Journal of Southern Medical University ›› 2026, Vol. 46 ›› Issue (1): 94-103.doi: 10.12122/j.issn.1673-4254.2026.01.10
Previous Articles Next Articles
Jinyan ZHAO1,2,3( ), Jiao PENG1, Minghe LIN1, Xiaoqin ZHU1,2,3, Bin HUANG1,2,3, Jiumao LIN1,2,3(
), Jiao PENG1, Minghe LIN1, Xiaoqin ZHU1,2,3, Bin HUANG1,2,3, Jiumao LIN1,2,3( )
)
Received:2025-05-17
Online:2026-01-20
Published:2026-01-16
Contact:
Jiumao LIN
E-mail:zhaojinyan0928@163.com;linjiumao@fjtcm.edu.cn
Supported by:Jinyan ZHAO, Jiao PENG, Minghe LIN, Xiaoqin ZHU, Bin HUANG, Jiumao LIN. Qingjie Fuzheng Granules alleviates 5-fluorouracil-induced skeletal muscle injury in tumor-bearing mice by inhibiting mitochondria-dependent apoptosis and activating the AMPK-PGC-1α pathway[J]. Journal of Southern Medical University, 2026, 46(1): 94-103.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2026.01.10
| Group | Tumor weight | Gastrocnemius weight |
|---|---|---|
| Normal | - | 0.31±0.02 |
| Control | 10.93±1.92 | 0.25±0.01** |
| Model | 2.83±0.40## | 0.21±0.01## |
| Treatment | 2.74±0.57 | 0.25±0.01△ |
| F | 63.88 | 25.57 |
| P | <0.001 | <0.001 |
Tab.1 Comparison of tumor weight and gastrocnemius muscle weight in each group (g, Mean±SD, n=4)
| Group | Tumor weight | Gastrocnemius weight |
|---|---|---|
| Normal | - | 0.31±0.02 |
| Control | 10.93±1.92 | 0.25±0.01** |
| Model | 2.83±0.40## | 0.21±0.01## |
| Treatment | 2.74±0.57 | 0.25±0.01△ |
| F | 63.88 | 25.57 |
| P | <0.001 | <0.001 |
| Group | Grip | Suspension score |
|---|---|---|
| Normal | 76.37±1.48 | 3 |
| Control | 63.45±1.66** | 2.33±0.47* |
| Model | 54.75±1.93## | 1.85±0.33# |
| Treatment | 69.12±2.96△△ | 2.67±0.27△△ |
| F | 76.63 | 9.74 |
| P | <0.001 | <0.01 |
Tab.2 Comparison of grip and suspension test scores in each group (Mean±SD, n=4)
| Group | Grip | Suspension score |
|---|---|---|
| Normal | 76.37±1.48 | 3 |
| Control | 63.45±1.66** | 2.33±0.47* |
| Model | 54.75±1.93## | 1.85±0.33# |
| Treatment | 69.12±2.96△△ | 2.67±0.27△△ |
| F | 76.63 | 9.74 |
| P | <0.001 | <0.01 |
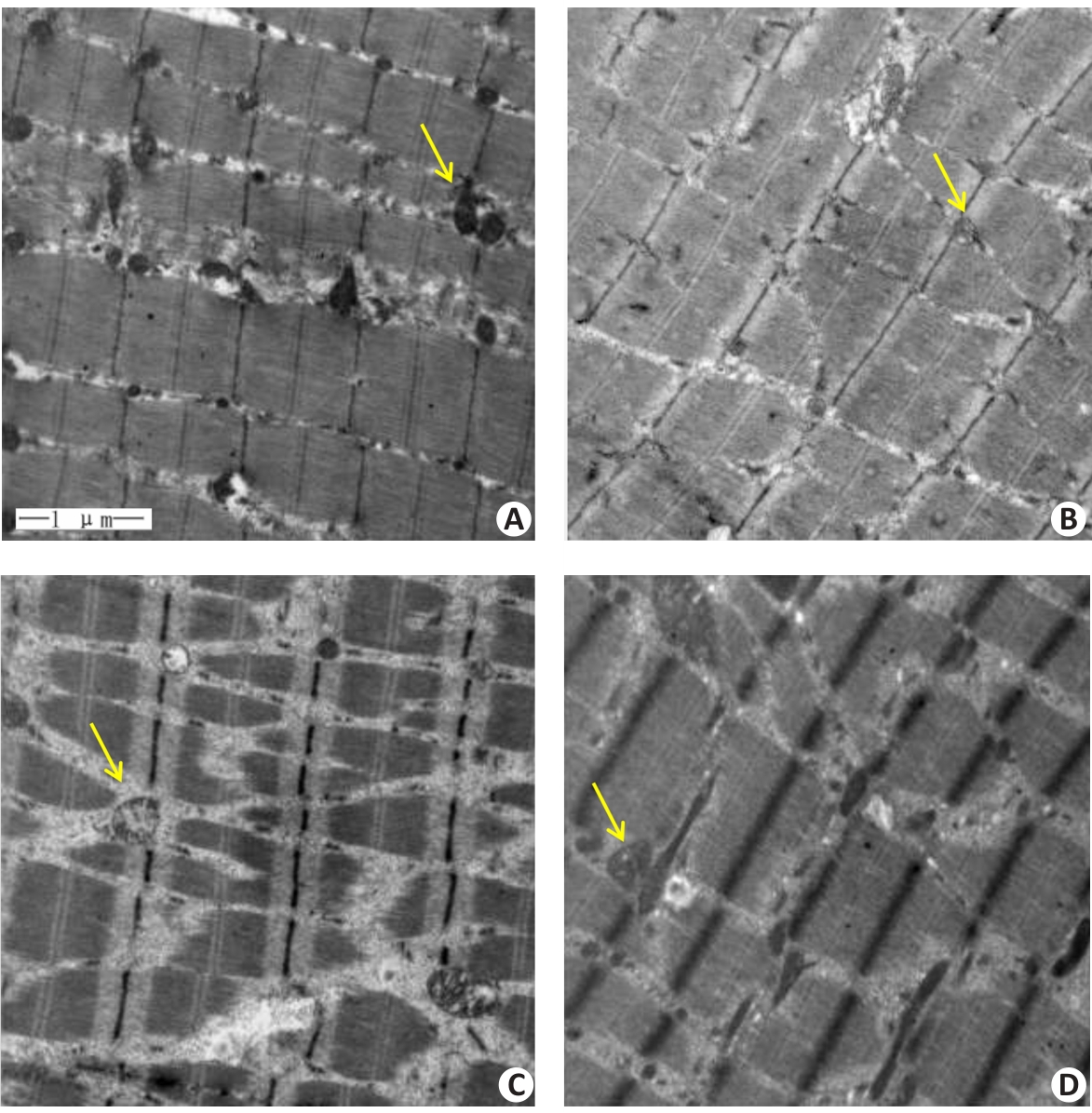
Fig.2 Transmission electron microscopy of the gastrocnemius muscle in each group (Original magnification: ×20 000). A: Normal group. B: Control group. C: Model group. D: Treatment group. Yellow arrows indicate the mitochondria.
| Group | Content of ATP |
|---|---|
| Normal | 3.31±0.25 |
| Control | 2.06±0.11** |
| Model | 0.89±0.12## |
| Treatment | 1.44±0.17△△ |
| F | 113.85 |
| P | <0.001 |
Tab.3 Comparison of ATP content in the gastrocnemius muscle in each group (μmol/g, Mean±SD, n=4)
| Group | Content of ATP |
|---|---|
| Normal | 3.31±0.25 |
| Control | 2.06±0.11** |
| Model | 0.89±0.12## |
| Treatment | 1.44±0.17△△ |
| F | 113.85 |
| P | <0.001 |
| Group | Apoptosis rate |
|---|---|
| Normal | 3.66±0.37 |
| Control | 14.13±0.60** |
| Model | 36.39±0.53## |
| Treatment | 13.61±1.06△△ |
| F | 1214.94 |
| P | <0.001 |
Tab.4 Comparison of cell apoptosis rate in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Apoptosis rate |
|---|---|
| Normal | 3.66±0.37 |
| Control | 14.13±0.60** |
| Model | 36.39±0.53## |
| Treatment | 13.61±1.06△△ |
| F | 1214.94 |
| P | <0.001 |
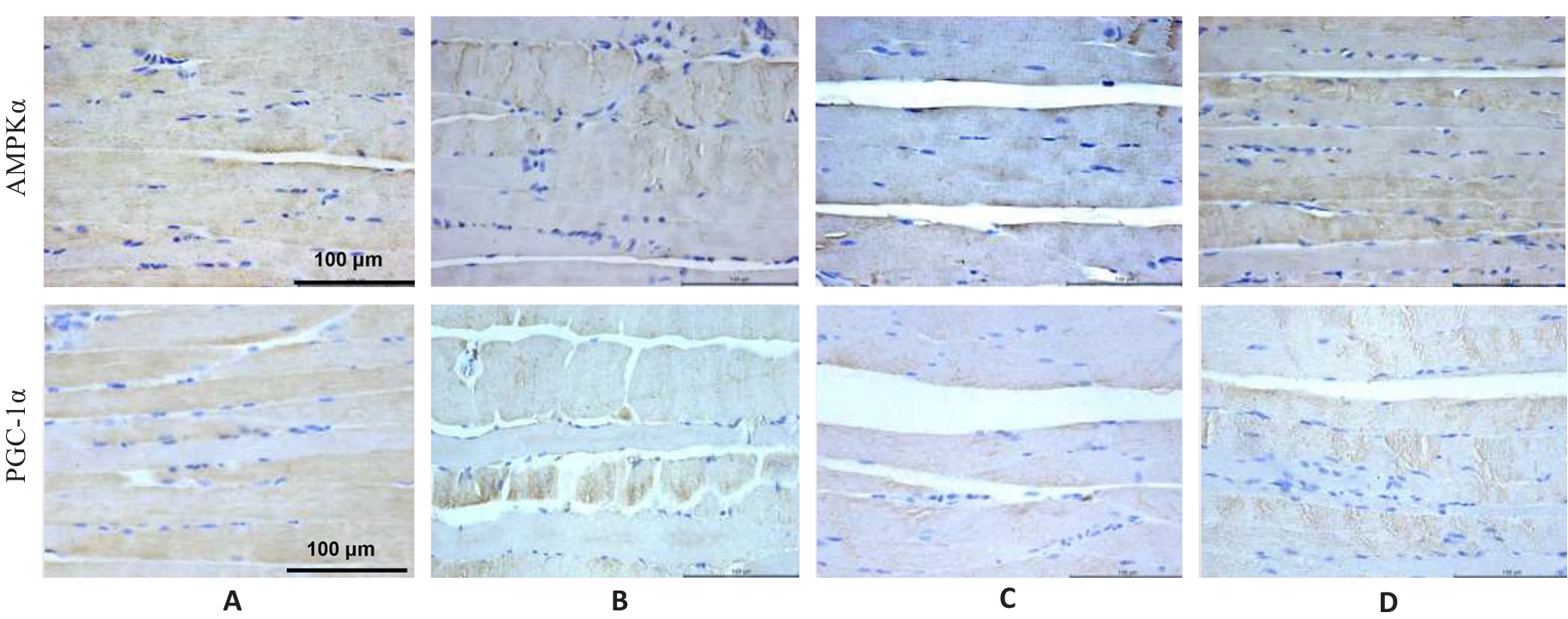
Fig.4 Immunohistochemistry for detecting AMPK and PGC-1α protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | AMPK | PGC-1α |
|---|---|---|
| Normal | 56.46±0.62 | 57.90±1.40 |
| Control | 42.48±1.88** | 25.67±1.01** |
| Model | 16.27±1.07## | 14.29±0.65## |
| Treatment | 21.8±1.17△△ | 20.67±2.00△△ |
| F | 648.10 | 406.17 |
| P | <0.001 | <0.01 |
Tab.5 Protein expression levels of AMPK and PGC-1α in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | AMPK | PGC-1α |
|---|---|---|
| Normal | 56.46±0.62 | 57.90±1.40 |
| Control | 42.48±1.88** | 25.67±1.01** |
| Model | 16.27±1.07## | 14.29±0.65## |
| Treatment | 21.8±1.17△△ | 20.67±2.00△△ |
| F | 648.10 | 406.17 |
| P | <0.001 | <0.01 |
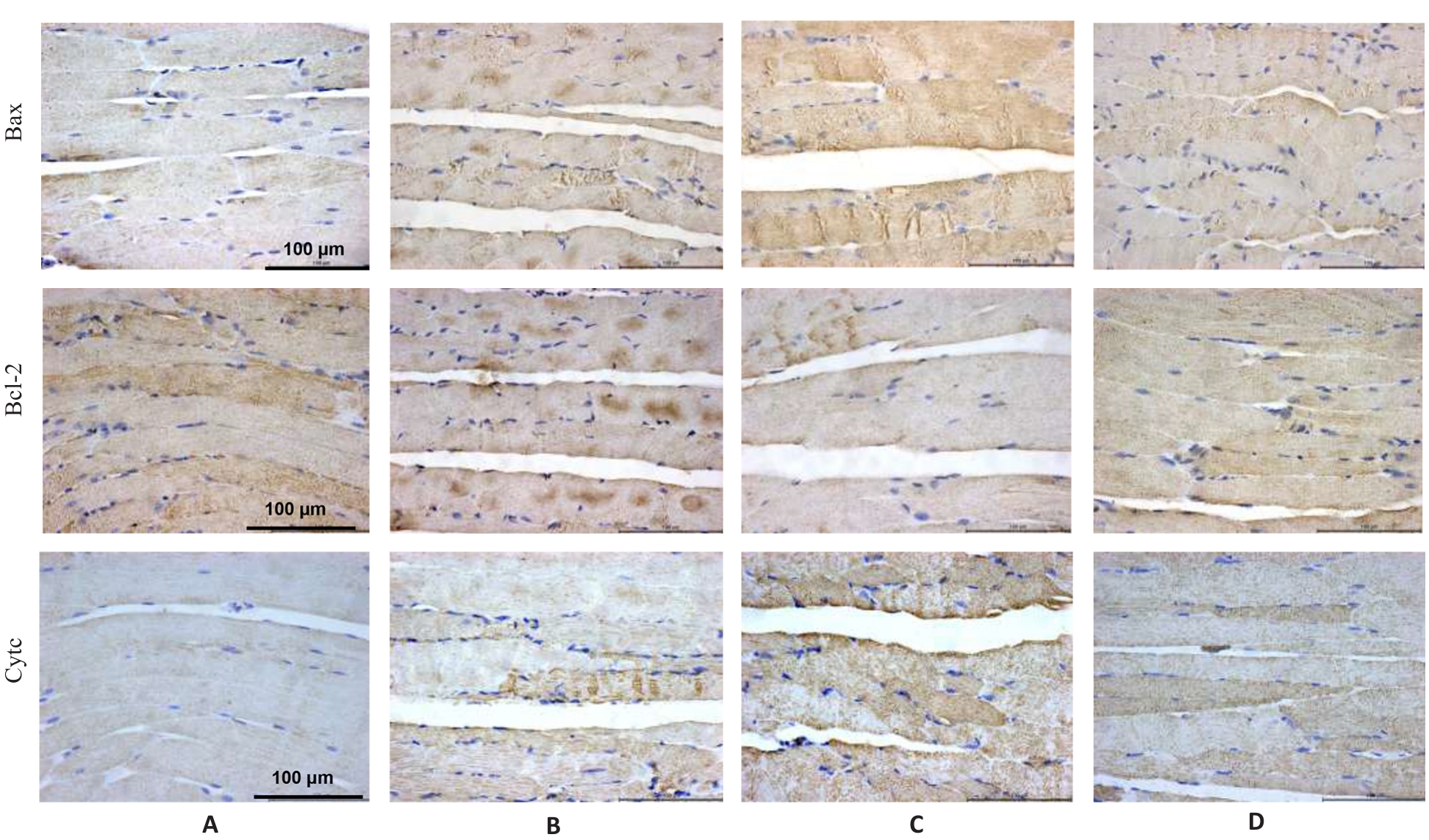
Fig.5 Immunohistochemistry for detecting Bax, Bcl-2 and Cyt c protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | Bax | Bcl-2 | Cyt c |
|---|---|---|---|
| Normal | 29.42±0.79 | 71.08±1.53 | 20.41±0.53 |
| Control | 51.03±1.74** | 55.40±0.92** | 43.32±1.55** |
| Model | 80.67±1.36## | 37.53±1.41## | 69.45±1.13## |
| Treatment | 33.38±1.0△△ | 57.27±1.07△△ | 38.02±0.96△△ |
| F | 661.12 | 293.84 | 674.29 |
| P | <0.001 | <0.001 | <0.001 |
Tab.6 Protein expression levels of Bax, Bcl-2 and Cyt c in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Bax | Bcl-2 | Cyt c |
|---|---|---|---|
| Normal | 29.42±0.79 | 71.08±1.53 | 20.41±0.53 |
| Control | 51.03±1.74** | 55.40±0.92** | 43.32±1.55** |
| Model | 80.67±1.36## | 37.53±1.41## | 69.45±1.13## |
| Treatment | 33.38±1.0△△ | 57.27±1.07△△ | 38.02±0.96△△ |
| F | 661.12 | 293.84 | 674.29 |
| P | <0.001 | <0.001 | <0.001 |
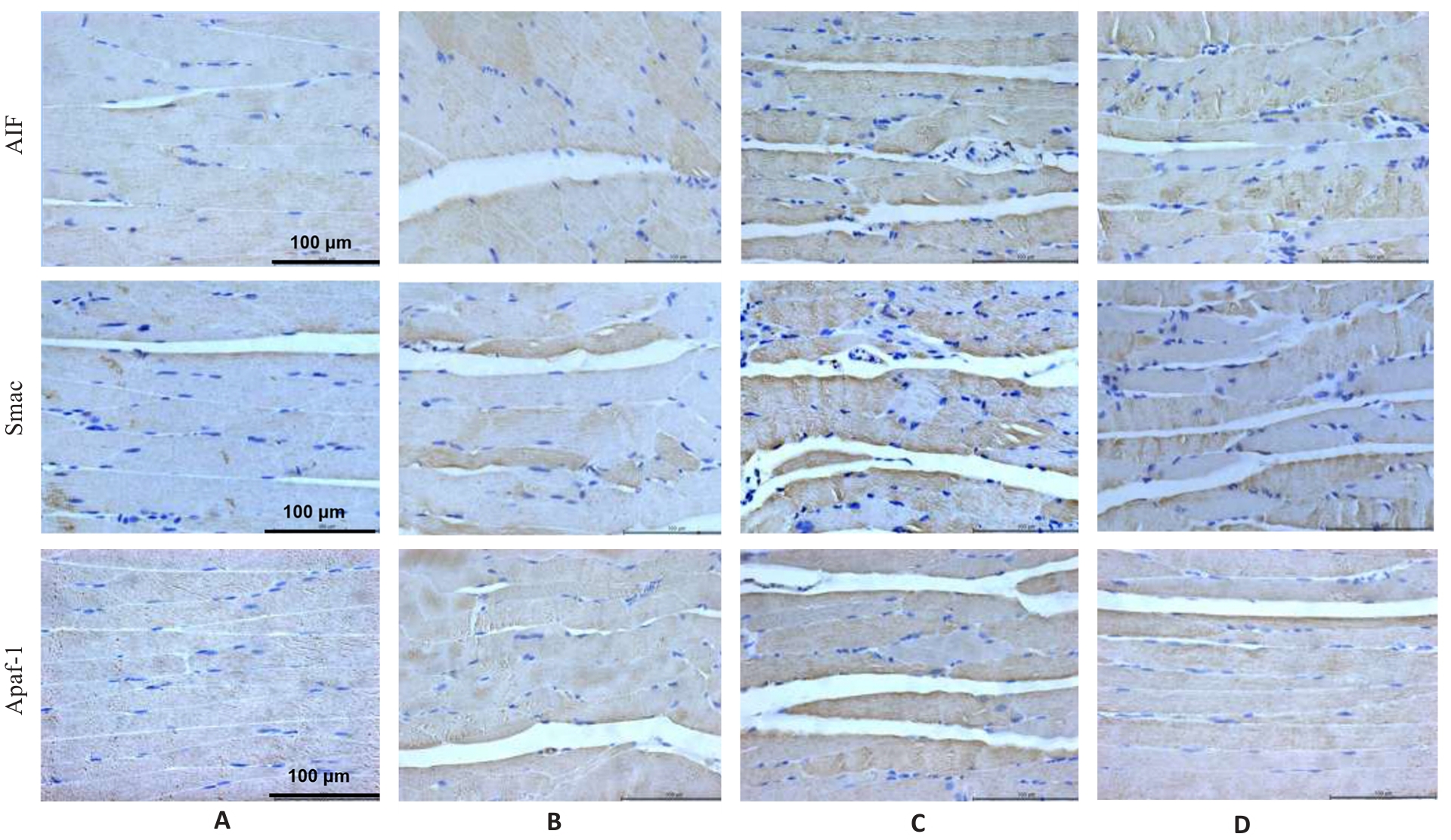
Fig.6 Immunohistochemistry for detecting AIF, Smac and Apaf1 protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | AIF | Smac | Apaf1 |
|---|---|---|---|
| Normal | 17.09±0.58 | 18.21±0.66 | 19.54±0.51 |
| Control | 36.43±0.50** | 36.41±0.69** | 39.32±0.69** |
| Model | 51.70±0.55## | 51.23±1.72## | 49.32±1.27## |
| Treatment | 27.24±0.32△△ | 23.59±1.59△△ | 29.63±1.68△△ |
| F | 1719.99 | 270.47 | 252.82 |
| P | <0.001 | <0.001 | <0.001 |
Tab.7 Protein expression levels of AIF, Smac and Apaf1 in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | AIF | Smac | Apaf1 |
|---|---|---|---|
| Normal | 17.09±0.58 | 18.21±0.66 | 19.54±0.51 |
| Control | 36.43±0.50** | 36.41±0.69** | 39.32±0.69** |
| Model | 51.70±0.55## | 51.23±1.72## | 49.32±1.27## |
| Treatment | 27.24±0.32△△ | 23.59±1.59△△ | 29.63±1.68△△ |
| F | 1719.99 | 270.47 | 252.82 |
| P | <0.001 | <0.001 | <0.001 |
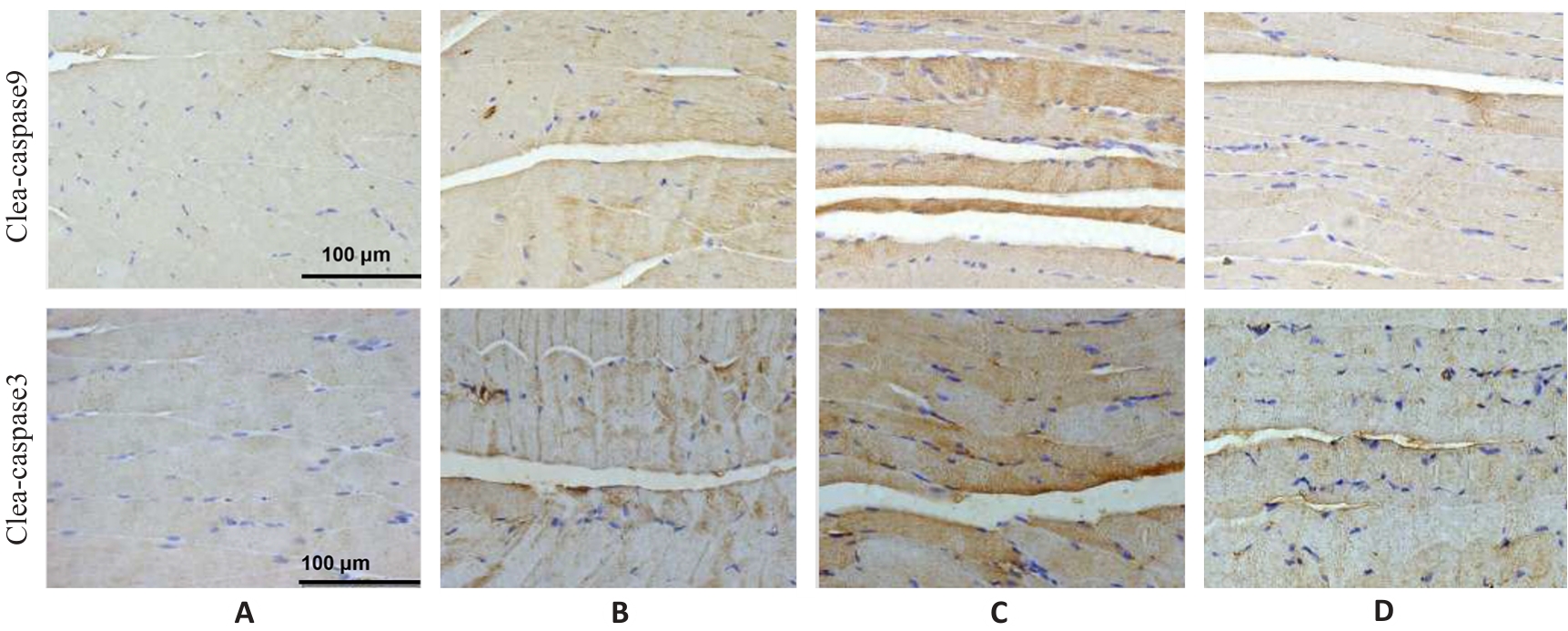
Fig.7 Immunohistochemistry for detecting cleaved caspase-9 and cleaved caspase-3 expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | Cleaved-caspase9 | Cleaved-caspase3 |
|---|---|---|
| Normal | 16.35±0.34 | 19.61±0.50 |
| Control | 44.50±0.84** | 29.94±1.60** |
| Model | 90.65±0.80## | 58.86±1.33## |
| Treatment | 20.08±0.43△△ | 20.67±2.00△△ |
| F | 2244.19 | 569.43 |
| P | <0.001 | <0.01 |
Tab.8 Protein expression levels of cleaved caspase-9 and cleaved caspase-3 in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Cleaved-caspase9 | Cleaved-caspase3 |
|---|---|---|
| Normal | 16.35±0.34 | 19.61±0.50 |
| Control | 44.50±0.84** | 29.94±1.60** |
| Model | 90.65±0.80## | 58.86±1.33## |
| Treatment | 20.08±0.43△△ | 20.67±2.00△△ |
| F | 2244.19 | 569.43 |
| P | <0.001 | <0.01 |
| [1] | Cao WY, Li JH, Yang KP, et al. An overview of autophagy: Mechanism, regulation and research progress[J]. Bull Cancer, 2021, 108(3): 304-22. doi:10.1016/j.bulcan.2020.11.004 |
| [2] | Beesley VL, Ross TL, King MT, et al. Evaluating patient-reported symptoms and late adverse effects following completion of first-line chemotherapy for ovarian cancer using the MOST (Measure of Ovarian Symptoms and Treatment concerns)[J]. Gynecol Oncol, 2022, 164(2): 437-45. doi:10.1016/j.ygyno.2021.12.006 |
| [3] | Fox RS, Ancoli-Israel S, Roesch SC, et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy[J]. Support Care Cancer, 2020, 28(2): 845-55. doi:10.1007/s00520-019-04834-w |
| [4] | Liu WM, Liu J, Ma L, et al. Effect of mindfulness Yoga on anxiety and depression in early breast cancer patients received adjuvant chemotherapy: a randomized clinical trial[J]. J Cancer Res Clin Oncol, 2022, 148(9): 2549-60. doi:10.1007/s00432-022-04167-y |
| [5] | Zhang H, Meng YT, Jiang RX, et al. Effect of multimodal exercise on cancer-related fatigue in patients undergoing simultaneous radiotherapy and chemotherapy: a randomized trial in patients with breast cancer[J]. Altern Ther Health Med, 2023, 29(5): 233-7. |
| [6] | Mallard J, Hucteau E, Charles AL, et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients[J]. J Cachexia Sarcopenia Muscle, 2022, 13(3): 1896-907. doi:10.1002/jcsm.12991 |
| [7] | Mallard J, Hucteau E, Bender L, et al. A single chemotherapy administration induces muscle atrophy, mitochondrial alterations and apoptosis in breast cancer patients[J]. J Cachexia Sarcopenia Muscle, 2024, 15(1): 292-305. doi:10.1002/jcsm.13414 |
| [8] | Jahnke VE, Peterson JM, Van Der Meulen JH, et al. Mitochondrial dysfunction and consequences in calpain-3-deficient muscle[J]. Skelet Muscle, 2020, 10(1): 37. doi:10.1186/s13395-020-00254-1 |
| [9] | 黄燕峰, 朱达坚, 鲁 路. 黄芪多糖对慢性疲劳小鼠骨骼肌线粒体功能的影响及作用机制[J]. 广东医学, 2017, 38(12): 1789-94. |
| [10] | 张 璐, 丁焕章, 许浩燃, 等. 参芪补中方通过激活AMPK/SIRT1/PGC-1α改善COPD肺脾气虚证大鼠线粒体功能障碍[J].南方医科大学学报,2025, 45(5): 969-976. |
| [11] | 关海燕, 张洪亮. 恶性肿瘤患者化疗引起癌因性疲乏的中西医研究进展[J]. 新疆中医药, 2018, 36(5): 119-21. |
| [12] | 华杭菊, 林久茂, 任丽萍, 等. 清解扶正方联合mFOLFOX4方案治疗晚期大肠癌的疗效观察[J]. 福建中医药, 2019, 50(1): 20-1, 24. |
| [13] | 王泽坤, 陈晓琦, 陈召起, 等. 癌因性疲乏的中西医研究进展[J]. 中华中医药杂志, 2023, 38(3): 1185-9. |
| [14] | 周 婷, 吴泳蓉, 熊家青, 等. 癌因性疲乏的中医病因探析[J]. 中华中医药杂志, 2022, 37(2): 982-5. |
| [15] | Wei XT, Xin JY, Chen W, et al. Astragalus polysaccharide ameliorated complex factor-induced chronic fatigue syndrome by modulating the gut microbiota and metabolites in mice[J]. Biomed Pharmacother, 2023, 163: 114862. doi:10.1016/j.biopha.2023.114862 |
| [16] | Dong J, Wang S, Gui YR, et al. Astragalus membranaceus (Huang Qi) for cancer-related fatigue: a protocol for systematic review and meta-analysis[J]. Medicine (Baltimore), 2022, 101(3): e28633. doi:10.1097/md.0000000000028633 |
| [17] | 余 意, 胡明华, 张丹丹, 等. 黄芪多糖对气虚大鼠的补气作用及其机制探讨[J]. 中药新药与临床药理, 2021, 32(4): 505-10. |
| [18] | 徐振秋, 李雪峰, 张海弢, 等. 麦芽油软胶囊缓解体力疲劳作用的研究[J]. 食品与发酵科技, 2015, 51(5): 17-9, 26. |
| [19] | 郜浩帆, 姚佳霖, 王宝亮, 等. 黄芪及其有效成分治疗重症肌无力的作用机制研究进展[J]. 中华中医药学刊, 2025, 43(10): 171-5. |
| [201] | 柴梦音, 寇卜心, 豆双双, 等. 黄芪超微粉抗急性肝损伤、抗疲劳作用及黄芪甲苷含量的变化[J]. 现代中医药, 2022, 42(5): 26-32. |
| [21] | 吴 娇, 仝芳超. 黄芪的化学成分、药理作用及临床应用[J]. 滨州医学院学报, 2024, 47(1): 68-75. |
| [22] | Wu ZH, Yin B, You FM. Molecular mechanism of anti-colorectal cancer effect of Hedyotis diffusa willd and its extracts[J]. Front Pharmacol, 2022, 13: 820474. doi:10.3389/fphar.2022.820474 |
| [23] | Zhu DY, Yuan SM, Chen C. Hedyotis diffusa-Sculellaria barbata (HD-SB) suppresses the progression of colorectal cancer cells via the hsa_circ_0039933/hsa-miR-204-5p/wnt11 axis[J]. Sci Rep, 2023, 13(1): 13331. doi:10.1038/s41598-023-40393-1 |
| [24] | Yang ZP, Lu S, Tang HZ, et al. Molecular targets and mechanisms of Hedyotis diffusa- Scutellaria barbata herb pair for the treatment of colorectal cancer based on network pharmacology and molecular docking[J]. Evid Based Complement Alternat Med, 2022, 2022: 6186662. doi:10.1155/2022/6186662 |
| [25] | Yang SW, Chu SF, Gao Y, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis[J]. Cells, 2019, 8(7): 738. doi:10.3390/cells8070738 |
| [26] | Deng XH, Zhang SW, Wu JZ, et al. Promotion of mitochondrial biogenesis via activation of AMPK-PGC1ɑ signaling pathway by ginger (Zingiber officinale Roscoe) extract, and its major active component 6-gingerol[J]. J Food Sci, 2019, 84(8): 2101-11. doi:10.1111/1750-3841.14723 |
| [27] | Sun SN, Yang SR, Cheng Y, et al. Jinlida granules alleviate podocyte apoptosis and mitochondrial dysfunction via the AMPK/PGC-1α pathway in diabetic nephropathy[J]. Int J Mol Med, 2025, 55(2): 26. doi:10.3892/ijmm.2024.5467 |
| [28] | Fontana F, Macchi C, Anselmi M, et al. PGC1-α-driven mitochondrial biogenesis contributes to a cancer stem cell phenotype in melanoma[J]. Biochim Biophys Acta Mol Basis Dis, 2024, 1870(1): 166897. doi:10.1016/j.bbadis.2023.166897 |
| [29] | 苏东东, 靳庆瑞, 赵 梦, 等. 参兰颗粒通过AMPK/PGC-1α/NRF1介导的线粒体保护改善阿霉素性心脏毒性[J].中药药理与临床, 2025, 41(9): 2-9. |
| [30] | Yang XF, Xue PP, Chen HR, et al. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis[J]. Theranostics, 2020, 10(3): 1415-32. doi:10.7150/thno.40857 |
| [31] | Piao LM, Huang Z, Inoue A, et al. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice[J]. Stem Cell Res Ther, 2022, 13(1): 226. doi:10.1186/s13287-022-02895-z |
| [32] | Cao YY, Wang Z, Yu T, et al. Sepsis induces muscle atrophy by inhibiting proliferation and promoting apoptosis via PLK1-AKT signalling[J]. J Cell Mol Med, 2021, 25(20): 9724-39. doi:10.1111/jcmm.16921 |
| [33] | Nguyen TT, Wei SB, Nguyen TH, et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease[J]. Exp Mol Med, 2023, 55(8): 1595-619. doi:10.1038/s12276-023-01046-5 |
| [34] | Gu J, Rauniyar S, Wang Y, et al. Chrysophanol induced glioma cells apoptosis via activation of mitochondrial apoptosis pathway[J]. Bioengineered, 2021, 12(1): 6855-68. doi:10.1080/21655979.2021.1972079 |
| [35] | Cosentino K, Hertlein V, Jenner A, et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation[J]. Mol Cell, 2022, 82(5): 933-49.e9. doi:10.1016/j.molcel.2022.01.008 |
| [36] | Zhang S, Rao SJ, Yang MW, et al. Role of mitochondrial pathways in cell apoptosis during He-patic ischemia/reperfusion injury[J]. Int J Mol Sci, 2022, 23(4): 2357. doi:10.3390/ijms23042357 |
| [37] | Wang HJ, Zhang CW, Li MN, et al. Antimicrobial peptides mediate apoptosis by changing mitochondrial membrane permeability[J]. Int J Mol Sci, 2022, 23(21): 12732. doi:10.3390/ijms232112732 |
| [38] | Flores-Romero H, Dadsena S, García-Sáez AJ. Mitochondrial pores at the crossroad between cell death and inflammatory signaling[J]. Mol Cell, 2023, 83(6): 843-56. doi:10.1016/j.molcel.2023.02.021 |
| [39] | Cetraro P, Plaza-Diaz J, MacKenzie A, et al. A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer[J]. Cancers (Basel), 2022, 14(7): 1671. doi:10.3390/cancers14071671 |
| [40] | Picca A, Calvani R, Coelho-Junior HJ, et al. Cell death and inflammation: the role of mitochondria in health and disease[J]. Cells, 2021, 10(3): 537. doi:10.3390/cells10030537 |
| [41] | Barroso T, Melo-Alvim C, Ribeiro LA, et al. Targeting inhibitor of apoptosis proteins to overcome chemotherapy resistance-a marriage between targeted therapy and cytotoxic chemotherapy[J]. Int J Mol Sci, 2023, 24(17): 13385. doi:10.3390/ijms241713385 |
| [1] | Lu ZHANG, Huanzhang DING, Haoran XU, Ke CHEN, Bowen XU, Qinjun YANG, Di WU, Jiabing TONG, Zegeng LI. Shenqi Buzhong Formula ameliorates mitochondrial dysfunction in a rat model of chronic obstructive pulmonary disease by activating the AMPK/SIRT1/PGC-1α pathway [J]. Journal of Southern Medical University, 2025, 45(5): 969-976. |
| [2] | Zhiliang CHEN, Yonggang YANG, Xia HUANG, Yan CHENG, Yuan QU, Qiqi HENG, Yujia FU, Kewei LI, Ning GU. Differential expressions of exosomal miRNAs in patients with chronic heart failure and hyperuricemia: diagnostic values of miR-27a-5p and miR-139-3p [J]. Journal of Southern Medical University, 2025, 45(1): 43-51. |
| [3] | WAN Lu, QIAN Yuchi, NI Wenjing, LU Yuxin, LI Wei, PAN Yan, CHEN Weidong. Linagliptin improves diabetic kidney disease in rats by promoting mitochondrial biogenesis through the AMPK/PGC-1α/TFAM pathway [J]. Journal of Southern Medical University, 2023, 43(12): 2053-2060. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||