Journal of Southern Medical University ›› 2026, Vol. 46 ›› Issue (1): 104-112.doi: 10.12122/j.issn.1673-4254.2026.01.11
Jianxiong AN1,2,3( ), Zhijia CHI3, Caiqun ZHAO3, Yongxiang LI3, Ruoguo WANG3, Yanan HU1,2(
), Zhijia CHI3, Caiqun ZHAO3, Yongxiang LI3, Ruoguo WANG3, Yanan HU1,2( )
)
Received:2025-07-15
Online:2026-01-20
Published:2026-01-16
Contact:
Yanan HU
E-mail:anjianxiong@yeah.net;huyanan1998@yeah.net
Jianxiong AN, Zhijia CHI, Caiqun ZHAO, Yongxiang LI, Ruoguo WANG, Yanan HU. Efficacy and safety of super electroconvulsive therapy for treatment-resistant depression: a retrospective analysis of 292 cases[J]. Journal of Southern Medical University, 2026, 46(1): 104-112.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2026.01.11
| Index | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
| Age (year, Mean±SD) | 27.97±12.83 | 30.43±10.48 | 27.84±12.02 | 0.299 |
Gender [n (%)] Male Female | 43 (48.31) 45 (51.14) | 54 (60.67) 35 (39.33) | 18 (46.15) 21 (53.85) | 0.469 0.513 |
| BMI (kg/m2, Mean±SD) | 28.23±5.35 | 29.23±8.22 | 27.23±7.46 | 0.423 |
Education [n (%)] None ≤6 years >6 years | 13 (14.77) 10 (11.36) 65 (73.86) | 11 (12.36) 18 (20.22) 60 (67.42) | 3 (7.69) 7 (17.95) 29 (74.36) | 0.348 0.472 0.621 |
Medications [n (%)] Antidepressant Sleep medicine | 88 (100) 67 (76.14) | 89 (100) 70 (78.65) | 38 (97.43) 31 (79.48) | 0.989 0.863 |
Suicide attempt [n (%)] Within 1 year No | 62 (70.45) 26 (29.55) | 54 (60.67) 35 (39.33) | 30 (76.92) 9 (23.08) | 0.437 0.265 |
Diagnosis [n (%)] Without psychotic features With psychotic features | 56 (63.64) 32 (36.36) | 61 (68.54) 28 (31.46) | 27 (69.23) 12 (30.77) | 0.652 0.537 |
Anesthesia induction (Mean±SD) Scopolamine (mg) Propofol (mg) Remifentanil (μg) | 0.32±0.07 52.21±5.15 198.31±22.22 | 0.30±0.08 55.34±21.45 202.26±24.14 | 0.30±0.09 51.67±4.49 200.26±24.97 | 0.831 0.243 0.529 |
| Duration between propofol and electric stimulus (min) | 12.11±1.53 | 11.88±1.71 | 12.18±1.75 | 0.522 |
Tab.1 General characteristics of the patients in the 3 groups
| Index | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
| Age (year, Mean±SD) | 27.97±12.83 | 30.43±10.48 | 27.84±12.02 | 0.299 |
Gender [n (%)] Male Female | 43 (48.31) 45 (51.14) | 54 (60.67) 35 (39.33) | 18 (46.15) 21 (53.85) | 0.469 0.513 |
| BMI (kg/m2, Mean±SD) | 28.23±5.35 | 29.23±8.22 | 27.23±7.46 | 0.423 |
Education [n (%)] None ≤6 years >6 years | 13 (14.77) 10 (11.36) 65 (73.86) | 11 (12.36) 18 (20.22) 60 (67.42) | 3 (7.69) 7 (17.95) 29 (74.36) | 0.348 0.472 0.621 |
Medications [n (%)] Antidepressant Sleep medicine | 88 (100) 67 (76.14) | 89 (100) 70 (78.65) | 38 (97.43) 31 (79.48) | 0.989 0.863 |
Suicide attempt [n (%)] Within 1 year No | 62 (70.45) 26 (29.55) | 54 (60.67) 35 (39.33) | 30 (76.92) 9 (23.08) | 0.437 0.265 |
Diagnosis [n (%)] Without psychotic features With psychotic features | 56 (63.64) 32 (36.36) | 61 (68.54) 28 (31.46) | 27 (69.23) 12 (30.77) | 0.652 0.537 |
Anesthesia induction (Mean±SD) Scopolamine (mg) Propofol (mg) Remifentanil (μg) | 0.32±0.07 52.21±5.15 198.31±22.22 | 0.30±0.08 55.34±21.45 202.26±24.14 | 0.30±0.09 51.67±4.49 200.26±24.97 | 0.831 0.243 0.529 |
| Duration between propofol and electric stimulus (min) | 12.11±1.53 | 11.88±1.71 | 12.18±1.75 | 0.522 |
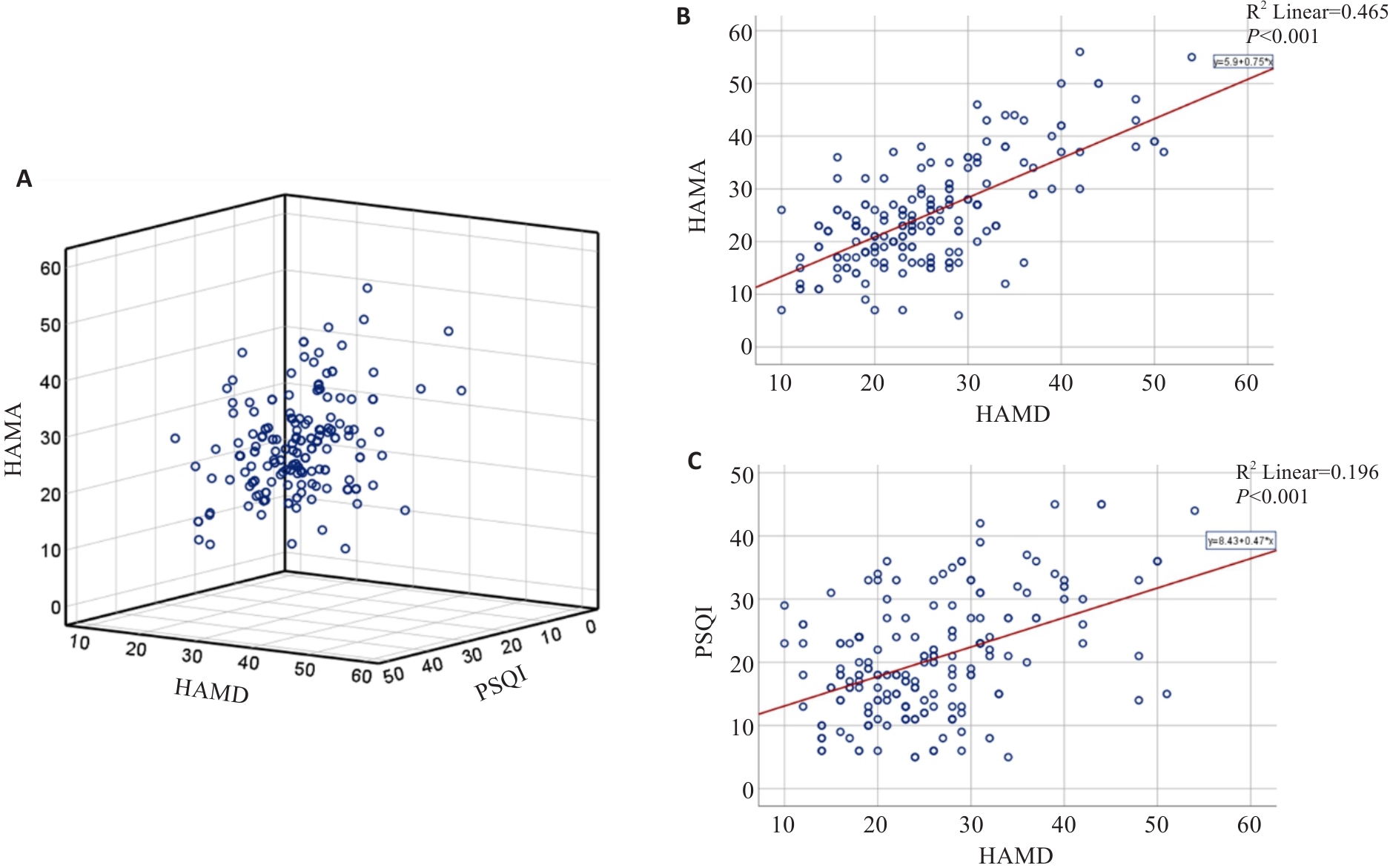
Fig.2 Correlation among HAMA, HAMD-17, and PSQI scores of the patients at baseline. A: 3-D scatterplot of the relation among HAMA, HAMD-17 and PSQI scores. B: Correlation between HAMA and HAMD-17 scores. C: Correlation between PSQI and HAMD-17 scores.
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
EEG seizure duration (s) Total First Second Third | 235.3±123.79 235.3±123.79† - - | 208.76±72.99 77.85±27.50* 112.26±62.01 - | 231.85±93.34 50.56±22.99* 64.33±13.20 115.15±75.08 | 0.171 <0.001 0.185 - |
| Super ECT sessions | 2.13±1.44 | 2.23±2.01 | 2.41±2.15 | 0.759 |
Race of hospitalizations [n(%)] 1 month 3 months 6 months | 44 (50.00) 8 (9.09) 13 (14.77) 19 (21.59) | 43 (48.31) 14 (15.73) 28 (31.46)* 32 (35.96)* | 17 (43.59) 7 (17.95) 10 (25.64) 11 (28.21) | 0.383 0.470 0.012 0.026 |
Tab.2 Seizure duration analysis and treatment conditions
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
EEG seizure duration (s) Total First Second Third | 235.3±123.79 235.3±123.79† - - | 208.76±72.99 77.85±27.50* 112.26±62.01 - | 231.85±93.34 50.56±22.99* 64.33±13.20 115.15±75.08 | 0.171 <0.001 0.185 - |
| Super ECT sessions | 2.13±1.44 | 2.23±2.01 | 2.41±2.15 | 0.759 |
Race of hospitalizations [n(%)] 1 month 3 months 6 months | 44 (50.00) 8 (9.09) 13 (14.77) 19 (21.59) | 43 (48.31) 14 (15.73) 28 (31.46)* 32 (35.96)* | 17 (43.59) 7 (17.95) 10 (25.64) 11 (28.21) | 0.383 0.470 0.012 0.026 |
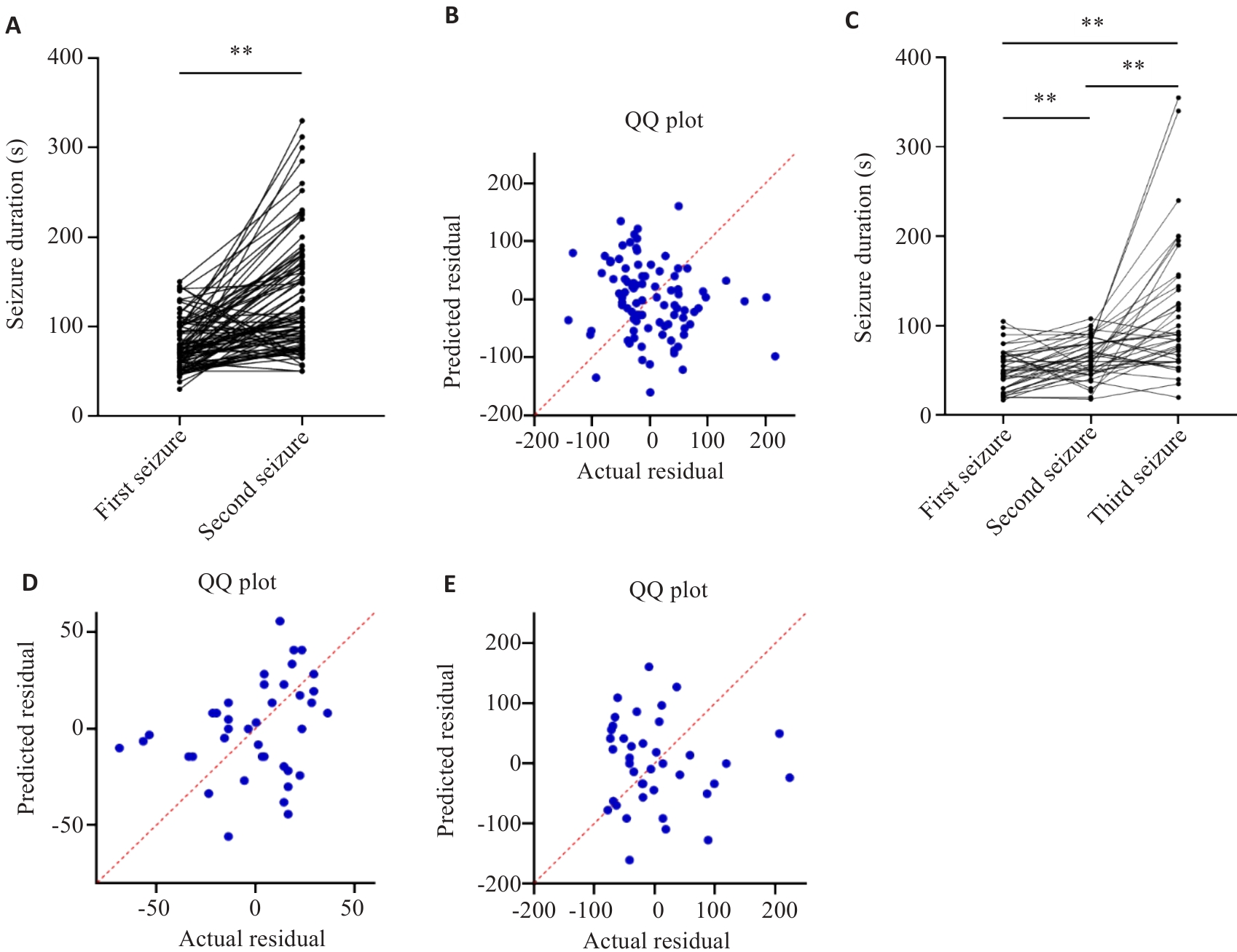
Fig.3 EEG seizure duration analysis. A, B: Correlation between the first and second seizure duration in E2 group. C-E: Correlation between the first, second and third seizure duration in E3 group. **P<0.01.
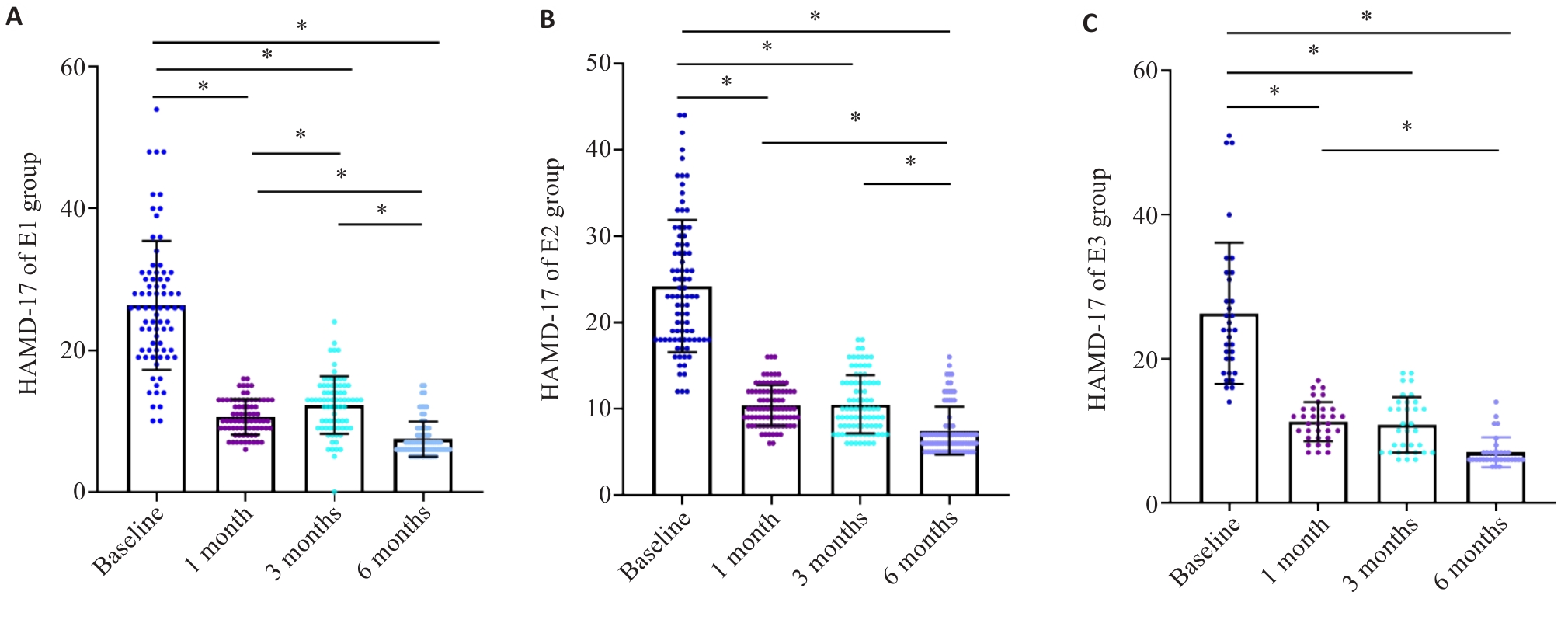
Fig.4 Response to super ECT assessed according to HAMD-17 scores relative to the baseline during the initial 6 months. A: HAMD-17 scores in E1 group. B: HAMD-17 scores in E2 group. C: HAMD-17 scores in E3 group. *P<0.05.
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
Total response 1 month 3 months 6 months | 74 (84.09) 72 (81.82) 74 (84.09) | 84 (88.76) 81 (79.78) 76 (76.40) | 31 (79.48) 31 (79.48) 32 (82.05) | 0.634 0.863 0.790 |
Recovered 1 month 3 months 6 months | 5 (5.68) 5 (5,68) 30 (34.09) | 6 (6.74) 9 (10.11) 28 (31.46) | 2 (5.13) 4 (10.26) 10 (25.64) | 0.781 0.632 0.459 |
Significant improved 1 month 3 months 6 months | 56 (63.64) 40 (45.45) 42 (47.73) | 52 (58.43) 43 (48.31) 30 (33.71) | 23 (58.97) 18 (46.15) 20 (51.28) | 0.702 0.693 0.764 |
Improved 1 month 3 months 6 months | 13 (14.77) 27 (30.68) 2 (2.27) | 21 (23.59) 19 (21.35) 10 (11.24) | 6 (15.38) 9 (23.08) 2 (5.13) | 0.603 0.534 0.357 |
Ineffective 1 month 3 months 6 months | 2 (2.27) 4 (4.55) 2 (2.27) | 2 (2.25) 5 (5.62) 1 (1.12) | 2 (5.13) 2 (5.13) 1 (2.56) | 0.913 0.893 0.796 |
Tab.3 Treatment response based on the reduction rate of HAMD-17 scores in 1 month, 3 months, and 6 months [n(%)]
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
Total response 1 month 3 months 6 months | 74 (84.09) 72 (81.82) 74 (84.09) | 84 (88.76) 81 (79.78) 76 (76.40) | 31 (79.48) 31 (79.48) 32 (82.05) | 0.634 0.863 0.790 |
Recovered 1 month 3 months 6 months | 5 (5.68) 5 (5,68) 30 (34.09) | 6 (6.74) 9 (10.11) 28 (31.46) | 2 (5.13) 4 (10.26) 10 (25.64) | 0.781 0.632 0.459 |
Significant improved 1 month 3 months 6 months | 56 (63.64) 40 (45.45) 42 (47.73) | 52 (58.43) 43 (48.31) 30 (33.71) | 23 (58.97) 18 (46.15) 20 (51.28) | 0.702 0.693 0.764 |
Improved 1 month 3 months 6 months | 13 (14.77) 27 (30.68) 2 (2.27) | 21 (23.59) 19 (21.35) 10 (11.24) | 6 (15.38) 9 (23.08) 2 (5.13) | 0.603 0.534 0.357 |
Ineffective 1 month 3 months 6 months | 2 (2.27) 4 (4.55) 2 (2.27) | 2 (2.25) 5 (5.62) 1 (1.12) | 2 (5.13) 2 (5.13) 1 (2.56) | 0.913 0.893 0.796 |
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
Side effects [n (%)] Fever Headache/dizziness General pain Dry mouth Nausea | 13 (14.77) 27 (30.68) 18 (20.45) 20 (22.73) 18 (20.45) | 21 (23.59) 28 (31.46) 9 (10.11) 17 (19.10) 19 (21.35) | 6 (15.38) 17 (43.58) 12 (30.76) 4 (10.26) 9 (23.08) | 0.764 0.213 0.179 0.452 0.812 |
Cognitive function assessment (Mean±SD) MMSE in pre-treatment MMSE in post-treatment | 27.13±1.24 27.3±3.79 | 26.83±2.03 26.76±2.99 | 27.41±2.15 26.85±3.73 | 0.559 0.794 |
Tab.4 Evaluation of the side effects of the treatment and cognitive function of the patients
| Variables | E1 (n=88) | E2 (n=89) | E3 (n=39) | P |
|---|---|---|---|---|
Side effects [n (%)] Fever Headache/dizziness General pain Dry mouth Nausea | 13 (14.77) 27 (30.68) 18 (20.45) 20 (22.73) 18 (20.45) | 21 (23.59) 28 (31.46) 9 (10.11) 17 (19.10) 19 (21.35) | 6 (15.38) 17 (43.58) 12 (30.76) 4 (10.26) 9 (23.08) | 0.764 0.213 0.179 0.452 0.812 |
Cognitive function assessment (Mean±SD) MMSE in pre-treatment MMSE in post-treatment | 27.13±1.24 27.3±3.79 | 26.83±2.03 26.76±2.99 | 27.41±2.15 26.85±3.73 | 0.559 0.794 |
| [1] | Friedrich MJ. Depression is the leading cause of disability around the world[J]. JAMA, 2017, 317(15): 1517. doi:10.1001/jama.2017.3826 |
| [2] | Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report[J]. Am J Psychiatry, 2006, 163(11): 1905-17. doi:10.1176/appi.ajp.163.11.1905 |
| [3] | Khin NA, Chen YF, Yang Y, et al. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications[J]. J Clin Psychiatry, 2011, 72(4): 464-72. doi:10.4088/jcp.10m06191 |
| [4] | Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE)[J]. Arch Gen Psychiatry, 2006, 63(12): 1337-44. doi:10.1001/archpsyc.63.12.1337 |
| [5] | Weiss A, Hussain S, Ng B, et al. Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy[J]. Aust N Z J Psychiatry, 2019, 53(7): 609-23. doi:10.1177/0004867419839139 |
| [6] | Espinoza RT, Kellner CH. Electroconvulsive therapy[J]. N Engl J Med, 2022, 386(7): 667-72. doi:10.1056/nejmra2034954 |
| [7] | Liu CC, Qian XY, An JX, et al. Electroconvulsive therapy under general anesthesia with cisatracurium, laryngeal mask airways, and bispectral index[J]. J ECT, 2016, 32(1): 17-9. doi:10.1097/yct.0000000000000251 |
| [8] | McClintock SM, Choi J, Deng ZD, et al. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy[J]. J ECT, 2014, 30(2): 165-76. doi:10.1097/yct.0000000000000137 |
| [9] | van Duist M, Spaans HP, Verwijk E, et al. ECT non-remitters: prognosis and treatment after 12 unilateral electroconvulsive therapy sessions for major depression[J]. J Affect Disord, 2020, 272: 501-7. doi:10.1016/j.jad.2020.03.134 |
| [10] | Pennings CH, Van Boxtel M, De Korte-De Boer D, et al. Anaesthesia as a risk factor for long-term cognitive decline: Results of the prospective MAAS cohort study[J]. Eur J Anaesthesiol, 2025, 42(5): 468-77. doi:10.1097/eja.0000000000002133 |
| [11] | Dandekar MP, Diaz AP, Rahman Z, et al. A narrative review on invasive brain stimulation for treatment-resistant depression[J]. Braz J Psychiatry, 2022, 44(3): 317-30. doi:10.1590/1516-4446-2021-1874 |
| [12] | Bewernick B, Schlaepfer TE. Update on neuromodulation for treatment-resistant depression[J]. F1000Research, 2015, 4: 1389. doi:10.12688/f1000research.6633.1 |
| [13] | Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage[J]. J ECT, 2010, 26(3): 159-74. doi:10.1097/yct.0b013e3181e48165 |
| [14] | Bakewell CJ, Russo J, Tanner C, et al. Comparison of clinical efficacy and side effects for bitemporal and bifrontal electrode placement in electroconvulsive therapy[J]. J ECT, 2004, 20(3): 145-53. doi:10.1097/00124509-200409000-00005 |
| [15] | Tor PC, Bautovich A, Wang MJ, et al. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression[J]. J Clin Psychiatry, 2015, 76(9): e1092-8. doi:10.4088/jcp.14r09145 |
| [16] | Luccarelli J, McCoy TH Jr, Seiner SJ, et al. Changes in seizure duration during acute course electroconvulsive therapy[J]. Brain Stimul, 2021, 14(4): 941-6. doi:10.1016/j.brs.2021.05.016 |
| [17] | McCabe GA, Smith MM, Widiger TA. Psychopathy and antisocial personality disorder in the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders: an attempted replication of Wygantet al. (2016)[J]. Pers Disord Theory Res Treat, 2023, 14(6): 636-48. doi:10.1037/per0000626 |
| [18] | Han CS, Wang G, Chan S, et al. Definition and identification of patients with treatment-resistant depression in real-world clinical practice settings across Asia[J]. Neuropsychiatr Dis Treat, 2020, 16: 2929-41. doi:10.2147/ndt.s264799 |
| [19] | Kronsell A, Nordenskjöld A, Bell M, et al. The effect of anaesthetic dose on response and remission in electroconvulsive therapy for major depressive disorder: nationwide register-based cohort study[J]. BJPsych Open, 2021, 7(2): e71. doi:10.1192/bjo.2021.31 |
| [20] | Weinger MB, Partridge BL, Hauger R, et al. Prevention of the cardiovascular and neuroendocrine response to electroconvulsive therapy: I. Effectiveness of pretreatment regimens on hemodynamics[J]. Anesth Analg, 1991, 73(5): 556-62. doi:10.1213/00000539-199111000-00008 |
| [21] | Maixner DF, Weiner R, Reti IM, et al. Electroconvulsive therapy is an essential procedure[J]. Am J Psychiatry, 2021, 178(5): 381-2. doi:10.1176/appi.ajp.2020.20111647 |
| [22] | Pluijms EM, Birkenhäger TK, Huijbrechts IPAM, et al. Influence of resistance to antidepressant pharmacotherapy on short-term response to electroconvulsive therapy[J]. J Affect Disord, 2002, 69(1/2/3): 93-9. doi:10.1016/s0165-0327(00)00378-5 |
| [23] | Kho KH, Zwinderman AH, Blansjaar BA. Predictors for the efficacy of electroconvulsive therapy: chart review of a naturalistic study[J]. J Clin Psychiatry, 2005, 66(7): 894-9. doi:10.4088/jcp.v66n0712 |
| [24] | Goldfarb S, Fainstein N, Ganz T, et al. Electric neurostimulation regulates microglial activation via retinoic acid receptor α signaling[J]. Brain Behav Immun, 2021, 96: 40-53. doi:10.1016/j.bbi.2021.05.007 |
| [25] | Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial[J]. JAMA, 2001, 285(10): 1299-307. doi:10.1001/jama.285.10.1299 |
| [26] | Fink M. What is an adequate treatment in convulsive therapy?[J]. Acta Psychiatr Scand, 1991, 84(5): 424-7. doi:10.1111/j.1600-0447.1991.tb03172.x |
| [27] | Brus O, Cao Y, Gustafsson E, et al. Self-assessed remission rates after electroconvulsive therapy of depressive disorders[J]. Eur Psychiatry, 2017, 45: 154-60. doi:10.1016/j.eurpsy.2017.06.015 |
| [28] | Haas S, Nash K, Lippmann SB. ECT-induced seizure durations[J]. J Ky Med Assoc, 1996, 94(6): 233-6. |
| [29] | Kales H, Raz J, Tandon R, et al. Relationship of seizure duration to antidepressant efficacy in electroconvulsive therapy[J]. Psychol Med, 1997, 27(6): 1373-80. doi:10.1017/s0033291797005564 |
| [30] | Nishikawa K, Yamakage M. Effects of the concurrent use of a reduced dose of propofol with divided supplemental remifentanil and moderate hyperventilation on duration and morphology of electroconvulsive therapy-induced electroencephalographic seizure activity: a randomized controlled trial[J]. J Clin Anesth, 2017, 37: 63-8. doi:10.1016/j.jclinane.2016.11.006 |
| [31] | O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report[J]. Am J Geriatr Psychiatry, 2001, 9(4): 382-90. doi:10.1176/appi.ajgp.9.4.382 |
| [32] | Zolezzi M. Medication management during electroconvulsant therapy[J]. Neuropsychiatr Dis Treat, 2016, 12: 931-9. doi:10.2147/ndt.s100908 |
| [33] | Radman T, Lisanby SH. New directions in the rational design of electrical and magnetic seizure therapies: individualized Low Amplitude Seizure Therapy (iLAST) and Magnetic Seizure Therapy (MST)[J]. Int Rev Psychiatry, 2017, 29(2): 63-78. doi:10.1080/09540261.2017.1304898 |
| [34] | Phutane VH, Thirthalli J, Muralidharan K, et al. Double-blind randomized controlled study showing symptomatic and cognitive superiority of bifrontal over bitemporal electrode placement during electroconvulsive therapy for schizophrenia[J]. Brain Stimul, 2013, 6(2): 210-7. doi:10.1016/j.brs.2012.04.002 |
| [35] | Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study[J]. Psychol Med, 2016, 46(3): 623-35. doi:10.1017/s0033291715002159 |
| [36] | Phillips JL, Norris S, Talbot J, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial[J]. Am J Psychiatry, 2019, 176(5): 401-9. doi:10.1176/appi.ajp.2018.18070834 |
| [37] | Erdil F, Ozgul U, Çolak C, et al. Effect of the addition of ketamine to sevoflurane anesthesia on seizure duration in electroconvulsive therapy[J]. J ECT, 2015, 31(3): 182-5. doi:10.1097/yct.0000000000000225 |
| [38] | Kranaster L, Kammerer-Ciernioch J, Hoyer C, et al. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study[J]. Eur Arch Psychiatry Clin Neurosci, 2011, 261(8): 575-82. doi:10.1007/s00406-011-0205-7 |
| [39] | Menon V, Varadharajan N, Faheem A, et al. Ketamine vs electroconvulsive therapy for major depressive episode: a systematic review and meta-analysis[J]. JAMA Psychiatry, 2023, 80(6): 639-42. doi:10.1001/jamapsychiatry.2023.0562 |
| [40] | Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis[J]. JAMA Psychiatry, 2022, 79(12): 1162-72. doi:10.1001/jamapsychiatry.2022.3352 |
| [41] | Read J, Harrop C, Geekie J. Time to acknowledge the bias of some electroconvulsive therapy researchers and defenders[J]. Lancet Psychiatry, 2022, 9(2): e9. doi:10.1016/s2215-0366(21)00506-x |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||