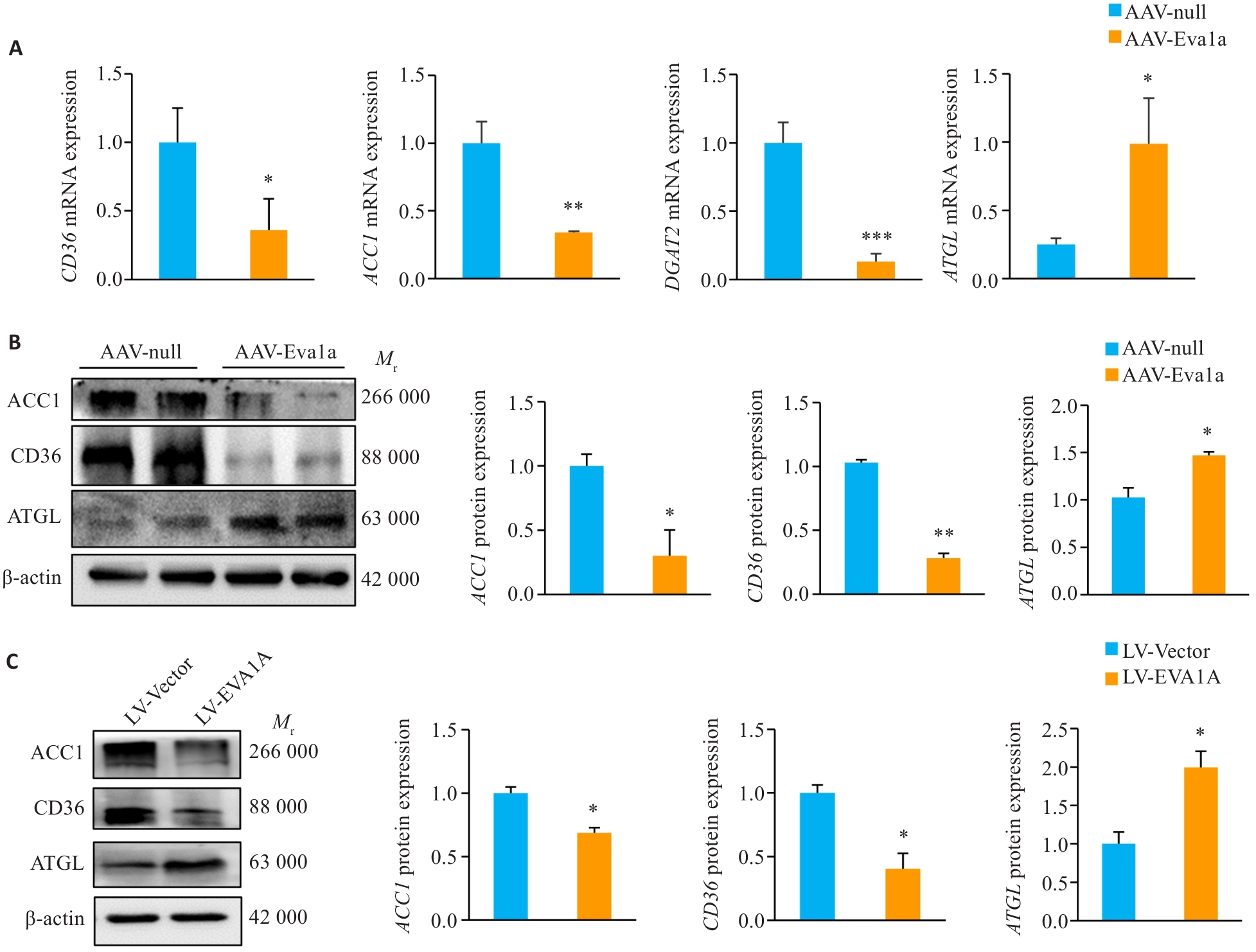
Fig.3 Effect of EVA1A overexpression on expression of lipid metabolism-related genes in the liver of ob/ob mice and in HepG2 cells induced by OA. A: Relative hepatic mRNA levels of CD36, ACC1, DGAT2 and ATGL. B: Protein expression levels of ACC1, CD36, and ATGL in the liver of ob/ob mice. C: Protein expression levels of ACC1, CD36, and ATGL in HepG2 cells treated with OA. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
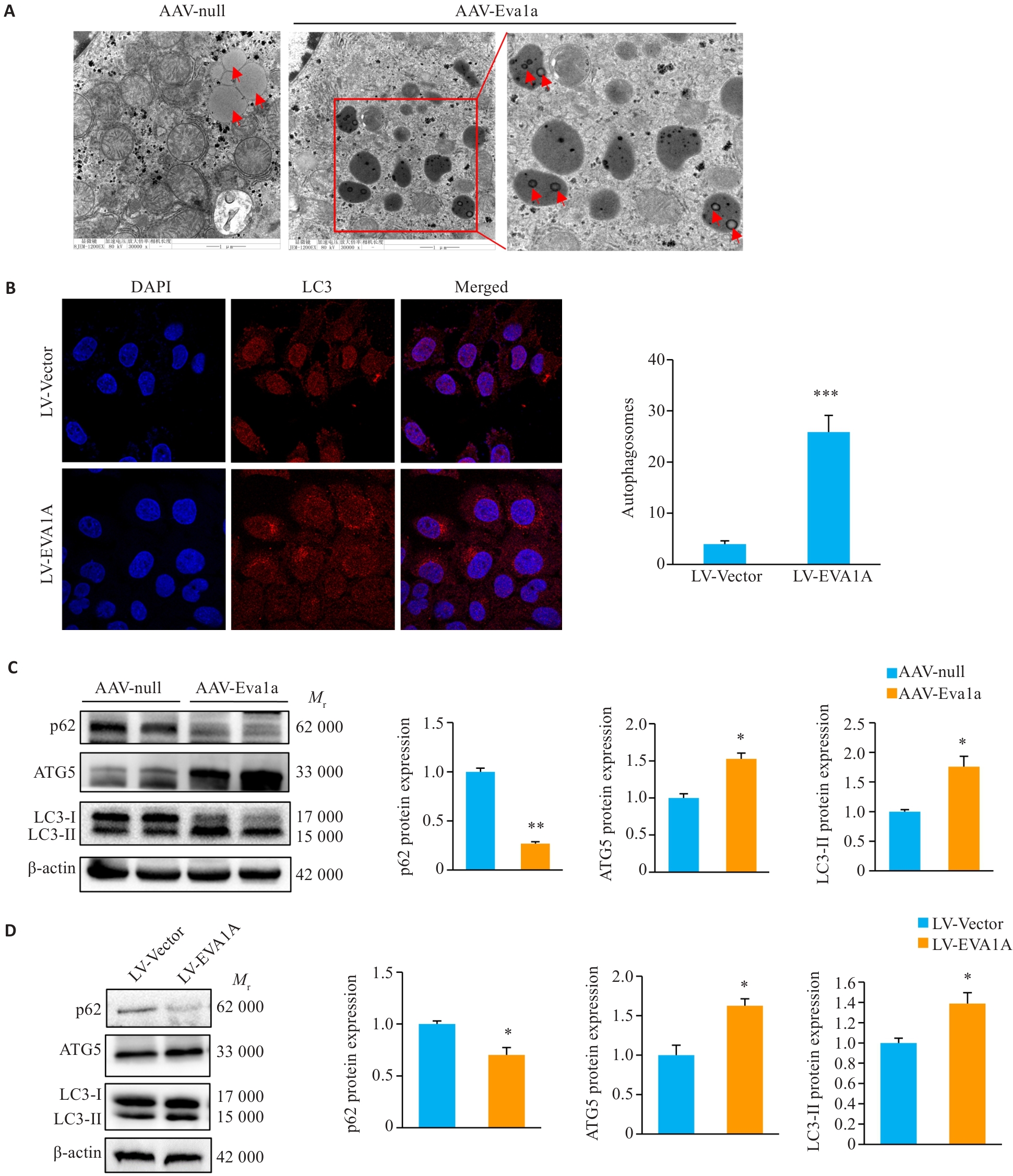
Fig.4 Effect of Eva1a overexpression on lipophagy in the liver of ob/ob mice and in OA-induced HepG2 cells. A: Lipophagy in mouse liver observed with transmission electron microscopy (×30 000). B: Immunofluorescence staining of LC3-labeled autophagosomes in HepG2 cells treated with OA (×1260). C: Protein expression levels of autophagy-related genes p62, LC3, and ATG5 in ob/ob mouse liver. D: Protein expression levels of autophagy-related genes p62, LC3, and ATG5 in HepG2 cells treated with OA. The red arrows indicate lipid droplets. *P<0.05, **P<0.01, ***P<0.001 vs AAV-null group or LV-Vector group.
| [1] | Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22(1): 63. doi:10.1186/s12902-022-00980-1 |
| [2] | Shi MR, Zhang H, Wang W, et al. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease[J]. J Diabetes Complicat, 2023, 37(10): 108610. doi:10.1016/j.jdiacomp.2023.108610 |
| [3] | Xu S, Wu X, Wang S, et al. TRIM56 protects against nonalcoholic fatty liver disease by promoting the degradation of fatty acid synthase[J]. J Clin Invest, 2024, 134(5): e166149. doi:10.1172/jci166149 |
| [4] | Wang L, Yu CF, Lu Y, et al. TMEM166, a novel transmembrane protein, regulates cell autophagy and apoptosis[J]. Apoptosis, 2007, 12(8): 1489-502. doi:10.1007/s10495-007-0073-9 |
| [5] | Hu J, Li G, Qu L, et al. TMEM166/EVA1A interacts with ATG16L1 and induces autophagosome formation and cell death[J]. Cell Death Dis, 2016, 7(8): e2323. doi:10.1038/cddis.2016.230 |
| [6] | De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights[J]. Curr Pharm Des, 2013, 19(29): 5239-49. doi:10.2174/13816128130303 |
| [7] | Lu GD, Ang YH, Zhou J, et al. CCAAT/enhancer binding protein α predicts poorer prognosis and prevents energy starvation-induced cell death in hepatocellular carcinoma[J]. Hepatology, 2015, 61(3): 965-78. doi:10.1002/hep.27593 |
| [8] | Xu Q, Liao Z, Gong Z, et al. Down-regulation of EVA1A by miR-103a-3p promotes hepatocellular carcinoma cells proliferation and migration[J]. Cell Mol Biol Lett, 2022, 27(1): 93. doi:10.1186/s11658-022-00388-8 |
| [9] | Yang JJ, Wang B, Xu Q, et al. TMEM166 inhibits cell proliferation, migration and invasion in hepatocellular carcinoma via upregulating TP53[J]. Mol Cell Biochem, 2021, 476(2): 1151-63. doi:10.1007/s11010-020-03979-1 |
| [10] | Zhen Y, Yuan Z, Zhang J, et al. Flubendazole induces mitochondrial dysfunction and DRP1-mediated mitophagy by targeting EVA1A in breast cancer[J]. Cell Death Dis, 2022, 13(4): 375. doi:10.1038/s41419-022-04823-8 |
| [11] | Lin X, Cui M, Xu D, et al. Liver-specific deletion of Eva1a/Tmem166 aggravates acute liver injury by impairing autophagy[J]. Cell Death Dis, 2018, 9(7): 768. doi:10.1038/s41419-018-0800-x |
| [12] | Li MT, Lu G, Hu J, et al. EVA1A/TMEM166 regulates embryonic neurogenesis by autophagy[J]. Stem Cell Rep, 2016, 6(3): 396-410. doi:10.1016/j.stemcr.2016.01.011 |
| [13] | Liu B, Liu B, Zhou Y, et al. EVA1A regulates hematopoietic stem cell regeneration via ER-mitochondria mediated apoptosis[J]. Cell Death Dis, 2023, 14(1): 71. doi:10.1038/s41419-023-05559-9 |
| [14] | Canham L, Sendac S, Diagbouga MR, et al. EVA1A (Eva-1 homolog A) promotes endothelial apoptosis and inflammatory activation under disturbed flow via regulation of autophagy[J]. Arterioscler Thromb Vasc Biol, 2023, 43(4): 547-61. doi:10.1161/atvbaha.122.318110 |
| [15] | Li J, Chen Y, Gao J, et al. Eva1a ameliorates atherosclerosis by promoting re-endothelialization of injured arteries via Rac1/Cdc42/Arpc1b[J]. Cardiovasc Res, 2021, 117(2): 450-61. doi:10.1093/cvr/cvaa011 |
| [16] | Liu XK, Gao X, Yang YL, et al. EVA1A reverses lenvatinib resistance in hepatocellular carcinoma through regulating PI3K/AKT/p53 signaling axis[J]. Apoptosis, 2024, 29(7): 1161-84. doi:10.1007/s10495-024-01967-0 |
| [17] | Li YX, Huang XG, Yang G, et al. CD36 favours fat sensing and transport to govern lipid metabolism[J]. Prog Lipid Res, 2022, 88: 101193. doi:10.1016/j.plipres.2022.101193 |
| [18] | Yang YX, Liu XK, Yang D, et al. Interplay of CD36, autophagy, and lipid metabolism: insights into cancer progression[J]. Metabolism, 2024, 155: 155905. doi:10.1016/j.metabol.2024.155905 |
| [19] | Zeng H, Qin H, Liao M, et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing[J]. Mol Metab, 2022, 57: 101428. doi:10.1016/j.molmet.2021.101428 |
| [20] | Zeng S, Wu F, Chen M, et al. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid β-oxidation by increasing its localization to mitochondria and interaction with long-chain acyl-CoA synthetase 1[J]. Antioxid Redox Signal, 2022, 36(16/17/18): 1081-100. doi:10.1089/ars.2021.0157 |
| [21] | Zhu HL, Zhao TM, Zhao S, et al. O-GlcNAcylation promotes the progression of nonalcoholic fatty liver disease by upregulating the expression and function of CD36[J]. Metabolism, 2024, 156: 155914. doi:10.1016/j.metabol.2024.155914 |
| [22] | Wilson CG, Tran JL, Erion DM, et al. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice[J]. Endocrinology, 2016, 157(2): 570-85. doi:10.1210/en.2015-1866 |
| [23] | Brownsey RW, Zhande R, Boone AN. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions[J]. Biochem Soc Trans, 1997, 25(4): 1232-8. doi:10.1042/bst0251232 |
| [24] | Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, et al. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2[J]. Science, 2001, 291(5513): 2613-6. doi:10.1126/science.1056843 |
| [25] | Akheruzzaman M, Hegde V, Shin AC, et al. Reducing endogenous insulin is linked with protection against hepatic steatosis in mice[J]. Nutr Diabetes, 2020, 10(1): 11. doi:10.1038/s41387-020-0114-9 |
| [26] | Gluchowski NL, Gabriel KR, Chitraju C, et al. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice[J]. Hepatology, 2019, 70(6): 1972-85. doi:10.1002/hep.30765 |
| [27] | Bai R, Rebelo A, Kleeff J, et al. Identification of prognostic lipid droplet-associated genes in pancreatic cancer patients via bioinformatics analysis[J]. Lipids Health Dis, 2021, 20(1): 58. doi:10.1186/s12944-021-01476-y |
| [28] | Fang QH, Shen QL, Li JJ, et al. Inhibition of microRNA-124a attenuates non-alcoholic fatty liver disease through upregulation of adipose triglyceride lipase and the effect of liraglutide intervention[J]. Hepatol Res, 2019, 49(7): 743-57. doi:10.1111/hepr.13330 |
| [29] | Reid BN, Ables GP, Otlivanchik OA, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis[J]. J Biol Chem, 2008, 283(19): 13087-99. doi:10.1074/jbc.m800533200 |
| [30] | Saadati S, Sadeghi A, Mansour A, et al. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial[J]. BMC Gastroenterol, 2019, 19(1): 133. doi:10.1186/s12876-019-1055-4 |
| [31] | Yu HY, Yang F, Zhong WT, et al. Secretory Galectin-3 promotes hepatic steatosis via regulation of the PPARγ/CD36 signaling pathway[J]. Cell Signal, 2021, 84: 110043. doi:10.1016/j.cellsig.2021.110043 |
| [32] | Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice[J]. J Hepatol, 2013, 58(5): 993-9. doi:10.1016/j.jhep.2013.01.011 |
| [33] | Blanchard PG, Festuccia WT, Houde VP, et al. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion [S[J]. J Lipid Res, 2012, 53(6): 1117-25. doi:10.1194/jlr.m021485 |
| [34] | Owen JL, Zhang Y, Bae SH, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase[J]. Proc Natl Acad Sci USA, 2012, 109(40): 16184-9. doi:10.1073/pnas.1213343109 |
| [35] | Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway[J]. Cell, 2011, 146(3): 408-20. doi:10.1016/j.cell.2011.06.034 |
| [36] | Chakrabarti P, English T, Shi J, et al. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage[J]. Diabetes, 2010, 59(4): 775-81. doi:10.2337/db09-1602 |
| [37] | Chakrabarti P, Kim JY, Singh M, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway[J]. Mol Cell Biol, 2013, 33(18): 3659-66. doi:10.1128/mcb.01584-12 |
| [38] | Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nat Cell Biol, 2011, 13(2): 132-41. doi:10.1038/ncb2152 |
| [1] | Qinjun YANG, Hongyu ZHU, Yuan GAO, Cheng YANG, Tong LIU, Lu ZHANG, Jiabing TONG, Zegeng LI. Sangma Zhike Formula alleviates airway inflammation and hyperresponsiveness in rats with postinfectious cough by inhibiting the TRPV1-SP/CGRP and pyroptosis pathways [J]. Journal of Southern Medical University, 2025, 45(9): 1830-1839. |
| [2] | Biyun LUO, Xin YI, Yijing CAI, Shiqing ZHANG, Peng WANG, Tong LI, Ken Kin Lam YUNG, Pingzheng ZHOU. Ching Shum Pills alleviates non-alcoholic fatty liver disease in mice by ameliorating lipid metabolism disorders [J]. Journal of Southern Medical University, 2025, 45(9): 1840-1849. |
| [3] | Jingjing ZHANG, Song FENG, Dali ZHANG, Jian XUE, Chao ZHOU, Pengcheng LIU, Shuangnan FU, Man GONG, Hui FENG, Ning ZHANG. Altered oral microbiome and metabolites are associated with improved lipid metabolism in HBV-infected patients with metabolic dysfunction-associated fatty liver disease [J]. Journal of Southern Medical University, 2025, 45(9): 2034-2045. |
| [4] | Zhengyuan FAN, Zihan SHEN, Ya LI, Tingting SHEN, Gaofeng LI, Suyun LI. Protective effect of Bufei Yishen Formula against cigarette smoke extract-induced human bronchial epithelial cell damage and its mechanism [J]. Journal of Southern Medical University, 2025, 45(7): 1372-1379. |
| [5] | Liming WANG, Hongrui CHEN, Yan DU, Peng ZHAO, Yujie WANG, Yange TIAN, Xinguang LIU, Jiansheng LI. Yiqi Zishen Formula ameliorates inflammation in mice with chronic obstructive pulmonary disease by inhibiting the PI3K/Akt/NF-κB signaling pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1409-1422. |
| [6] | Ruimin HAN, Manke ZHAO, Junfang YUAN, Zhenhong SHI, Zhen WANG, Defeng WANG. Live combined Bacillus subtilis and Enterococcus faecium improves glucose and lipid metabolism in type 2 diabetic mice with circadian rhythm disruption via the SCFAs/GPR43/GLP-1 pathway [J]. Journal of Southern Medical University, 2025, 45(7): 1490-1497. |
| [7] | Xinheng WANG, Xiaohan SHAO, Tongtong LI, Lu ZHANG, Qinjun YANG, Weidong YE, Jiabing TONG, Zegeng LI, Xiangming FANG. Pingchuanning Formula suppresses airway inflammation in a rat model of asthmatic cold syndrome by regulating the HMGB1/Beclin-1 axis-mediated autophagy [J]. Journal of Southern Medical University, 2025, 45(6): 1153-1162. |
| [8] | Dandan LI, Jiaxin CHU, Yan YAN, Wenjun XU, Xingchun ZHU, Yun SUN, Haofeng DING, Li REN, Bo ZHU. Curcumin inhibits lipid metabolism in non-small cell lung cancer by downregulating the HIF-1α pathway [J]. Journal of Southern Medical University, 2025, 45(5): 1039-1046. |
| [9] | Yang YANG, Kai WANG, Jianxiu LIU, Zhimo ZHOU, Wen JIA, Simou WU, Jinxing LI, Fang HE, Ruyue CHENG. Early life Bifidobacterium bifidum BD-1 intervention alleviates hyperactivity of juvenile female rats with attention deficit hyperactivity disorder [J]. Journal of Southern Medical University, 2025, 45(4): 702-710. |
| [10] | Jinshui ZHANG, Shuo LI, Dongdong WEI, Xin CHENG, Yun DENG, Youzhi ZHANG. Protective effect of graphene heating film far-infrared hyperthermia against frostbite in mice [J]. Journal of Southern Medical University, 2025, 45(3): 522-530. |
| [11] | Mengyao YUAN, Xianghan RUAN, Yang LI, Ting ZHANG, Chunxiang HAO, Hao LI, Jingsheng LOU, Jiangbei CAO, Yanhong LIU, Weidong MI, Xiaoying ZHANG. Preoperative serum magnesium as a biomarker for predicting delirium following non-cardiac surgery in elderly patients: a retrospective cohort study [J]. Journal of Southern Medical University, 2025, 45(12): 2616-2627. |
| [12] | Du SHANG, Wen LI, Lihua CUI, Ming CHEN. Hugan Decoction alleviates non-alcoholic fatty liver disease in rats by activating the AMPK/m-TOR signaling pathway and reducing lipid synthesis [J]. Journal of Southern Medical University, 2025, 45(12): 2667-2678. |
| [13] | Qing SHI, Suye RAN, Lingyu SONG, Hong YANG, Wenjuan WANG, Hanlin LIU, Qi LIU. NLRP6 overexpression improves nonalcoholic fatty liver disease by promoting lipid oxidation and decomposition in hepatocytes through the AMPK/CPT1A/PGC1A pathway [J]. Journal of Southern Medical University, 2025, 45(1): 118-125. |
| [14] | Xianheng ZHANG, Jian LIU, Qi HAN, Yiming CHEN, Xiang DING, Xiaolu CHEN. Huangqin Qingrechubi Capsule alleviates inflammation and uric acid and lipid metabolism imbalance in rats with gouty arthritis by inhibiting the PTEN/PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(8): 1450-1458. |
| [15] | Yuming ZHANG, Shicheng XIA, Linlin ZHANG, Mengxi CHEN, Xiaojing LIU, Qin GAO, Hongwei YE. Protective effect of Lonicerae japonicae flos extract against doxorubicin-induced liver injury in mice [J]. Journal of Southern Medical University, 2024, 44(8): 1571-1581. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||